Automated Prediction of Neoadjuvant Chemoradiotherapy Response in Locally Advanced Cervical Cancer Using Hybrid Model-Based MRI Radiomics
Abstract
:1. Introduction
2. Materials and Methods
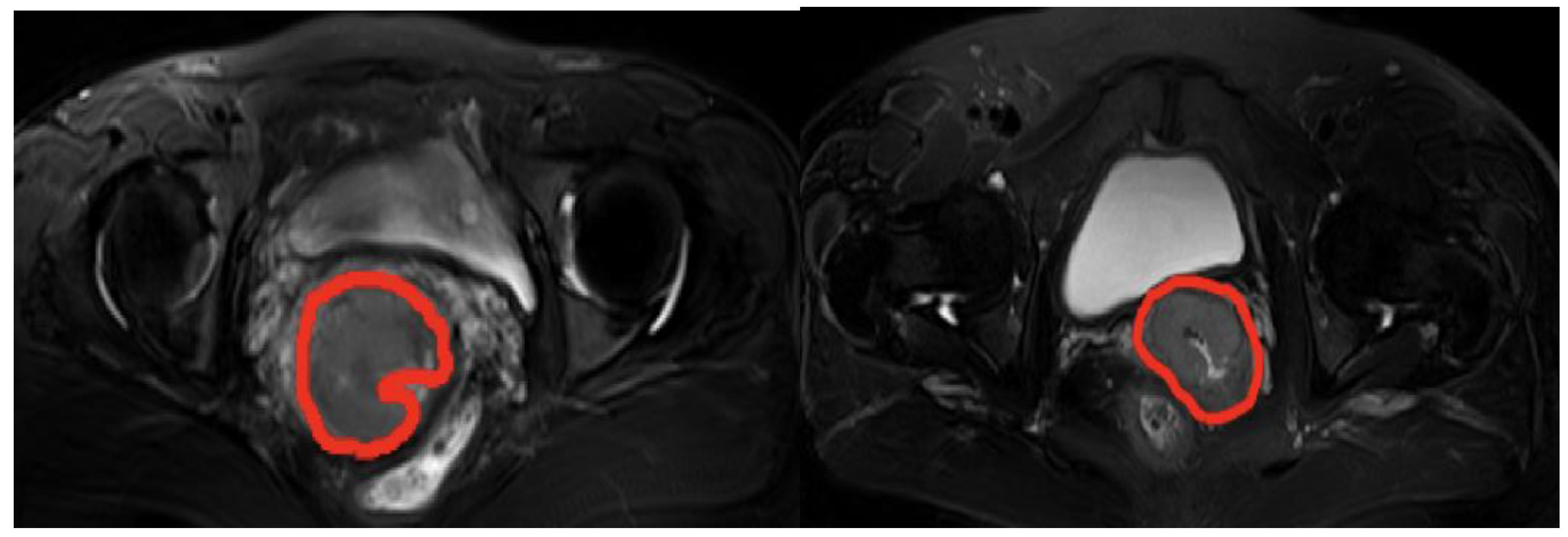
2.1. Materials
2.2. Methods
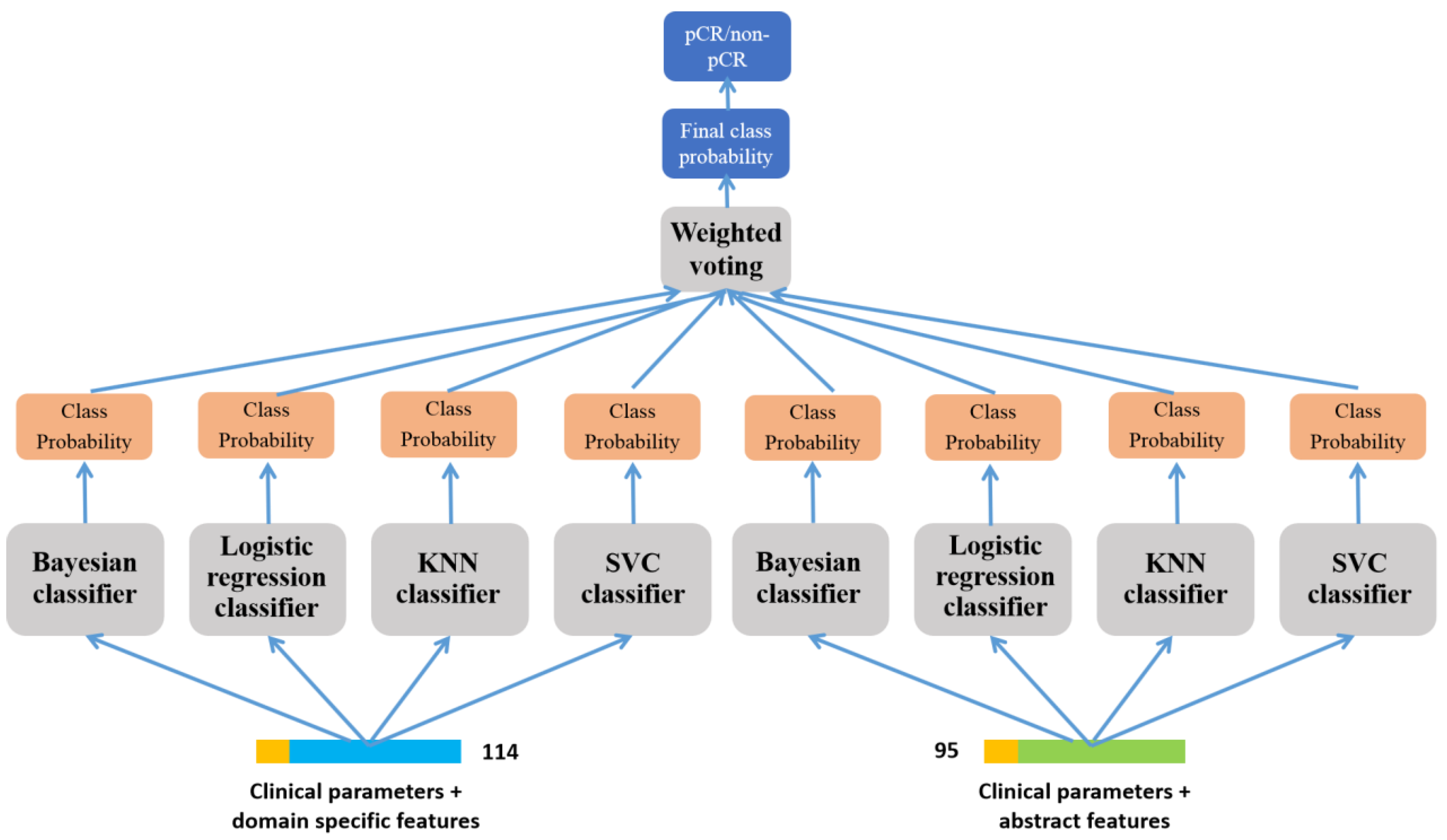
2.2.1. Methodological Framework
2.2.2. Data Preprocessing
2.2.3. Extraction of Domain-Specific Features
2.2.4. Extraction of Abstract Features
2.2.5. Prediction of pCR-Based on Ensemble Learning
2.2.6. Evaluation Metrics
2.2.7. Training and Validation
3. Results
3.1. Clinical Characteristics of the Patients
3.2. The Features Extracted from MRI and Feature Vectors
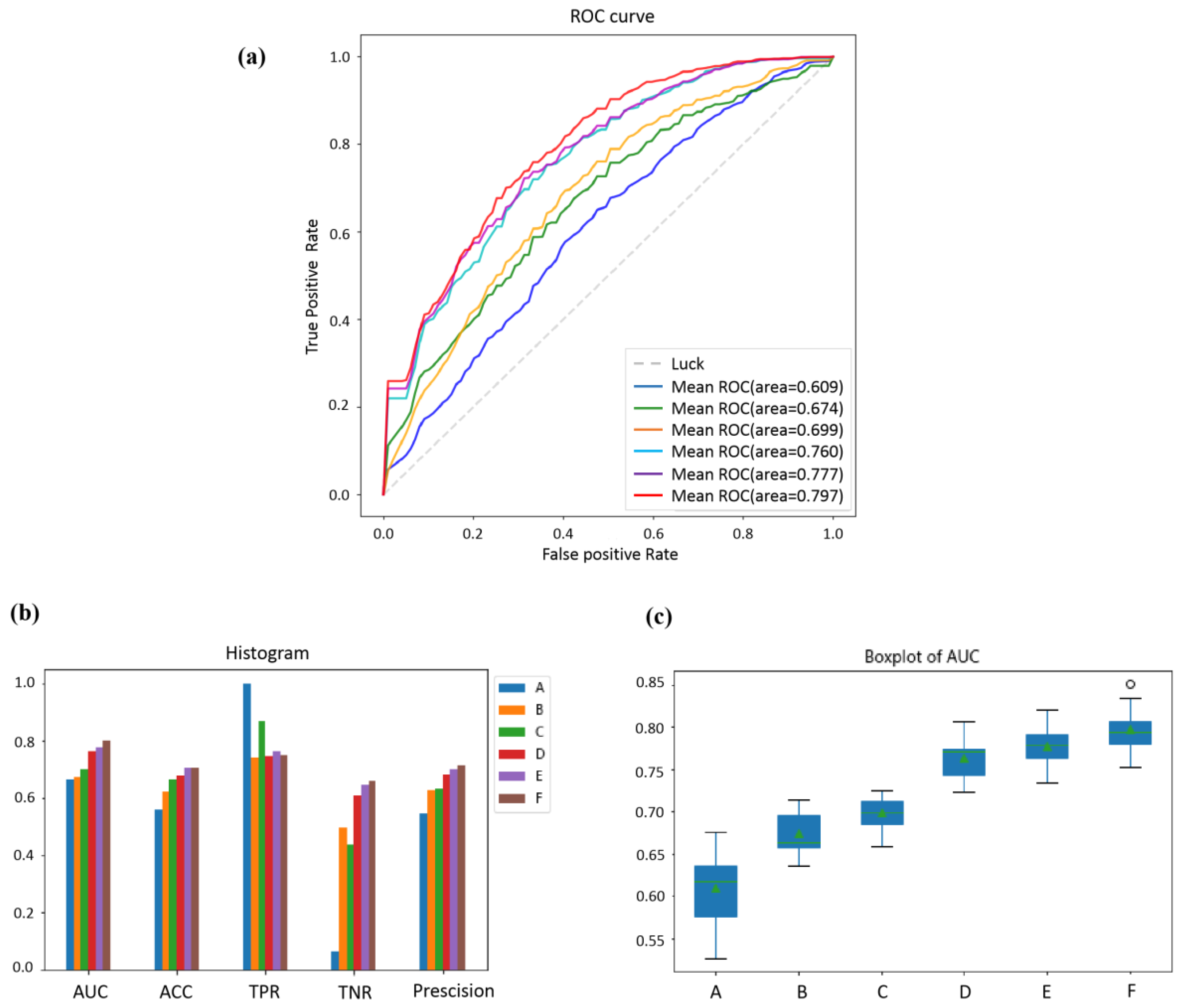
3.3. Comparisons of the Different Features of Prediction Performance
3.4. Comparisons of the Effect of Different Classifiers on Prediction Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Tanderup, K.; Schmid, M.P.; Jurgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Ferrall, L.; Lin, K.Y.; Roden, R.B.S.; Hung, C.F.; Wu, T.C. Cervical Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2021, 27, 4953–4973. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Enomoto, T.; Kast, W.M.; McCormack, M.; Tan, D.S.P.; Wu, X.; Gonzalez-Martin, A. Integration of immunotherapy into treatment of cervical cancer: Recent data and ongoing trials. Cancer Treat. Rev. 2022, 106, 102385. [Google Scholar] [CrossRef] [PubMed]
- Pfaendler, K.S.; Tewari, K.S. Changing paradigms in the systemic treatment of advanced cervical cancer. Am. J. Obstet. Gynecol. 2016, 214, 22–30. [Google Scholar] [CrossRef]
- Skelton, W.P.; Castagno, J.; Cardenas-Goicoechea, J.; Daily, K.; Yeung, A.; Markham, M.J. Bevacizumab Eligibility in Patients with Metastatic and Recurrent Cervical Cancer: A Retrospective Review. Clin. Med. Insights Oncol. 2018, 12, 1179554918779587. [Google Scholar] [CrossRef]
- Wendel Naumann, R.; Leath, C.A., 3rd. Advances in immunotherapy for cervical cancer. Curr. Opin. Oncol. 2020, 32, 481–487. [Google Scholar] [CrossRef]
- Marret, G.; Borcoman, E.; Le Tourneau, C. Pembrolizumab for the treatment of cervical cancer. Expert Opin. Biol. Ther. 2019, 19, 871–877. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.J.; Crispin-Ortuzar, M.; Chin, S.F.; Provenzano, E.; Bardwell, H.A.; Ma, W.; Cope, W.; Dariush, A.; Dawson, S.J.; Abraham, J.E.; et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 2022, 601, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, Z.; Li, C.; Li, Z.; Lou, X.; Shao, L.; Wang, Y.; Huang, Y.; Chen, H.; Pang, X.; et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A multicentre observational study. Lancet Digit. Health 2022, 4, e8–e17. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.M.; Aherne, E.A.; Ellenson, L.; Nikolovski, I.; Alghamdi, M.; Vazquez-Garcia, I.; Zamarin, D.; Long Roche, K.; Liu, Y.; Patel, D.; et al. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat. Cancer 2022, 3, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yu, H.; Ke, J.; Ding, P.; Yi, Y.; Jiang, X.; Duan, X.; Tang, J.; Chang, D.T.; Wu, X.; et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat. Commun. 2021, 12, 1851. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Xia, C.; Huang, Z.; Zuo, P.; Stemmer, A.; Song, B. Quantitative free-breathing dynamic contrast-enhanced MRI in hepatocellular carcinoma using gadoxetic acid: Correlations with Ki67 proliferation status, histological grades, and microvascular density. Abdom. Radiol. 2018, 43, 1393–1403. [Google Scholar] [CrossRef]
- Altazi, B.A.; Fernandez, D.C.; Zhang, G.G.; Hawkins, S.; Naqvi, S.M.; Kim, Y.; Hunt, D.; Latifi, K.; Biagioli, M.; Venkat, P.; et al. Investigating multi-radiomic models for enhancing prediction power of cervical cancer treatment outcomes. Phys. Med. 2018, 46, 180–188. [Google Scholar] [CrossRef]
- Gui, B.; Autorino, R.; Micco, M.; Nardangeli, A.; Pesce, A.; Lenkowicz, J.; Cusumano, D.; Russo, L.; Persiani, S.; Boldrini, L.; et al. Pretreatment MRI Radiomics Based Response Prediction Model in Locally Advanced Cervical Cancer. Diagnostics 2021, 11, 631. [Google Scholar] [CrossRef]
- Yaniv, Z.; Lowekamp, B.C.; Johnson, H.J.; Beare, R. SimpleITK Image-Analysis Notebooks: A Collaborative Environment for Education and Reproducible Research. J. Digit. Imaging 2018, 31, 290–303. [Google Scholar] [CrossRef]
- Lowekamp, B.C.; Chen, D.T.; Ibanez, L.; Blezek, D. The Design of SimpleITK. Front. Neuroinform. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Antropova, N.; Huynh, B.Q.; Giger, M.L. A deep feature fusion methodology for breast cancer diagnosis demonstrated on three imaging modality datasets. Med. Phys. 2017, 44, 5162–5171. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Cui, Y.; Wang, P.; Ren, J.; Wang, L.; Ma, Y.; Jia, Y.; Ma, X.; Zhao, L. Multi-Parametric Magnetic Resonance Imaging-Based Radiomics Analysis of Cervical Cancer for Preoperative Prediction of Lymphovascular Space Invasion. Front. Oncol. 2021, 11, 663370. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, W.L.; Zhang, G.W.; Sun, Z.; Zhong, J.M.; Wei, M.Q.; Yang, H.; Wei, L.C.; Huan, Y. Changes in magnetic resonance T2-weighted imaging signal intensity correlate with concurrent chemoradiotherapy response in cervical cancer. J. Contemp. Brachytherapy 2019, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Y.; Yang, J.J.; Zhao, J.; Xiang, Y.; Xia, C.; Cao, Y.; Chen, B.; Guan, H.; Qi, Y.F.; et al. MRI-based radiomics analysis improves preoperative diagnostic performance for the depth of stromal invasion in patients with early stage cervical cancer. Insights Imaging 2022, 13, 17. [Google Scholar] [CrossRef]
- Jajodia, A.; Gupta, A.; Prosch, H.; Mayerhoefer, M.; Mitra, S.; Pasricha, S.; Mehta, A.; Puri, S.; Chaturvedi, A. Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer. Tomography 2021, 7, 344–357. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, S.; Dong, D.; Yin, F.; Chen, A.; Wang, S.; Zhao, G.; Fang, M.; Tian, J.; et al. MR-Based Radiomics Nomogram of Cervical Cancer in Prediction of the Lymph-Vascular Space Invasion preoperatively. J. Magn. Reson. Imaging 2019, 49, 1420–1426. [Google Scholar] [CrossRef]
- Huynh, B.Q.; Li, H.; Giger, M.L. Digital mammographic tumor classification using transfer learning from deep convolutional neural networks. J. Med. Imaging 2016, 3, 034501. [Google Scholar] [CrossRef]
- Lao, J.; Chen, Y.; Li, Z.C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef]
- Kocher, M.; Ruge, M.I.; Galldiks, N.; Lohmann, P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther. Onkol. 2020, 196, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Basulto-Martinez, M.; Heinze, A.; Gratzke, C.; Miernik, A. Radiomics Applications in Renal Tumor Assessment: A Comprehensive Review of the Literature. Cancers 2020, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Hein, S.; Reis, G.; Gratzke, C.; Miernik, A. Current and future applications of machine and deep learning in urology: A review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World J. Urol. 2020, 38, 2329–2347. [Google Scholar] [CrossRef] [PubMed]
- Smedley, N.F.; Aberle, D.R.; Hsu, W. Using deep neural networks and interpretability methods to identify gene expression patterns that predict radiomic features and histology in non-small cell lung cancer. J. Med. Imaging 2021, 8, 031906. [Google Scholar] [CrossRef]
- Shi, R.; Chen, W.; Yang, B.; Qu, J.; Cheng, Y.; Zhu, Z.; Gao, Y.; Wang, Q.; Liu, Y.; Li, Z.; et al. Prediction of KRAS, NRAS and BRAF status in colorectal cancer patients with liver metastasis using a deep artificial neural network based on radiomics and semantic features. Am. J. Cancer Res. 2020, 10, 4513–4526. [Google Scholar]



| Parameters | pCR Group n (%) n = 74 | Non-pCR Group n (%) n = 64 | p Value |
|---|---|---|---|
| Age (years) | |||
| median(range) | 48 (25–64) | 47 (27–65) | 0.401 |
| Tumor diameter (cm) | 0.787 | ||
| <4 | 20 (27.0) | 16 (25.0) | |
| ≥4 | 54 (73.0) | 48 (75.0) | |
| Lymph node | 0.068 | ||
| positive | 24 (32.4) | 12 (18.8) | |
| negative | 50 (67.2) | 52 (81.2) | |
| 2018 FIGO stage | 0.846 | ||
| IB | 3 (4.1) | 4 (6.3) | |
| IIA-IIB | 39 (52.7) | 32 (50) | |
| IIIA-IIIC1 | 32 (43.2) | 28 (43.8) | |
| Radiotherapy technology | 0.886 | ||
| 3DRT | 54 (73.0) | 46 (71.9) | |
| IMRT | 20 (27.0) | 18 (28.1) | |
| Pathological type | 0.116 | ||
| Squamous carcinoma | 73 (98.6) | 59 (92.2) | |
| Adenocarcinoma | 1 (1.4) | 4 (6.3) | |
| Other | 0 | 1 (1.6) | |
| SCC | 0.117 | ||
| <1.5 | 25 (33.8) | 13 (20.3) | |
| 1.5–5 | 16 (21.6) | 12 (18.8) | |
| >5 | 11 (14.9) | 19 (29.7) | |
| Unclear | 22 (29.7) | 20 (31.2) | |
| OTT (days) | 0.707 | ||
| Median (range) | 35 (30–46) | 34 (30–42) |
| Classifier | AUC | ACC | TPR | TNR | Precision |
|---|---|---|---|---|---|
| A | 0.609 ± 0.037 | 0.582 ± 0.029 | 0.649 ± 0.047 | 0.517 ± 0.053 | 0.599 ± 0.028 |
| B | 0.674 ± 0.023 | 0.622 ± 0.027 | 0.739 ± 0.034 | 0.494 ± 0.033 | 0.625 ± 0.026 |
| C | 0.699 ± 0.019 | 0.663 ± 0.019 | 0.866 ± 0.024 | 0.435 ± 0.032 | 0.633 ± 0.017 |
| D | 0.760 ± 0.022 | 0.678 ± 0.024 | 0.765 ± 0.031 | 0.611 ± 0.026 | 0.683 ± 0.022 |
| E | 0.777 ± 0.021 | 0.704 ± 0.019 | 0.763 ± 0.033 | 0.645 ± 0.032 | 0.702 ± 0.021 |
| F | 0.797 ± 0.023 | 0.705 ± 0.018 | 0.750 ± 0.026 | 0.660 ± 0.025 | 0.711 ± 0.020 |
| Classification Method | AUC | ACC | TPR | TNR | Precision |
|---|---|---|---|---|---|
| Bayesian | 0.688 ± 0.041 | 0.620 ± 0.015 | 0.674 ± 0.015 | 0.594 ± 0.052 | 0.634 ± 0.024 |
| Logistic Regression | 0.757 ± 0.021 | 0.687 ± 0.021 | 0.749 ± 0.025 | 0.625 ± 0.031 | 0.694 ± 0.018 |
| KNN | 0.748 ± 0.017 | 0.691 ± 0.035 | 0.740 ± 0.030 | 0.650 ± 0.049 | 0.683 ± 0.027 |
| SVC | 0.732 ± 0.015 | 0.669 ± 0.047 | 0.715 ± 0.066 | 0.602 ± 0.019 | 0.687 ± 0.026 |
| Ensemble classifier (ours) | 0.797 ± 0.023 | 0.705 ± 0.018 | 0.750 ± 0.026 | 0.660 ± 0.025 | 0.711 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Xu, Y.; Dong, M.; Zhang, Y.; Gong, J.; Huang, D.; He, J.; Wei, L.; Huang, S.; Zhao, L. Automated Prediction of Neoadjuvant Chemoradiotherapy Response in Locally Advanced Cervical Cancer Using Hybrid Model-Based MRI Radiomics. Diagnostics 2024, 14, 5. https://doi.org/10.3390/diagnostics14010005
Yang H, Xu Y, Dong M, Zhang Y, Gong J, Huang D, He J, Wei L, Huang S, Zhao L. Automated Prediction of Neoadjuvant Chemoradiotherapy Response in Locally Advanced Cervical Cancer Using Hybrid Model-Based MRI Radiomics. Diagnostics. 2024; 14(1):5. https://doi.org/10.3390/diagnostics14010005
Chicago/Turabian StyleYang, Hua, Yinan Xu, Mohan Dong, Ying Zhang, Jie Gong, Dong Huang, Junhua He, Lichun Wei, Shigao Huang, and Lina Zhao. 2024. "Automated Prediction of Neoadjuvant Chemoradiotherapy Response in Locally Advanced Cervical Cancer Using Hybrid Model-Based MRI Radiomics" Diagnostics 14, no. 1: 5. https://doi.org/10.3390/diagnostics14010005





