Hereditary Thoracic Aortic Diseases
Abstract
:1. Introduction
2. Clinical Conditions
3. Genetics in HTADs
3.1. Marfan Syndrome
3.2. Loeys–Dietz Syndrome
3.3. Ehlers–Danlos Syndrome
3.4. Turner Syndrome
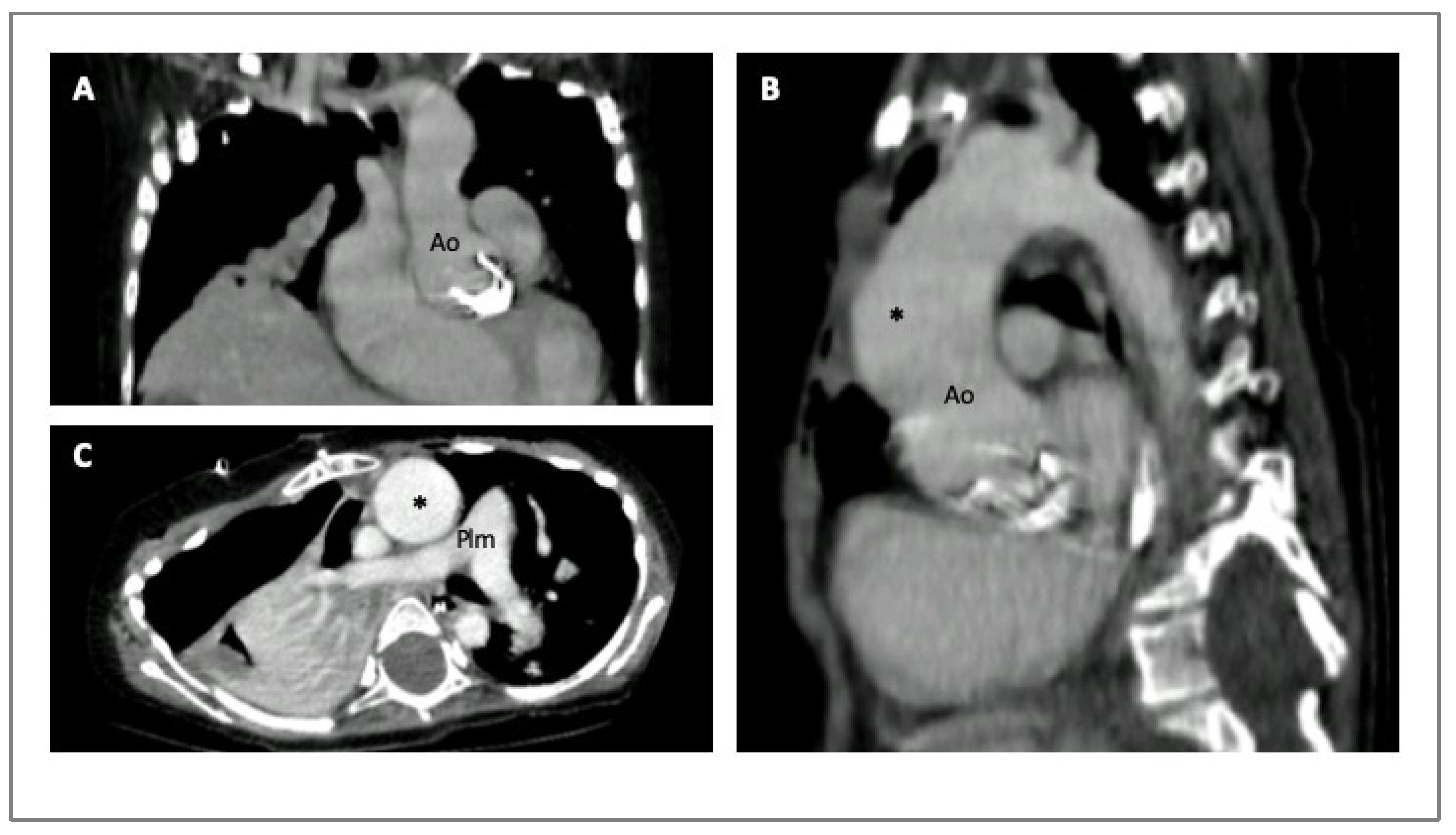
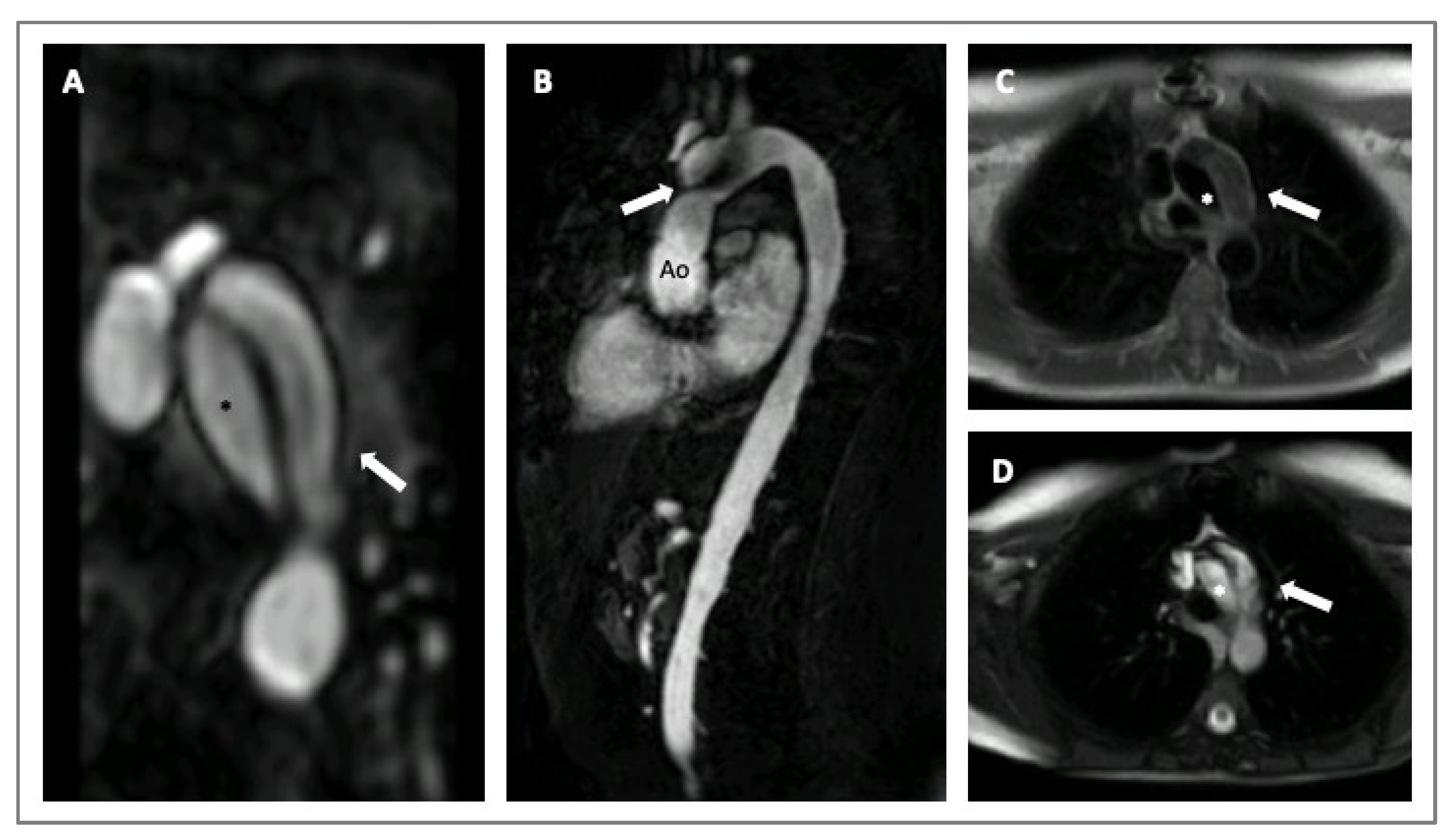
4. Imaging in HTADs
5. Medical Treatment in HTADs
5.1. β-Blockers
5.2. Angiotensin II Type I Receptor Blockers (ARBs)
5.3. Other Drugs
6. Surgery in HTADs
7. Pregnancy in HTADs
8. Sport Activity in HTADs
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. The Task Force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.; Schüler, H.; Alawi, M.; Kolbe, V.; Rybczynski, M.; Woitschach, R.; Sheikhzadeh, S.; Stark, V.C.; Olfe, J.; Roser, E.; et al. Next-generation sequencing of 32 genes associated with hereditary aortopathies and related disorders of connective tissue in a cohort of 199 patients. Gen. Med. 2019, 21, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, A.; Luyckx, I.; Loeys, B. Aetiology and management of hereditary aortopathy. Nat. Rev. Cardiol. 2017, 14, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Bathen, T.; Krohg-Sørensen, K.; Lidal, I.B. Multidisciplinary aortopathy clinics: A systematic scoping review of the literature and evaluation of patient experiences from a newly started clinic in Norway. Am. J. Med. Genet. A 2020, 182, 2552–2569. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: A report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e483. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.W.; Markwardt, S.; Eagle, K.A.; Devereux, B.B.; Weinsaft, J.W.; Asch, F.M.; LeMaire, S.A.; Maslen, C.L.; Song, H.K.; Milewicz, D.M.; et al. Cardiovascular Outcomes in Aortopathy: GenTAC Registry of Genetically Triggered Aortic Aneurysms and Related Conditions. J. Am. Coll. Cardiol. 2022, 79, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; Lindsay, M.E. Role of Clinical Genetic Testing in the Management of Aortopathies. Curr. Cardiol. Rep. 2021, 23, 10. [Google Scholar] [CrossRef]
- Zeigler, S.M.; Sloan, B.; Jones, J.A. Pathophysiology and Pathogenesis of Marfan Syndrome. Adv. Exp. Med. Biol. 2021, 1348, 185–206. [Google Scholar]
- Rigelsk, C.M.; Moran, R.T. Genetics of syndromic and nonsyndromic aortopathies. Curr. Opin. Pediatr. 2019, 31, 694–701. [Google Scholar] [CrossRef]
- Gouda, P.; Kay, R.; Habib, M.; Aziz, A.; Aziza, E.; Welsh, R. Clinical features and complications of Loeys-Dietz syndrome: A systematic review. Int. J. Cardiol. 2022, 362, 158–167. [Google Scholar] [CrossRef]
- Meccanici, F.; Shotte, M.H.; Snoeren, M.; Bons, L.R.; van den Hoven, A.T.; Kardys, I.; Budde, R.P.; van den Bosch, A.E.; Duijnhouwer, A.L.; Roos-Hesselink, J. Aortic dilatation and growth in women with Turner syndrome. Heart 2022, 109, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yetman, A.T.; Bisselou, K.S.M.; Sanmann, J.N.; Karz, R.J.; Steingraeber, C.J.; Wilde, M.; Murray, M.; Starr, L.J. Vascular dissection in women with Turner syndrome. Int. J. Cardiol. 2021, 325, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Duijnhouwer, A.L.; Bons, L.R.; Timmers, H.J.L.M.; van Kimmenade, R.R.L.; Snoeren, M.; Timmermans, J.; van den Hovern, A.T.; Kempers, M.; van Dijk, A.P.J.; Fleischer, K.; et al. Aortic dilatation and outcome in women with Turner syndrome. Heart 2019, 105, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.L.; Lechich, K.M.; Tierney, E.S.S.; Collins, R.T., II. Cardiac involvement in classical or hypermobile Ehlers-Danlos syndrome is uncommon. Gen. Med. 2020, 22, 1583–1588. [Google Scholar] [CrossRef]
- Bhandari, R.; Aatre, R.A.; Kanthi, Y. Diagnostic approach and management of genetic aortopathies. Vasc. Med. 2020, 25, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.S.; Sillence, D.O. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. A 2014, 164, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Folkestad, L.; Dahl Hald, J.; Gram, J.; Langdahl, B.L.; Hermann, A.P.; Diederichsen, A.C.; Abrahamsen, B.; Brixen, K. Cardiovascular disease in patients with osteogenesis imperfecta—A nationwide, register-based cohort study. Int. J. Cardiol. 2016, 225, 250–257. [Google Scholar] [CrossRef]
- Robinson, P.N.; Neumann, L.M.; Demuth, S.; Enders, H.; Jung, U.; Konig, R.; Mitulla, B.; Muller, D.; Muschke, P.; Pfeiffer, L.; et al. Shprintzen-Goldberg syndrome: Fourteen new patients and a clinical analysis. Am. J. Med. Genet. 2005, 135A, 251–262. [Google Scholar] [CrossRef]
- Carmignac, V.; Thevenon, J.; Adès, L.; Allewaert, B.; Julia, S.; Thauvin-Robinet, C.; Gueneau, L.; Courcet, J.B.; Lopez, E.; Holman, K.; et al. IN-frame mutations in exon 1 of SKI cause dominant Shprintzen-Globerg syndrome. J. Hum. Genet. 2012, 91, 950–957. [Google Scholar] [CrossRef]
- Fusco, A.; Mauriello, A.; Lioncino, M.; Palmiero, G.; Fratta, F.; Granato, C.; Cirillo, A.; Caiazza, M.; Monda, E.; Credendino, A.; et al. The Heart Muscle and Valve Involvement in Marfan Syndrome, Loeys-Dietz Syndrome and Collagenopathies. Heart Fail. Clin. 2022, 18, 165–175. [Google Scholar] [CrossRef]
- Renard, M.; Francis, C.; Ghosh, R.; Scott, A.F.; Dane Witmer, P.; Ades, L.C.; Andelfinger, G.U.; Arnaud, P.; Coileau, C.; Callewaert, B.; et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018, 72, 605–615. [Google Scholar] [CrossRef]
- Takeda, N.; Komuro, I. Genetic basis of hereditary thoracic aortic aneurysm and dissection. J. Cardiol. 2019, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, E.; Frentz, S.; Morgan, T.; Garcia-Minaur, S.; Leventer, R.J.; McGillvray, G.; Pariani, M.; van der Steen, A.; Pope, M.; Holter-Espinasse, M.; et al. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in filamin A. Eur. J. Hum. Genet. 2013, 21, 494–502. [Google Scholar] [CrossRef]
- Milewicz, D.; Hostetler, E.; Wallace, S.; Mellor-Crummey, L.; Gong, L.; Pannu, H.; Guo, D.C.; Ragalado, E. Precision medical and surgical management for thoracic aortic aneurysms and acute aortic dissections based on the causative mutant gene. J. Cardiovasc. Surg. 2016, 57, 172–177. [Google Scholar]
- Sakai, L.Y.; Keene, D.R.; Renard, M.; De Backer, J. FBN1, the disease-causing gene for Marfan syndrome and other genetic disorder. Gene 2016, 591, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, L.; Dietz, H.; Loyes, B. Loyes-Dietz syndrome. Adv. Exp. Med. Biol. 2014, 802, 95–105. [Google Scholar]
- Prakash, S.K. The impact of somatic mosaicism on bicuspid aortic valve and aortic dissection in Turner Syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Warncke, M.; Wright, F.; Weinrich, J.M.; Schoennagel, B.P.; Henes, F.O.; Adam, G.; von Kodolitsch, Y.; Schoen, G.; Bannas, P. Longitudinal follow-up by MR angiography reveals progressive dilatation of the distal aorta after aortic root replacement in Marfan syndrome. Eur. Radiol. 2023, 33, 6984–6992. [Google Scholar] [CrossRef]
- Evangelista, A.; Sitges, M.; Jondeau, G.; Nijveldt, R.; Pepi, M.; Cuellar, H.; Pontone, G.; Bossone, E.; Groenink, M.; Dweck, M.R.; et al. Multimodality imaging in thoracic aortic diseases: A clinical consensus statement from the European Association of Cardiovascular Imaging and the European Society of Cardiology working group on aorta and peripheral vascular diseases. Eur. Heart J. Cardiovasc. Imaging 2023, 24, e65–e85. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Kaemmerer, H.; von Kodolitsch, Y. Unravelling the Pathogenetic Mechanisms in Congenital Aortopathies: Need for an Integrative Translational Approach. J. Clin. Med. 2020, 9, 204. [Google Scholar] [CrossRef]
- Zentner, D.; James, P.; Bannon, P.; Jeremy, R. Familial Aortopathies—State of the Art Review. Heart Lung Circ. 2020, 29, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, K.L.; Marquis, K.M.; Braverman, A.C.; Ohman, J.W.; Bhalla, S.; Lin, C.Y.; Naeem, M.; Raptis, C.A. Imaging of Genetic Thoracic Aortopathy. Radiographics 2022, 42, 1283–1302. [Google Scholar] [CrossRef]
- Mazur, W.; Siegel, M.J.; Miszalski-Jamka, T.; Pelberg, R. CT Atlas of Adult Congenital Heart Disease; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Van Andel, M.M.; van Ooij, P.; de Waard, V.; Gottwald, L.M.; van Kimmenade, R.R.J.; Scholte, A.J.; Dickinson, M.G.; Zwinderman, A.H.; Mulder, B.J.M.; Nederveen, A.J.; et al. Abnormal aortic hemodynamics are associated with risk factors for aortic complications in patients with marfan syndrome. Int. J. Cardiol. Heart Vasc. 2022, 43, 101128. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.A.; Alpert, B.S.; Ward, J.C.; Pyeritz, R.E. Effect of beta-adrenergic blockade on aortic root rate of dilation in the Marfan syndrome. Am. J. Cardiol. 1994, 74, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Boodhwani, M.; Andelfinger, G.; Leipsic, J.; Lindsay, T.; McMurtry, M.S.; Therrien, J.; Siu, S.C. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can. J. Cardiol. 2014, 30, 577–589. [Google Scholar] [CrossRef]
- Borger, M.A.; Fedak, P.W.M.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D.; et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Executive summary. J. Thorac. Cardiovasc. Surg. 2018, 156, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; McLeod, C.; O’Gara, P.T.; et al. 2020 ACC/AHA Guidelines for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [PubMed]
- Sivasubramanian, R.; Meyers, K.E. Hypertension in Children and Adolescents with Turner Syndrome (TS), Neurofibromatosis 1 (NF1), and Williams Syndrome (WS). Curr. Hypertens. Rep. 2021, 23, 18. [Google Scholar] [CrossRef]
- Bramel, E.E.; Bagirzadeh, R.; Saqib, M.; Creamer, T.J.; Espinoza Camejo, W.; Roker, L.A.; Habashi, J.P.; Dietz, H.C.; MacFarlane, E.G. Distinct Contribution of Global and Regional Angiotensin II Type 1a Receptor Inactivation to Amelioration of Aortopathy in Tgfbr1M318R/+ Mice. Front. Cardiovasc. Med. 2022, 9, 936142. [Google Scholar] [CrossRef]
- Sandor, G.G.; Alghamdi, M.H.; Raffin, L.A.; Potts, M.T.; Williams, L.D.; Potts, J.E.; Kiess, M.; van Breemen, C. A randomized, double blind pilot study to assess the effects of losartan vs. atenolol on the biophysical properties of the aorta in patients with Marfan and Loeys-Dietz syndromes. Int. J. Cardiol. 2015, 179, 470–475. [Google Scholar] [CrossRef]
- Von Kodolitsch, Y.; Robinson, P.N. Marfan syndrome: An update of genetics, medical and surgical management. Heart 2007, 93, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, V.; Vilacosta, I.; Bruna, I.; Fuster, V. Marfan syndrome. Part 2: Treatment and management of patients. Nat. Rev. Cardiol. 2010, 7, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.H. An update of medical care in Marfan syndrome. Tzu Chi Med. J. 2021, 34, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.T.; Perdu, J.; De Backer, J.; Bozec, E.; Collignon, P.; Emmerich, J.; Fauret, A.L.; Fiessinger, J.N.; Germain, D.P.; Georgesco, G.; et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: A prospective randomised, open, blinded-endpoints trial. Lancet 2010, 376, 1476–1484, Erratum in: Lancet 2016, 388, 564. [Google Scholar] [CrossRef]
- Loeys, B.L. Angiotensin receptor blockers: A panacea for Marfan syndrome and related disorders? Drug Discov. Today 2015, 20, 262–266. [Google Scholar] [CrossRef] [PubMed]
- DeGette, R.L.; Grant, R.W.; Mph, M.D. Observational study design challenges-the case of fluoroquinolones and aortic disease. JAMA Intern. Med. 2020, 180, 1605–1606. [Google Scholar] [CrossRef]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 483–488. [Google Scholar] [CrossRef]
- Chung, A.W.; Yang, H.H.; Radomski, M.W.; van Breemen, C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ. Res. 2008, 102, e73–e85. [Google Scholar] [CrossRef]
- Williams, A.; Kenny, D.; Wilson, D.; Fagenello, G.; Nelson, M.; Dunstan, F.; Cockcroft, J.; Stuart, G.; Fraser, A.G. Effects of atenolol, perindopril and verapamil on haemodynamic and vascular function in Marfan syndrome—A randomised, double-blind, crossover trial. Eur. J. Clin. Investig. 2012, 42, 891–899. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, A.J.; Wilson, N.K.; Habaschi, J.P.; Bedjia, D.; Whitworth, R.R.; Lindsay, M.E.; Schoenhoff, F.; Meyers, L.; Huso, N.; et al. A deleterious gene-by-environment interaction imposed by calcium channel blockers in Marfan syndrome. Elife 2015, 4, e08648. [Google Scholar] [CrossRef]
- Crisby, M.; Nordin-Fredriksson, G.; Shah, P.K.; Yano, J.; Zhu, J.; Nilsson, J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: Implications for plaque stabilization. Circulation 2001, 103, 926–933. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, D.; McGuinness, J.; Byrne, J.; Terzo, E.; Huuskonen, V.; McAllister, H.; Black, A.; Kearney, S.; Kay, E.; Hill, A.D.K.; et al. Pravastatin reduces Marfan aortic dilation. Circulation 2011, 124 (Suppl. S11), S168–S173. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.D.; Alejo, D.; Crawford, T.; Hibino, N.; Dietz, H.C.; Cameron, D.E.; Vricella, L.A. Aortic Root replacement for children with Loeys-Dietz Syndrome. Ann. Thor. Surg. 2017, 103, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.D., 3rd; Liu, H.R.; Zhou, X.; Patel, N.D.; Lui, C.; Suarez Pierre, A.; Jacobs, M.L.; Dietz, H.C.; Habashi, J.; Hibino, N.; et al. Valve sparing aortic root replacement in children: Outcomes from 100 consecutive cases. J. Thorac. Cardiovasc. Surg. 2019, 157, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Massih, T.A.; Vouhé, P.R.; Mauriat, P.; Mousseaux, E.; Sidi, D.; Bonnet, D. Replacement of the ascending aorta in children: A series of fourteen patients. J. Thorac. Cardiovasc. Surg. 2002, 124, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Berdat, P.; Pavlovic, M.; Shukanov, S.; Englberger, L.; Pfamatter, J.P. Surgery of the dilated aortic root and ascending aorta in pediatric patients: Techniques and results. EJCTS 2003, 24, 249–254. [Google Scholar] [CrossRef]
- Everitt, M.D.; Pinto, N.; Hawkins, J.A.; Mitchell, M.B.; Kouretas, P.C.; Yetman, A.T. Cardiovascular surgery in children with Marfan syndrome or Loeys-Dietz syndrome. J. Thorac. Cardiovasc. Surg. 2009, 137, 1327–1333. [Google Scholar] [CrossRef]
- Ivanov, Y.; Drury, N.E.; Stickley, J.; Botha, P.; Khan, N.E.; Jones, T.J.; Brawn, W.; Barron, D.J. Strategies to minimize need for prosthetic aortic valve replacement in congenital aortic stenosis-value of the Ross procedure. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 509–519. [Google Scholar] [CrossRef]
- Danial, P.; Neily, A.; Pontallier, M.; Gaudin, R.; Khraiche, D.; Osborne-Pellegrin, M.; Vouhe, P.; Raisky, O. Ross procedure or complex aortic valve repair using pericardium in children: A real dilemma. J. Thorac. Cardiovasc. Surg. 2022, 163, 1180–1191. [Google Scholar] [CrossRef]
- Pacini, D.; Parolari, A.; Berretta, P.; Di Bartolomeo, R.; Alamanni, F.; Bavaria, J. Endovascular treatment for type B dissection in Marfan syndrome: Is it worthwhile? Ann. Thorac. Surg. 2013, 95, 737–749. [Google Scholar] [CrossRef]
- Huu, L.A.; Olive, J.K.; Cekmecelioglu, D.; Chatterjee, S.; Amarasekara, H.S.; Green, S.Y. Endovascular therapy for patients with heritable thoracic aortic disease. Ann. Cardiothorac. Surg. 2022, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Qato, K.; Conway, A.; Lu, E.; Tran, N.n.; Giangola, G.; Carroccio, A. Outcomes of Thoracic Endovascular Aneurysm Repair (TEVAR) in Patients with Connective Tissue Disorders. Vasc. Endovasc. Surg. 2020, 54, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.L.; Swan, L. Aortopathy in pregnancy. Heart 2022, 108, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.C.; Mittauer, E.; Harris, K.M.; Evangelista, A.; Pyeritz, R.; Brinster, D.; Conklin, L.; Suzuki, T.; Fanola, C.; Ouzounian, M.; et al. Clinical features and outcomes of pregnancy-related acute aortic dissection. JAMA Cardiol. 2021, 6, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lameijer, H.; Shutte, J.M.; Shuitemaker, N.E.W.; van Roosmalen, J.J.M.; Pieper, P.G. Maternal mortality due to cardiovascular disease in the Netherlands: A 21-year experience. Neth. Heart J. 2020, 28, 27–36. [Google Scholar] [CrossRef]
- McKellar, S.H.; MacDonald, R.J.; Michelena, H.I.; Connolly, H.M.; Sundt, T.M., 3rd. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am. J. Cardiol. 2011, 107, 96–99. [Google Scholar] [CrossRef]
- Campens, L.; Baris, L.; Scott, S.N. Pregnancy outcome in thoracic aortic disease; data from the Registry of Pregnancy and Cardiac disease. Heart 2021, 107, 1704–1709. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Ross-Hesselinl, J.W.; Bauersachs, J.; Blomstrom-Lundqvist, C.; Cifkova, R.; De Bonis, M.; Iung, B.; Johnson, M.K.; Kintscher, U.; Borghi, C.; et al. 2018 ESC guidelines on the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 32, 3147–3197. [Google Scholar] [CrossRef]
- Cauldwell, M.; Steer, P.J.; Curtis, S.; Mohan, A.R.; Dockree, S.; Mackillop, L.; Parry, H.; Oliver, J.; Sterrenburg, M.; Bolteg, A.; et al. Maternal and fetal outcomes in pregnancies complicated by the inherited aortopathy Loeys-Dietz syndrome. BJOG 2019, 126, 1025–1031. [Google Scholar] [CrossRef]
- Murray, M.L.; Pepin, M.; Peterson, S.; Byers, P.H. Pregnancy-related death and complications in women with vascular Ehlers-Danlos syndrome. Genet. Med. 2014, 16, 874–880. [Google Scholar] [CrossRef]
- Thijssen, C.G.E.; Bons, L.R.; Gökalp, A.L.; Van Kimmenade, R.R.J.; Mokhles, M.M.; Pelliccia, A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Exercise and Sports Partecipation in Patients with Thoracic Aortic Disease: A Review. Expert. Rev. Cardiovasc. Ther. 2019, 17, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Boraita, A.; Heras, M.E.; Morales, F.; Marina-Breysse, M.; Canda, A.; Rabadan, M.; Barriopedro, M.I.; Varela, A.; de la Rosa, A.; Tunon, J. Reference Values of Aortic Root in Male and Female White Elite Athletes According to Sport. Circ. Cardiovasc. Imaging 2016, 9, e005292. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Back, M.; Borjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Verrillo, F.; Rubino, M.; Palmiero, G.; Fusco, A.; Cirillo, A.; Caiazza, M.; Guarnaccia, N.; Mauriello, A.; Lioncinio, M.; et al. Thoracic Aortic Dilation: Implications for Physical Activity and Sport Participation. Diagnostics 2022, 12, 35741202. [Google Scholar] [CrossRef] [PubMed]
- Galanti, G.; Stefani, L.; Toncelli, L.; Vono, M.C.R.; Mercuri, R.; Maffulli, N. Effects of Sports Activity in Athletes with Bicuspid Aortic Valve and Mild Aortic Regurgitation. Br. J. Sports Med. 2010, 44, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.; Berger, K.R.; Murphy, E.A.; Pyeritz, R.E. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N. Engl. J. Med. 1994, 330, 1335–1341. [Google Scholar] [CrossRef]
- Yetman, A.T.; Bornemeier, R.A.; McCrindle, B.W. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am. J. Cardiol. 2005, 95, 1125–1127. [Google Scholar] [CrossRef]
- Selamet Tierney, E.S.; Feingold, B.; Printz, B.F.; Park, S.C.; Graham, D.; Kleinman, C.S.; Mahnke, C.B.; Timchak, D.M.; Neches, W.H.; Gersony, W.M. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J. Pediatr. 2007, 150, 77–82. [Google Scholar] [CrossRef]
- Chiu, H.H.; Wu, M.H.; Wang, J.K.; Lu, C.W.; Chiu, S.N.; Chen, C.A.; Lin, M.T.; Hu, F.C. Losartan added to β-blockade therapy for aortic root dilation in Marfan syndrome: A randomized, open-label pilot study. Mayo Clin. Proc. 2013, 88, 271–276. [Google Scholar] [CrossRef]
- Mullen, M.; Jin, X.Y.; Child, A.; Stuart, A.G.; Dodd, M.; Aragon-Martin, J.A.; Gaze, D.; Kiotsekoglou, A.; Yuan, L.; Hu, J.; et al. Irbesartan in Marfan syndrome (AIMS): A double-blind, placebo-controlled randomised trial. Lancet 2019, 394, 2263–2270. [Google Scholar] [CrossRef]
- Frank, M.; Adham, S.; Seigle, S.; Legrand, A.; Mirault, T.; Henneton, P.; Albuisson, J.; Denarié, N.; Mazzella, J.M.; Mousseaux, E.; et al. Vascular Ehlers-Danlos Syndrome: Long-Term Observational Study. J. Am. Coll. Cardiol. 2019, 73, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Van Driest, S.L.; Sleeper, L.A.; Gelb, B.D.; Morris, S.A.; Dietz, H.C.; Forbus, G.A.; Goldmuntz, E.; Hoskoppal, A.; James, J.; Lee, T.M.; et al. Variants in ADRB1 and CYP2C9: Association with Response to Atenolol and Losartan in Marfan Syndrome. J. Pediatr. 2020, 222, 213–220.e5. [Google Scholar] [CrossRef] [PubMed]
- Olfe, J.; Kanitz, J.J.; Stark, V.C.; Stute, F.; von Kodolitsch, Y.; Biermann, D.; Huebler, M.; Kozlik-Feldmann, R.; Mir, T.S. Prophylactic effect of angiotensin receptor blockers in children with genetic aortopathies: The early bird catches the worm. Clin. Res. Cardiol. 2023; Epub ahead of print. [Google Scholar] [CrossRef]


| Aortopathy | Clinical Red Flags | Aortic Segment Involved |
|---|---|---|
| Marfan | Family history Mitral prolapse Dural ectasia Lens ectopia Iridodesis Skeletal abnormalities Pneumothorax–pulmonary emphysema | Sinus Valsalva |
| Ehlers–Danlos syndrome type IV | Fragile, translucent, premature aging of the skin Characteristic facial appearance Hyperextensibility of joint Extensive bruising | Thoracic and abdominal aorta |
| Loeys–Dietz syndrome | Hypertelorism Bifid uvula Craniofacial features (cleft palate, proptosis, etc.) | Distal to the aortic root |
| Turner syndrome | Short stature, webbed neck, broad chest, obesity Congenital lymphedema Ovarian failure/infertility Metabolic and hormonal alterations (hyperlipidemia, impaired glucose tolerance, diabetes) Congenital heart disease (BAV, AC) |
|
| BAV | Family history Aortic regurgitation/stenosis Congenital heart disease (AC) |
|
| Gene Symbol | Disease | OMIM |
|---|---|---|
| EFEMP2 | Cutix laxa, autosomal recessive, type 1B | 604633 |
| ELN | Cutix laxa, autosomal dominant | 123700 |
| FBN2 | Congenital contractural arachnodactyly | 121050 |
| FLNA | Periventricular nodular heterotopia | 300049 |
| NOTCH1 | Bicuspid aortic valve with aneurysm | 109730 |
| SLC2A10 | Arterial tortuosity syndrome | 208050 |
| SMAD4 | Juvenile polyposis/hereditary hemorrhagic telangiectasia syndrome | 175050 |
| SKI | Shprintzen–Goldberg syndrome | 182212 |
| BGN * | LDS-like and MS-like | 301870 |
| FOXE3 * | Aortic aneurysm, familial thoracic 11 | 617349 |
| HCN4 * | Sick sinus syndrome 2 | 163800 |
| MAT2A * | Aortic dilatation, bicuspid aortic valve | - |
| MFAP5 * | Aortic aneurysm, familial thoracic 9 | 616166 |
| TGFB3/SMAD2 * | LDS type 5 and 6 | 615582, unassigned |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaziani, G.; Surace, F.C.; Girolami, F.; Bianco, F.; Bucciarelli, V.; Bonanni, F.; Bennati, E.; Arcieri, L.; Favilli, S. Hereditary Thoracic Aortic Diseases. Diagnostics 2024, 14, 112. https://doi.org/10.3390/diagnostics14010112
Spaziani G, Surace FC, Girolami F, Bianco F, Bucciarelli V, Bonanni F, Bennati E, Arcieri L, Favilli S. Hereditary Thoracic Aortic Diseases. Diagnostics. 2024; 14(1):112. https://doi.org/10.3390/diagnostics14010112
Chicago/Turabian StyleSpaziani, Gaia, Francesca Chiara Surace, Francesca Girolami, Francesco Bianco, Valentina Bucciarelli, Francesca Bonanni, Elena Bennati, Luigi Arcieri, and Silvia Favilli. 2024. "Hereditary Thoracic Aortic Diseases" Diagnostics 14, no. 1: 112. https://doi.org/10.3390/diagnostics14010112






