Optical Coherence Tomography Angiography in Retinal Vascular Disorders
Abstract
:1. Introduction
2. OCTA versus Traditional Angiography
3. Limitations and Artifacts in OCTA Imaging
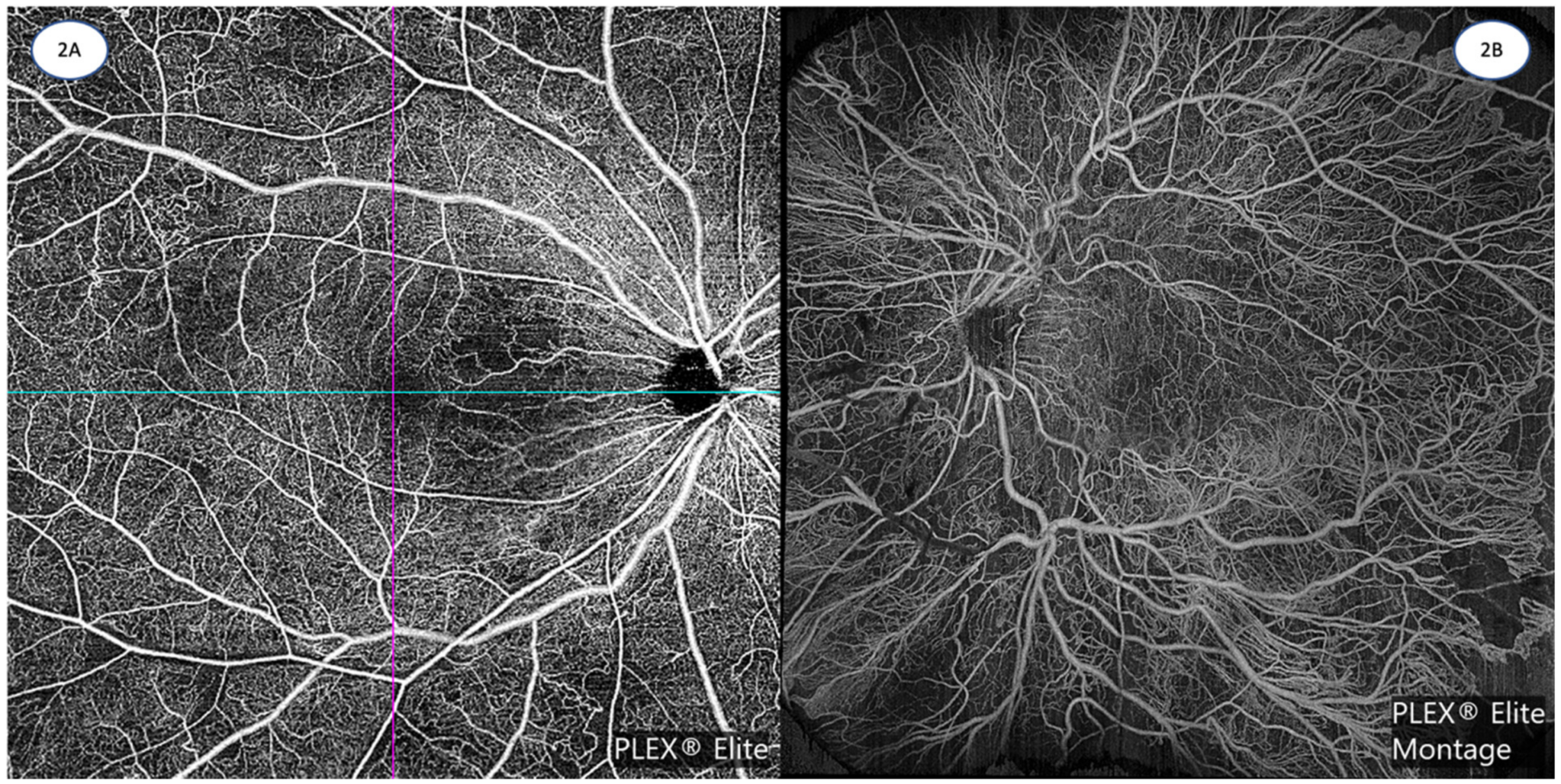
4. Standardization of Reporting of OCTA Findings
5. Diabetic Retinopathy (DR)
5.1. Pre-Clinical DR on OCTA
5.2. Clinical DR Lesions on OCTA
5.3. Diabetic Macular Ischemia on OCTA
5.4. Non-Perfusion in OCTA
5.5. OCTA Changes following Treatment in DR
6. Retinal Vein Occlusion (RVO)
6.1. Correlation between OCTA and FA in RVO
6.2. Structure-Function Correlation in RVO
6.3. OCTA Changes following Treatment in RVO
7. Retinal Artery Occlusion (RAO)
8. Artificial Intelligence in OCTA
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, S.S.; Jia, Y.; Zhang, M.; Su, J.P.; Liu, G.; Hwang, T.S.; Bailey, S.T.; Huang, D. Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT27–OCT36. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Tan, A.C.S.; Cheung, C.M.G.; Keane, P.A.; Dolz-Marco, R.; Sng, C.C.A.; Schmetterer, L. Optical coherence tomography angiography: A review of current and future clinical applications. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.S.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An overview of the clinical applications of optical coherence tomography angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, D.R.; Yi, J.J.; De Koo, L.O.; Ameri, H.; Puliafito, C.A.; Kashani, A.H. Optical Coherence Tomography Angiography of Diabetic Retinopathy in Human Subjects. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 796–805. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Van Velthoven, M.E.; Verbraak, F.D.; Yannuzzi, L.A.; Rosen, R.B.; Podoleanu, A.G.; de Smet, M.D. Imaging the retina by en face optical coherence tomography. Retina 2006, 26, 129–136. [Google Scholar] [CrossRef]
- Maloca, P.M.; Feu-Basilio, S.; Schottenhamml, J.; Valmaggia, P.; Scholl, H.P.N.; Rosinés-Fonoll, J.; Marin-Martinez, S.; Inglin, N.; Reich, M.; Lange, C.; et al. Reference database of total retinal vessel surface area derived from volume-rendered optical coherence tomography angiography. Sci. Rep. 2022, 12, 3695. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J.; Yannuzzi, L.A.; Balaratnasingam, C.; Dansingani, K.K.; Suzuki, M. Volume-Rendering Optical Coherence Tomography Angiography of Macular Telangiectasia Type 2. Ophthalmology 2015, 122, 2261–2269. [Google Scholar] [CrossRef]
- Kaizu, Y.; Nakao, S.; Wada, I.; Arima, M.; Yamaguchi, M.; Ishikawa, K.; Akiyama, M.; Kishimoto, J.; Hisatomi, T.; Sonoda, K.-H. Microaneurysm Imaging Using Multiple En Face OCT Angiography Image Averaging. Ophthalmol. Retin. 2020, 4, 175–186. [Google Scholar] [CrossRef]
- Parravano, M.; De Geronimo, D.; Scarinci, F.; Querques, L.; Virgili, G.; Simonett, J.M.; Varano, M.; Bandello, F.; Querques, G. Diabetic Microaneurysms Internal Reflectivity on Spectral-Domain Optical Coherence Tomography and Optical Coherence Tomography Angiography Detection. Am. J. Ophthalmol. 2017, 179, 90–96. [Google Scholar] [CrossRef]
- Soares, M.; Neves, C.; Marques, I.P.; Pires, I.; Schwartz, C.; Costa, M.; Santos, T.; Durbin, M.; Cunha-Vaz, J. Comparison of diabetic retinopathy classification using fluorescein angiography and optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 62–68. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Bonini Filho, M.A.; Chin, A.T.; Adhi, M.; Ferrara, D.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Duker, J.S.; Waheed, N.K. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 2015, 122, 1228–1238. [Google Scholar] [CrossRef]
- Hwang, T.S.; Jia, Y.; Gao, S.S.; Bailey, S.T.; Lauer, A.K.; Flaxel, C.J.; Wilson, D.J.; Huang, D. Optical Coherence Tomography Angiography Features of Diabetic Retinopathy. Retina 2015, 35, 2371–2376. [Google Scholar] [CrossRef]
- Liu, L.; Gao, S.S.; Bailey, S.T.; Huang, D.; Li, D.; Jia, Y. Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomed. Opt. Express 2015, 6, 3564–3576. [Google Scholar] [CrossRef]
- Zeimer, M.; Gutfleisch, M.; Heimes, B.; Spital, G.; Lommatzsch, A.; Pauleikhoff, D. Association between Changes in Macular Vasculature in Optical Coherence Tomography- and Fluorescein- Angiography and Distribution of Macular Pigment in Type 2 Idiopathic Macular Telangiectasia. Retina 2015, 35, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Shahlaee, A.; Adam, M.K.; Khan, M.A.; Chiang, A.; Maguire, J.I.; Hsu, J.; Ho, A.C. Quantification of Diabetic Macular Ischemia Using Optical Coherence Tomography Angiography and Its Relationship with Visual Acuity. Ophthalmology 2017, 124, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, C.; Carnevali, A.; Cicinelli, M.V.; Querques, L.; Querques, G.; Bandello, F. Optical Coherence Tomography Angiography of Venous Loops in Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA 2015, 112, E2395–E2402. [Google Scholar] [CrossRef] [PubMed]
- Bonini Filho, M.A.; Adhi, M.; de Carlo, T.E.; Ferrara, D.; Baumal, C.R.; Witkin, A.J.; Reichel, E.; Kuehlewein, L.; Sadda, S.R.; Sarraf, D.; et al. Optical Coherence Tomography Angiography in Retinal Artery Occlusion. Retina 2015, 35, 2339–2346. [Google Scholar] [CrossRef]
- Nobre Cardoso, J.; Keane, P.A.; Sim, D.A.; Bradley, P.; Agrawal, R.; Addison, P.K.; Egan, C.; Tufail, A. Systematic Evaluation of Optical Coherence Tomography Angiography in Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 163, 93–107.e6. [Google Scholar] [CrossRef]
- Keane, P.A.; Sadda, S.R. Retinal imaging in the twenty-first century: State of the art and future directions. Ophthalmology 2014, 121, 2489–2500. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse reactions due to indocyanine green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef] [PubMed]
- López-Sáez, M.P.; Ordoqui, E.; Tornero, P.; Baeza, A.; Sainza, T.; Zubeldia, J.M.; Baeza, M.L. Fluorescein-induced allergic reaction. Ann. Allergy Asthma. Immunol. 1998, 81, 428–430. [Google Scholar] [CrossRef]
- Mendis, K.R.; Balaratnasingam, C.; Yu, P.; Barry, C.J.; McAllister, I.L.; Cringle, S.J.; Yu, D.Y. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5864–5869. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina by Optical Coherence Tomography Angiography Instruments in Normal Eyes. JAMA Ophthalmol. 2017, 135, 259–262. [Google Scholar] [CrossRef]
- La Mantia, A.; Kurt, R.A.; Mejor, S.; Egan, C.A.; Tufail, A.; Keane, P.A.; Sim, D.A. Comparing Fundus Fluorescein Angiography and Swept-Source Optical Coherence Tomography Angiography in the Evaluation of Diabetic Macular Perfusion. Retina 2019, 39, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.A.; Keane, P.A.; Rajendram, R.; Karampelas, M.; Selvam, S.; Powner, M.B.; Fruttiger, M.; Tufail, A.; Egan, C.A. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am. J. Ophthalmol. 2014, 158, 144–153.e1. [Google Scholar] [CrossRef]
- Choudhry, N.; Duker, J.S.; Freund, K.B.; Kiss, S.; Querques, G.; Rosen, R.; Sarraf, D.; Souied, E.H.; Stanga, P.E.; Staurenghi, G.; et al. Classification and Guidelines for Widefield Imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol. Retin. 2019, 3, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.M.; Silva, P.S.; Liu, D.; Aiello, L.P.; Antoszyk, A.; Elman, M.; Friedman, S.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; et al. Association of Predominantly Peripheral Lesions on Ultra-Widefield Imaging and the Risk of Diabetic Retinopathy Worsening Over Time. JAMA Ophthalmol. 2022, 140, 946–954. [Google Scholar] [CrossRef]
- Silva, P.S.; Marcus, D.M.; Liu, D.; Aiello, L.P.; Antoszyk, A.; Elman, M.; Friedman, S.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; et al. Association of Ultra-Widefield Fluorescein Angiography-Identified Retinal Nonperfusion and the Risk of Diabetic Retinopathy Worsening Over Time. JAMA Ophthalmol. 2022, 140, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Tsui, I.; Kaines, A.; Havunjian, M.A.; Hubschman, S.; Heilweil, G.; Prasad, P.S.; Oliver, S.C.N.; Yu, F.; Bitrian, E.; Hubschman, J.-P.; et al. Ischemic index and neovascularization in central retinal vein occlusion. Retina 2011, 31, 105–110. [Google Scholar] [CrossRef]
- Singer, M.; Tan, C.S.; Bell, D.; Sadda, S.R. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina 2014, 34, 1736–1742. [Google Scholar] [CrossRef]
- Tan, C.S.; Li, K.Z.; Sadda, S.R. Wide-field angiography in retinal vein occlusions. Int. J. Retin. Vitr. 2019, 5, 18. [Google Scholar] [CrossRef]
- Poddar, R.; Migacz, J.V.; Schwartz, D.M.; Werner, J.S.; Gorczynska, I. Challenges and advantages in wide-field optical coherence tomography angiography imaging of the human retinal and choroidal vasculature at 1.7-MHz A-scan rate. J. Biomed. Opt. 2017, 22, 1–14. [Google Scholar] [PubMed]
- Choi, W.; Moult, E.M.; Waheed, N.K.; Adhi, M.; Lee, B.; Lu, C.D.; de Carlo, T.E.; Jayaraman, V.; Rosenfeld, P.J.; Duker, J.S.; et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology 2015, 122, 2532–2544. [Google Scholar] [CrossRef]
- Wang, X.N.; Cai, X.; Li, S.W.; Li, T.; Long, D.; Wu, Q. Wide-field swept-source OCTA in the assessment of retinal microvasculature in early-stage diabetic retinopathy. BMC Ophthalmol. 2022, 22, 473. [Google Scholar] [CrossRef] [PubMed]
- Couturier, A.; Rey, P.-A.; Erginay, A.; Lavia, C.; Bonnin, S.; Dupas, B.; Gaudric, A.; Tadayoni, R. Widefield OCT-Angiography and Fluorescein Angiography Assessments of Nonperfusion in Diabetic Retinopathy and Edema Treated with Anti–Vascular Endothelial Growth Factor. Ophthalmology 2019, 126, 1685–1694. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Wang, E.; Xia, S.; Chen, Y. Ultra-wide field swept-source optical coherence tomography angiography in patients with diabetes without clinically detectable retinopathy. BMC Ophthalmol. 2021, 21, 192. [Google Scholar] [CrossRef]
- Suzuki, N.; Hirano, Y.; Tomiyasu, T.; Esaki, Y.; Uemura, A.; Yasukawa, T.; Yoshida, M.; Ogura, Y. Retinal Hemodynamics Seen on Optical Coherence Tomography Angiography Before and After Treatment of Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5681. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Al-Sheikh, M.; Akil, H.; Sadda, S.R. Image artefacts in swept-source optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 564–568. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image Artifacts in Optical Coherence Tomography Angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Chen, C.L.; Wang, R.K. Methods and algorithms for optical coherence tomography-based angiography: A review and comparison. J. Biomed. Opt. 2015, 20, 100901. [Google Scholar] [CrossRef]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef]
- White, B.; Pierce, M.; Nassif, N.; Cense, B.; Park, B.; Tearney, G.; Bouma, B.; Chen, T.; de Boer, J. In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical coherence tomography. Opt. Express 2003, 11, 3490–3497. [Google Scholar] [CrossRef]
- Kraus, M.F.; Liu, J.J.; Schottenhamml, J.; Chen, C.L.; Budai, A.; Branchini, L.; Ko, T.; Ishikawa, H.; Wollstein, G.; Schuman, J.; et al. Quantitative 3D-OCT motion correction with tilt and illumination correction, robust similarity measure and regularization. Biomed. Opt. Express 2014, 5, 2591–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Y.; Zhang, T.; Kubach, S.; An, L.; Laron, M.; Sharma, U.; Wang, R.K. Wide-field imaging of retinal vasculature using optical coherence tomography-based microangiography provided by motion tracking. J. Biomed. Opt. 2015, 20, 066008. [Google Scholar] [CrossRef]
- Camino, A.; Zhang, M.; Gao, S.S.; Hwang, T.S.; Sharma, U.; Wilson, D.J.; Huang, D.; Jia, Y. Evaluation of artifact reduction in optical coherence tomography angiography with real-time tracking and motion correction technology. Biomed. Opt. Express 2016, 7, 3905–3915. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Q.; Wang, R.K. Efficient method to suppress artifacts caused by tissue hyper-reflections in optical microangiography of retina in vivo. Biomed. Opt. Express 2015, 6, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Dansingani, K.K.; Tan, A.C.S.; Gilani, F.; Phasukkijwatana, N.; Novais, E.; Querques, L.; Waheed, N.K.; Duker, J.S.; Querques, G.; Yannuzzi, L.A.; et al. Subretinal Hyperreflective Material Imaged With Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 169, 235–248. [Google Scholar] [CrossRef]
- Hormel, T.T.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Huang, D.; Jia, Y. Artificial intelligence in OCT angiography. Prog. Retin. Eye Res. 2021, 85, 100965. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, H.; Wahle, A.; Abràmoff, M.D.; Sonka, M. Multi-Layer 3D Simultaneous Retinal OCT Layer Segmentation: Just-Enough Interaction for Routine Clinical Use; Tavares, J.M.R.S., Natal Jorge, R.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 862–871. [Google Scholar]
- Corvi, F.; Pellegrini, M.; Erba, S.; Cozzi, M.; Staurenghi, G.; Giani, A. Reproducibility of Vessel Density, Fractal Dimension, and Foveal Avascular Zone Using 7 Different Optical Coherence Tomography Angiography Devices. Am. J. Ophthalmol. 2018, 186, 25–31. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Jia, Y.; Wang, J.; Patel, R.C.; Lauer, A.K.; Huang, D.; Bailey, S.T. Projection-resolved optical coherence tomography angiography exhibiting early flow prior to clinically observed retinal angiomatous proliferation. Am. J. Ophthalmol. Case Rep. 2017, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.W.; Nesper, P.L.; Jampol, L.M.; Mirza, R.G. Solitary retinal hemangioblastoma findings in OCTA pre- and post-laser therapy. Am. J. Ophthalmol. Case Rep. 2018, 10, 59–61. [Google Scholar] [CrossRef]
- Dansingani, K.K.; Inoue, M.; Engelbert, M.; Freund, K.B. Optical coherence tomographic angiography shows reduced deep capillary flow in paracentral acute middle maculopathy. Eye 2015, 29, 1620–1624. [Google Scholar] [CrossRef]
- Rochepeau, C.; Kodjikian, L.; Garcia, M.-A.; Coulon, C.; Burillon, C.; Denis, P.; Delaunay, B.; Mathis, T. Optical Coherence Tomography Angiography Quantitative Assessment of Choriocapillaris Blood Flow in Central Serous Chorioretinopathy. Am. J. Ophthalmol. 2018, 194, 26–34. [Google Scholar] [CrossRef]
- You, Q.S.; Chan, J.C.H.; Ng, A.L.K.; Choy, B.K.N.; Shih, K.C.; Cheung, J.J.C.; Wong, J.K.W.; Shum, J.W.H.; Ni, M.Y.; Lai, J.S.M.; et al. Macular Vessel Density Measured With Optical Coherence Tomography Angiography and Its Associations in a Large Population-Based Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4830–4837. [Google Scholar] [CrossRef]
- Tan, B.; Chua, J.; Lin, E.; Cheng, J.; Gan, A.; Yao, X.; Wong, D.W.K.; Sabanayagam, C.; Wong, D.; Chan, C.M.; et al. Quantitative Microvascular Analysis With Wide-Field Optical Coherence Tomography Angiography in Eyes With Diabetic Retinopathy. JAMA Netw. Open 2020, 3, e1919469. [Google Scholar] [CrossRef] [PubMed]
- Kaizu, Y.; Nakao, S.; Arima, M.; Hayami, T.; Wada, I.; Yamaguchi, M.; Sekiryu, H.; Ishikawa, K.; Ikeda, Y.; Sonoda, K.-H. Flow Density in Optical Coherence Tomography Angiography is Useful for Retinopathy Diagnosis in Diabetic Patients. Sci. Rep. 2019, 9, 8668. [Google Scholar] [CrossRef]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.-F.; Wolf, S.; Pichi, F.; Tian, M. Standardization of OCT Angiography Nomenclature in Retinal Vascular Diseases: First Survey Results. Ophthalmol. Retin. 2021, 5, 981–990. [Google Scholar] [CrossRef]
- Munk, M.R.; Kashani, A.H.; Tadayoni, R.; Korobelnik, J.-F.; Wolf, S.; Pichi, F.; Koh, A.; Ishibazawa, A.; Gaudric, A.; Loewenstein, A.; et al. Recommendations for OCT Angiography Reporting in Retinal Vascular Disease. Ophthalmol. Retin. 2022, 6, 753–761. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Prim. 2016, 2, 16012. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, D.; Tang, Z.; Ng, D.S.; Cheung, C.Y. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye 2021, 35, 149–161. [Google Scholar] [CrossRef]
- Shiihara, H.; Terasaki, H.; Sonoda, S.; Kakiuchi, N.; Shinohara, Y.; Tomita, M.; Sakamoto, T. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci. Rep. 2018, 8, 10143. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Sampani, K.; Clermont, A.; Abu-Qamar, O.; Rhee, J.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Vascular Density of Deep, Intermediate and Superficial Vascular Plexuses Are Differentially Affected by Diabetic Retinopathy Severity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, M.; Roh, Y.J. Evaluation of Foveal and Parafoveal Microvascular Changes Using Optical Coherence Tomography Angiography in Type 2 Diabetes Patients without Clinical Diabetic Retinopathy in South Korea. J. Diabetes Res. 2020, 2020, 6210865. [Google Scholar] [CrossRef]
- Thompson, I.A.; Durrani, A.K.; Patel, S. Optical coherence tomography angiography characteristics in diabetic patients without clinical diabetic retinopathy. Eye 2019, 33, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, D.; Yang, X.; Zou, R.; Zhao, K.; Cheng, D.; Huang, S.; Zhou, T.; Yang, Y.; Chen, F. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 192, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Filho, M.B.; Rebhun, C.B.; Moult, E.M.; Lee, B.; Alibhai, Y.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G.; et al. Analyzing Relative Flow Speeds in Diabetic Retinopathy Using Variable Interscan Time Analysis OCT Angiography. Ophthalmol. Retin. 2021, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Dela Cruz, A.J.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.D.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Wang, R.K.; Puliafito, C.A.; Kashani, A.H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT362. [Google Scholar] [CrossRef]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T.; et al. Determinants of Quantitative Optical Coherence Tomography Angiography Metrics in Patients with Diabetes. Sci. Rep. 2017, 7, 2575. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Kim, S.M.; Bae, Y.H.; Ma, D.J. A Study of the Association Between Retinal Vessel Geometry and Optical Coherence Tomography Angiography Metrics in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 14. [Google Scholar] [CrossRef]
- Suzuki, N.; Hirano, Y.; Yoshida, M.; Tomiyasu, T.; Uemura, A.; Yasukawa, T.; Ogura, Y. Microvascular Abnormalities on Optical Coherence Tomography Angiography in Macular Edema Associated With Branch Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 161, 126–132.e1. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, S.Y.; Yan, Y.; Yang, J.; Zhou, W.; Jonas, J.B.; Wei, W.B. Optical coherence tomography angiography in retinal vein occlusions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1615–1622. [Google Scholar] [CrossRef]
- Glacet-Bernard, A.; Miere, A.; Houmane, B.; Tilleul, J.; Souied, E. NONPERFUSION ASSESSMENT IN RETINAL VEIN OCCLUSION: Comparison Between Ultra-widefield Fluorescein Angiography and Widefield Optical Coherence Tomography Angiography. Retina 2021, 41, 1202–1209. [Google Scholar]
- Chen, L.; Yuan, M.; Sun, L.; Wang, Y.; Chen, Y. Evaluation of microvascular network with optical coherence tomography angiography (OCTA) in branch retinal vein occlusion (BRVO). BMC Ophthalmol. 2020, 20, 154. [Google Scholar] [CrossRef]
- Ouederni, M.; Khalifa, M.B.H.; Sassi, H.; Nefaa, F.; Ayed, O.; Cheour, M. Quantitative Analysis of Microvascular Network with Optical Coherence Tomography Angiography and its Correlation with Visual Acuity in Retinal Vein Occlusion. J. Curr. Ophthalmol. 2021, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.T.; Herman, I.M. Microvascular Modifications in Diabetic Retinopathy. Curr. Diabetes Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yang, D.; Huang, Z.; Zeng, Y.; Wang, J.; Hu, Y.; Zhang, L. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018, 55, 469–477. [Google Scholar] [CrossRef]
- Dimitrova, G.; Chihara, E.; Takahashi, H.; Amano, H.; Okazaki, K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients With Diabetes Without Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 190. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Tomasso, L.; Querques, L.; Zerbini, G.; Scorcia, V.; Bandello, F.; Querques, G. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017, 54, 695–702. [Google Scholar] [CrossRef]
- Simonett, J.M.; Scarinci, F.; Picconi, F.; Giorno, P.; De Geronimo, D.; Di Renzo, A.; Varano, M.; Frontoni, S.; Parravano, M. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017, 95, e751–e755. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Sim, R.; Tan, B.; Wong, D.; Yao, X.; Liu, X.; Ting, D.S.W.; Schmidl, D.; Ang, M.; Garhöfer, G.; et al. Optical Coherence Tomography Angiography in Diabetes and Diabetic Retinopathy. JCM 2020, 9, 1723. [Google Scholar] [CrossRef]
- Nesper, P.L.; Roberts, P.K.; Onishi, A.C.; Chai, H.; Liu, L.; Jampol, L.M.; Fawzi, A.A. Quantifying Microvascular Abnormalities With Increasing Severity of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO307. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, H.; Chu, Z.; Zhang, Q.; Chao, J.R.; Rezaei, K.A.; Wang, R.K. Microvascular Changes in the Choriocapillaris of Diabetic Patients Without Retinopathy Investigated by Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 50. [Google Scholar] [CrossRef]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef]
- Conti, F.F.; Qin, V.L.; Rodrigues, E.B.; Sharma, S.; Rachitskaya, A.V.; Ehlers, J.P.; Singh, R.P. Choriocapillaris and retinal vascular plexus density of diabetic eyes using split-spectrum amplitude decorrelation spectral-domain optical coherence tomography angiography. Br. J. Ophthalmol. 2019, 103, 452–456. [Google Scholar] [CrossRef]
- McLeod, D.S.; Lutty, G.A. High-resolution histologic analysis of the human choroidal vasculature. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3799–3811. [Google Scholar]
- Choi, W.; Waheed, N.K.; Moult, E.M.; Adhi, M.; Lee, B.; De Carlo, T.; Jayaraman, V.; Baumal, C.R.; Duker, J.S.; Fujimoto, J.G. Ultrahigh Speed Swept Source Optical Coherence Tomography Angiography of Retinal And Choriocapillaris Alterations in Diabetic Patients with and Without Retinopathy. Retina 2017, 37, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Fingler, J.; Kim, D.Y.; Zawadzki, R.J.; Morse, L.S.; Park, S.S.; Fraser, S.E.; Werner, J.S. Phase-Variance Optical Coherence Tomography. Ophthalmology 2014, 121, 180–187. [Google Scholar] [CrossRef]
- Miwa, Y.; Murakami, T.; Suzuma, K.; Uji, A.; Yoshitake, S.; Fujimoto, M.; Yoshitake, T.; Tamura, Y.; Yoshimura, N. Relationship between Functional and Structural Changes in Diabetic Vessels in Optical Coherence Tomography Angiography. Sci. Rep. 2016, 6, 29064. [Google Scholar] [CrossRef]
- Nakao, S.; Yoshida, S.; Kaizu, Y.; Yamaguchi, M.; Wada, I.; Ishibashi, T.; Sonoda, K. Microaneurysm Detection in Diabetic Retinopathy Using OCT Angiography May Depend on Intramicroaneurysmal Turbulence. Ophthalmol. Retin. 2018, 2, 1171–1173. [Google Scholar] [CrossRef]
- Archer, D.B. Neovascularization of the retina. Trans. Ophthalmol. Soc. UK (1962) 1976, 96, 471–493. [Google Scholar]
- Arya, M.; Sorour, O.; Chaudhri, J.; Alibhai, Y.; Waheed, N.K.; Duker, J.S.; Baumal, C.R. Distinguishing Intraretinal Microvascular Abnormalities from Retinal Neovascularization Using Optical Coherence Tomography Angiography. Retina 2020, 40, 1686–1695. [Google Scholar] [CrossRef]
- Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991, 98, 807–822. [CrossRef]
- Khalid, H.; Schwartz, R.; Nicholson, L.; Huemer, J.; El-Bradey, M.H.; Sim, D.A.; Patel, P.J.; Balaskas, K.; Hamilton, R.D.; Keane, P.A.; et al. Widefield optical coherence tomography angiography for early detection and objective evaluation of proliferative diabetic retinopathy. Br. J. Ophthalmol. 2021, 105, 118–123. [Google Scholar] [CrossRef]
- Mansour, A.M.; Schachat, A.; Bodiford, G.; Haymond, R. Foveal Avascular Zone in Diabetes Mellitus. Retina 1993, 13, 125–128. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Fawzi, A.; Teo, K.Y.C.; Fukuyama, H.; Sen, S.; Tsai, W.-S.; Sivaprasad, S. Diabetic macular ischaemia- a new therapeutic target? Prog. Retin. Eye Res. 2022, 89, 101033. [Google Scholar] [CrossRef]
- Nicholson, L.; Ramu, J.; Chan, E.W.; Bainbridge, J.W.; Hykin, P.G.; Talks, S.J.; Sivaprasad, S. Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes With Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2019, 137, 626. [Google Scholar] [CrossRef]
- Pellegrini, M.; Cozzi, M.; Staurenghi, G.; Corvi, F. Comparison of wide field optical coherence tomography angiography with extended field imaging and fluorescein angiography in retinal vascular disorders. PLoS ONE 2019, 14, e0214892. [Google Scholar] [CrossRef]
- Kawai, K.; Uji, A.; Miyazawa, T.; Yamada, T.; Amano, Y.; Miyagi, S.; Seo, R.; Miyata, M.; Kadomoto, S.; Tsujikawa, A. Prevention of Image Quality Degradation in Wider Field Optical Coherence Tomography Angiography Images Via Image Averaging. Trans. Vis. Sci. Technol. 2021, 10, 16. [Google Scholar] [CrossRef]
- Amato, A.; Nadin, F.; Borghesan, F.; Cicinelli, M.V.; Chatziralli, I.; Sadiq, S.; Mirza, R.; Bandello, F. Widefield Optical Coherence Tomography Angiography in Diabetic Retinopathy. J. Diabetes Res. 2020, 2020, 8855709. [Google Scholar] [CrossRef]
- Lin, Z.; Deng, A.; Hou, N.; Gao, L.; Zhi, X. Advances in targeted retinal photocoagulation in the treatment of diabetic retinopathy. Front. Endocrinol. 2023, 14, 1108394. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Eichenbaum, D.A.; Roth, D.B.; Hill, L.; Fung, A.E.; Haskova, Z. Ranibizumab Induces Regression of Diabetic Retinopathy in Most Patients at High Risk of Progression to Proliferative Diabetic Retinopathy. Ophthalmol. Retin. 2018, 2, 997–1009. [Google Scholar] [CrossRef]
- Mitchell, P.; McAllister, I.; Larsen, M.; Staurenghi, G.; Korobelnik, J.-F.; Boyer, D.S.; Do, D.V.; Brown, D.M.; Katz, T.A.; Berliner, A.; et al. Evaluating the Impact of Intravitreal Aflibercept on Diabetic Retinopathy Progression in the VIVID-DME and VISTA-DME Studies. Ophthalmol. Retin. 2018, 2, 988–996. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Alam, M.N.; Le, D.; Hsiao, C.-C.; Yang, C.-H.; Chao, D.L.; Yao, X. OCT Angiography Biomarkers for Predicting Visual Outcomes after Ranibizumab Treatment for Diabetic Macular Edema. Ophthalmol. Retin. 2019, 3, 826–834. [Google Scholar] [CrossRef]
- Ghasemi Falavarjani, K.; Iafe, N.A.; Hubschman, J.-P.; Tsui, I.; Sadda, S.R.; Sarraf, D. Optical Coherence Tomography Angiography Analysis of the Foveal Avascular Zone and Macular Vessel Density After Anti-VEGF Therapy in Eyes With Diabetic Macular Edema and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 30. [Google Scholar] [CrossRef]
- Sorour, O.A.; Sabrosa, A.S.; Yasin Alibhai, A.; Arya, M.; Ishibazawa, A.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Waheed, N.K. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int. Ophthalmol. 2019, 39, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.F.; Song, W.; Rodrigues, E.B.; Singh, R.P. Changes in retinal and choriocapillaris density in diabetic patients receiving anti-vascular endothelial growth factor treatment using optical coherence tomography angiography. Int. J. Retin. Vitr. 2019, 5, 41. [Google Scholar] [CrossRef]
- Acar, O.P.A.; Onur, I.U. Effect of panretinal photocoagulation on retina and choroid in diabetic retinopathy: An optical coherence tomography angiography study. Photodiagnosis Photodyn. Ther. 2022, 40, 103166. [Google Scholar] [CrossRef]
- Mirshahi, A.; Ghassemi, F.; Fadakar, K.; Mirshahi, R.; Bazvand, F.; Riazi-Esfahani, H. Effects of panretinal photocoagulation on retinal vasculature and foveal avascular zone in diabetic retinopathy using optical coherence tomography angiography: A pilot study. J. Curr. Ophthalmol. 2019, 31, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, H.; Riazi-Esfahani, H.; Khodabande, A.; Khalili Pour, E.; Mirshahi, A.; Ghassemi, F.; Mirshahi, R.; Khojasteh, H.; Bazvand, F.; Hashemi, A.; et al. Effect of panretinal photocoagulation on macular vasculature using optical coherence tomography angiography. Eur. J. Ophthalmol. 2021, 31, 1877–1884. [Google Scholar] [CrossRef]
- Fawzi, A.A.; Fayed, A.E.; Linsenmeier, R.A.; Gao, J.; Yu, F. Improved Macular Capillary Flow on Optical Coherence Tomography Angiography After Panretinal Photocoagulation for Proliferative Diabetic Retinopathy. Am. J. Ophthalmol. 2019, 206, 217–227. [Google Scholar] [CrossRef]
- Tsai, G.; Banaee, T.; Conti, F.; Singh, R. Optical coherence tomography angiography in eyes with retinal vein occlusion. J. Ophthalmic Vis. Res. 2018, 13, 315–332. [Google Scholar]
- An, W.; Han, J. Research progress of UWFFA and OCTA in retinal vein occlusion: A review. Eur. J. Ophthalmol. 2021, 31, 2850–2855. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Toto, L.; Di Antonio, L.; Borrelli, E.; Senatore, A.; Di Nicola, M.; Di Martino, G.; Ciancaglini, M.; Carpineto, P. Optical coherence tomography angiography microvascular findings in macular edema due to central and branch retinal vein occlusions. Sci. Rep. 2017, 7, 40763. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Shahlaee, A.; Sridhar, J.; Khan, M.A.; Ho, A.C.; Hsu, J. Quantitative Optical Coherence Tomography Angiography Features and Visual Function in Eyes With Branch Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 166, 76–83. [Google Scholar] [CrossRef]
- Coscas, F.; Glacet-Bernard, A.; Miere, A.; Caillaux, V.; Uzzan, J.; Lupidi, M.; Coscas, G.; Souied, E.H. Optical Coherence Tomography Angiography in Retinal Vein Occlusion: Evaluation of Superficial and Deep Capillary Plexa. Am. J. Ophthalmol. 2016, 161, 160–171.e2. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Leila, M.; Bessa, A.S.; Lolah, M.; Abou Shousha, M.; El Hennawi, H.M.; Hafez, T.A. Grading of macular perfusion in retinal vein occlusion using en-face swept-source optical coherence tomography angiography: A retrospective observational case series. BMC Ophthalmol 2019, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, M.; Savastano, M.C.; Lumbroso, B. Capillary Network Anomalies in Branch Retinal Vein Occlusion on Optical Coherence Tomography Angiography. Retina 2015, 35, 2332–2338. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lee, S.Y.; Moshfeghi, A.; Durbin, M.K.; Puliafito, C.A. Optical Coherence Tomography Angiography of Retinal Venous Occlusion. Retina 2015, 35, 2323–2331. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, A.; Sakimoto, S.; Tsuboi, K.; Wakabayashi, T.; Hara, C.; Fukushima, Y.; Sayanagi, K.; Nishida, K.; Sakaguchi, H.; Nishida, K. Evaluation of retinal nonperfusion in branch retinal vein occlusion using wide-field optical coherence tomography angiography. Acta Ophthalmol. 2019, 97, e913–e918. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Muraoka, Y.; Uji, A.; Tamiya, R.; Oritani, Y.; Kawai, K.; Ooto, S.; Murakami, T.; Iida-Miwa, Y.; Tsujikawa, A. Nonperfusion Area Quantification in Branch Retinal Vein Occlusion: A Widefield Optical Coherence Tomography Angiography Study. Retina 2021, 41, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Seknazi, D.; Coscas, F.; Sellam, A.; Rouimi, F.; Coscas, G.; Souied, E.H.; Glacet-Bernard, A. Optical Coherence Tomography Angiography in Retinal Vein Occlusion: Correlations Between Macular Vascular Density, Visual Acuity, and Peripheral Nonperfusion Area on Fluorescein Angiography. Retina 2018, 38, 1562–1570. [Google Scholar] [CrossRef]
- Cavalleri, M.; Sacconi, R.; Parravano, M.; Costanzo, E.; Pezzella, M.; Bandello, F.; Querques, G. Optical Coherence Tomography Angiography in Central Retinal Vein Occlusion: Macular Changes and Their Correlation with Peripheral Nonperfusion at Ultra-Widefield Fluorescein Angiography. Ophthalmologica 2022, 245, 275–284. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Gu, X.; Zheng, B.; Chen, T. Correlation between the Nonperfusion Area on Ultra-Widefield Fluorescein Angiography and Nonflow Area on Optical Coherence Tomographic Angiography in Retinal Vein Occlusion. J. Ophthalmol. 2021, 2021, 5581319. [Google Scholar] [CrossRef]
- Siying, L.; Qiaozhu, Z.; Xinyao, H.; Linqi, Z.; Mingwei, Z.; Jinfeng, Q. Comparison of widefield swept-source optical coherence tomography angiography with ultra-widefield fluorescein angiography for the evaluation of lesions in retinal vein occlusion. BMC Ophthalmol. 2022, 22, 422. [Google Scholar] [CrossRef]
- Kang, J.-W.; Yoo, R.; Jo, Y.H.; Kim, H.C. Correlation of Microvascular Structures on Optical Coherence Tomography Angiography with Visual Acuity in Retinal Vein Occlusion. Retina 2017, 37, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Osaka, R.; Nakano, Y.; Takasago, Y.; Fujita, T.; Shiragami, C.; Hirooka, K.; Muraoka, Y.; Tsujikawa, A. Association Between Parafoveal Capillary Nonperfusion and Macular Function in Eyes With Branch Retinal Vein Occlusion. Retina 2017, 37, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Sellam, A.; Glacet-Bernard, A.; Coscas, F.; Miere, A.; Coscas, G.; Souied, E.H. Qualitative and Quantitative Follow-Up Using Optical Coherence Tomography Angiography of Retinal Vein Occlusion Treated with Anti-Vegf: Optical Coherence Tomography Angiography Follow-up of Retinal Vein Occlusion. Retina 2017, 37, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Marco, R.D.; Goldhardt, R.; Modi, Y. Central Retinal Artery Occlusion: Acute Management and Treatment. Curr. Ophthalmol. Rep. 2017, 5, 149–159. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Li, H.; Xu, J.; Wang, F. Optical coherence tomography angiography characteristics of acute retinal arterial occlusion. BMC Ophthalmol. 2019, 19, 147. [Google Scholar] [CrossRef]
- Baumal, C.R. Optical Coherence Tomography Angiography of Retinal Artery Occlusion. In Developments in Ophthalmology; Bandello, F., Souied, E.H., Querques, G., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 56, pp. 122–131. ISBN 978-3-318-05829-1. [Google Scholar]
- Abdellah, M.M. Multimodal Imaging of Acute Central Retinal Artery Occlusion. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 283–290. [Google Scholar]
- Alam, M.; Zhang, Y.; Lim, J.I.; Chan, R.V.P.; Yang, M.; Yao, X. Quantitative Optical Coherence Tomography Angiography Features for Objective Classification and Staging of Diabetic Retinopathy. Retina 2020, 40, 322–332. [Google Scholar] [CrossRef]
- Ran, A.; Cheung, C.Y. Deep Learning-Based Optical Coherence Tomography and Optical Coherence Tomography Angiography Image Analysis: An Updated Summary. Asia Pac. J. Ophthalmol. 2021, 10, 253–260. [Google Scholar] [CrossRef]
- Heisler, M.; Karst, S.; Lo, J.; Mammo, Z.; Yu, T.; Warner, S.; Maberley, D.; Beg, M.F.; Navajas, E.V.; Sarunic, M.V. Ensemble Deep Learning for Diabetic Retinopathy Detection Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2020, 9, 20. [Google Scholar] [CrossRef]
- Le, D.; Alam, M.; Yao, C.K.; Lim, J.I.; Hsieh, Y.T.; Chan, R.V.P.; Toslak, D.; Yao, X. Transfer Learning for Automated OCTA Detection of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2020, 9, 35. [Google Scholar] [CrossRef]
- Zang, P.; Hormel, T.T.; Hwang, T.S.; Bailey, S.T.; Huang, D.; Jia, Y. Deep-Learning-Aided Diagnosis of Diabetic Retinopathy, Age-Related Macular Degeneration, and Glaucoma Based on Structural and Angiographic OCT. Ophthalmol. Sci. 2023, 3, 100245. [Google Scholar] [CrossRef]
- Nagasato, D.; Tabuchi, H.; Masumoto, H.; Enno, H.; Ishitobi, N.; Kameoka, M.; Niki, M.; Mitamura, Y. Automated detection of a nonperfusion area caused by retinal vein occlusion in optical coherence tomography angiography images using deep learning. PLoS ONE 2019, 14, e0223965. [Google Scholar] [CrossRef]
- Mirshahi, R.; Anvari, P.; Riazi-Esfahani, H.; Sardarinia, M.; Naseripour, M.; Falavarjani, K.G. Foveal avascular zone segmentation in optical coherence tomography angiography images using a deep learning approach. Sci. Rep. 2021, 11, 1031. [Google Scholar] [CrossRef]
- Wang, J.; Hormel, T.T.; You, Q.; Guo, Y.; Wang, X.; Chen, L.; Hwang, T.S.; Jia, Y. Robust non-perfusion area detection in three retinal plexuses using convolutional neural network in OCT angiography. Biomed. Opt. Express 2020, 11, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sun, Z.; Shi, J.; Ran, A.; Tang, F.; Tang, Z.; Lok, J.; Szeto, S.; Chan, J.; Yip, F.; et al. A Multitask Deep-Learning System for Assessment of Diabetic Macular Ischemia on Optical Coherence Tomography Angiography Images. Retina 2022, 42, 184–194. [Google Scholar]
- Jiang, Z.; Huang, Z.; Qiu, B.; Meng, X.; You, Y.; Liu, X.; Geng, M.; Liu, G.; Zhou, C.; Yang, K.; et al. Weakly Supervised Deep Learning-Based Optical Coherence Tomography Angiography. IEEE Trans. Med. Imaging 2021, 40, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bi, L.; Xu, Y.; Feng, D.; Kim, J.; Xu, X. Robust deep learning method for choroidal vessel segmentation on swept source optical coherence tomography images. Biomed. Opt. Express 2019, 10, 1601–1612. [Google Scholar] [CrossRef]
- Gao, M.; Guo, Y.; Hormel, T.T.; Sun, J.; Hwang, T.S.; Jia, Y. Reconstruction of high-resolution 6×6-mm OCT angiograms using deep learning. Biomed. Opt. Express 2020, 11, 3585–3600. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Uji, A.; Muraoka, Y.; Akagi, T.; Tsujikawa, A. Enhanced Visualization of Retinal Microvasculature in Optical Coherence Tomography Angiography Imaging via Deep Learning. J. Clin. Med. 2020, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Jia, Y.; Liu, G.; Wang, J.; Huang, D. Regression-based algorithm for bulk motion subtraction in optical coherence tomography angiography. Biomed. Opt. Express 2017, 8, 3053–3066. [Google Scholar] [CrossRef] [PubMed]
- Camino, A.; Jia, Y.; Yu, J.; Wang, J.; Liu, L.; Huang, D. Automated detection of shadow artifacts in optical coherence tomography angiography. Biomed. Opt. Express 2019, 10, 1514–1531. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Bu, W.; Zheng, Y.; Wu, X. Automated layer segmentation of macular OCT images via graph-based SLIC superpixels and manifold ranking approach. Comput. Med Imaging Graph. 2017, 55, 42–53. [Google Scholar] [CrossRef]
- Guo, Y.; Hormel, T.T.; Xiong, H.; Wang, J.; Hwang, T.S.; Jia, Y. Automated Segmentation of Retinal Fluid Volumes From Structural and Angiographic Optical Coherence Tomography Using Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Liu, X.; Nath, S.; Korot, E.; Faes, L.; Wagner, S.K.; Keane, P.A.; Sebire, N.J.; Burton, M.J.; Denniston, A.K. A global review of publicly available datasets for ophthalmological imaging: Barriers to access, usability, and generalisability. Lancet Digit. Health 2021, 3, e51–e66. [Google Scholar] [CrossRef]
- Lo, J.; Yu, T.T.; Ma, D.; Zang, P.; Owen, J.P.; Zhang, Q.; Wang, R.K.; Beg, M.F.; Lee, A.Y.; Jia, Y.; et al. Federated Learning for Microvasculature Segmentation and Diabetic Retinopathy Classification of OCT Data. Ophthalmol. Sci. 2021, 1, 100069. [Google Scholar] [CrossRef] [PubMed]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The future of digital health with federated learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]


| OCTA System | AngioVue | SS OCT Angio | AngioPlex |
|---|---|---|---|
| Manufacturer | Optovue, Fremont, California, USA | Topcon Corporation, Tokyo, Japan | Carl Zeiss Meditec, Dublin, California, USA |
| OCT platform | RTVue XR AVANTI SD-OCT | DRI Triton SS-OCT | Cirrus 6000 SD-OCT |
| Light source | 840 nm | 1050 nm | 840 nm |
| Algorithm | Split-spectrum amplitude-decorrelation angiography (SSADA) | OCTA-Ratio Analysis (full spectrum amplitude) | Optical microangiography (OMAG) |
| Scanning speed | 70,000 scans/s | 100,000 scans/s | 100,000 scans/s |
| Scan area | 3 × 3, 6 × 6, 8 × 8 mm | 3 × 3, 4.5 × 4.5, 6 × 6, 9 × 9 mm | 3 × 3, 6 × 6, 8 × 8, 12 × 12, 14 × 10 (montage), 14 × 14 (montage) mm |
| Axial optical resolution | 3 microns | 8 microns | 5 microns |
| Lateral optical resolution | 15 microns | 20 microns | 15 microns |
| Axial imaging depth | 2.0–3.0 mm | 2.6 mm | 2.9 mm |
| Diabetic Retinopathy OCTA Abnormalities | |
|---|---|
| Qualitative | Microaneurysms [70,71] Intraretinal microvascular abnormalities (IRMAs) [72] Venous beading and loops [73] Neovascularization at the disc (NVD) and elsewhere (NVE) [15,72] |
| Quantitative | Increased areas of capillary non-perfusion [74] Reduced SCP and DCP vessel density [18,75] Reduced fractal dimension [75] Increased FAZ size [75] Reduced FAZ circularity [76] Reduced SCP and DCP vessel length density [77] Reduced vascular area density [77] Wider venular calibre [77] |
| Retinal Vein Occlusion OCTA Abnormalities | |
| Qualitative [78,79] | Microaneurysms Vessel tortuosity and dilatation Telangiectatic vessels Collateral vessels Optic disc collaterals Neovascularization |
| Quantitative | Increased areas of capillary non-perfusion, [80] Reduced SCP and DCP vessel density [79,81] Reduced fractal dimension [82] Increased FAZ size [79,81] Reduced FAZ circularity [81] Increased lacunarity [82] |
| Disease Entity | Key OCTA Insights |
|---|---|
| Diabetic Retinopathy | Provides quantitative, depth resolved vascular and foveal avascular zone parameters that allow assessment of different capillary plexuses Derivation of novel metrics from vascular parameters Detection of microvasculature abnormalities before the onset of visible clinical signs Allows further quantification and characterization of diabetic macular ischaemia as well as known diabetic retinopathy signs Allows better differentiation between intra-retinal microvascular abnormalities and neovascularisation Quantification of retinal non-perfusion that could potentially guide treatment and may be more reliable than fluorescein angiogram Determine impact of treatment on perfusion status |
| Retinal Vein Occlusion | Provides quantitative, depth resolved vascular and foveal avascular zone parameters that allow assessment of different capillary plexuses Derivation of novel metrics from vascular parameters Quantification of retinal non-perfusion that may guide further management Determine impact of treatment on perfusion status |
| Retinal Artery Occlusion | Provides quantitative, depth resolved vascular and foveal avascular zone parameter that allows assessment of different capillary plexuses Derivation of novel metrics from vascular parameters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, C.J.T.; Wong, M.Y.Z.; Cheong, K.X.; Zhao, J.; Teo, K.Y.C.; Tan, T.-E. Optical Coherence Tomography Angiography in Retinal Vascular Disorders. Diagnostics 2023, 13, 1620. https://doi.org/10.3390/diagnostics13091620
Ong CJT, Wong MYZ, Cheong KX, Zhao J, Teo KYC, Tan T-E. Optical Coherence Tomography Angiography in Retinal Vascular Disorders. Diagnostics. 2023; 13(9):1620. https://doi.org/10.3390/diagnostics13091620
Chicago/Turabian StyleOng, Charles Jit Teng, Mark Yu Zheng Wong, Kai Xiong Cheong, Jinzhi Zhao, Kelvin Yi Chong Teo, and Tien-En Tan. 2023. "Optical Coherence Tomography Angiography in Retinal Vascular Disorders" Diagnostics 13, no. 9: 1620. https://doi.org/10.3390/diagnostics13091620





