Clinical Role of Upfront F-18 FDG PET/CT in Determining Biopsy Sites for Lung Cancer Diagnosis
Abstract
:1. Introduction
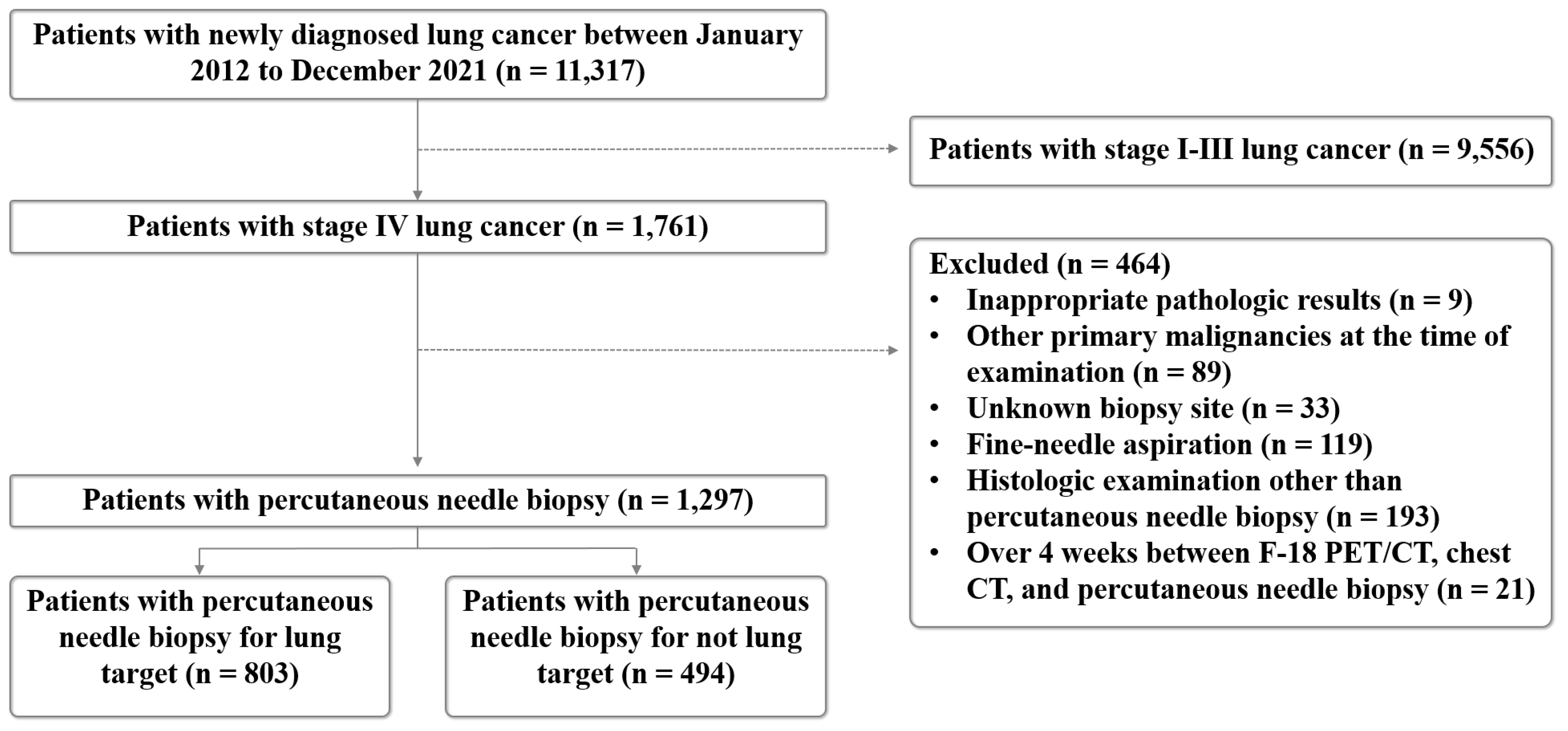
2. Materials and Methods
2.1. Study Population
2.2. Chest CT and F-18 FDG PET/CT Image Acquisition
2.3. PNB
2.4. CT-Guided PNB
2.5. C-Arm Cone-Beam CT-Guided PNB
2.6. US-Guided PNB
2.7. Pathological Analysis
2.8. Diagnostic Assessment and Complication Analysis
2.9. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Biopsy Characteristics and Outcomes
3.3. Complications
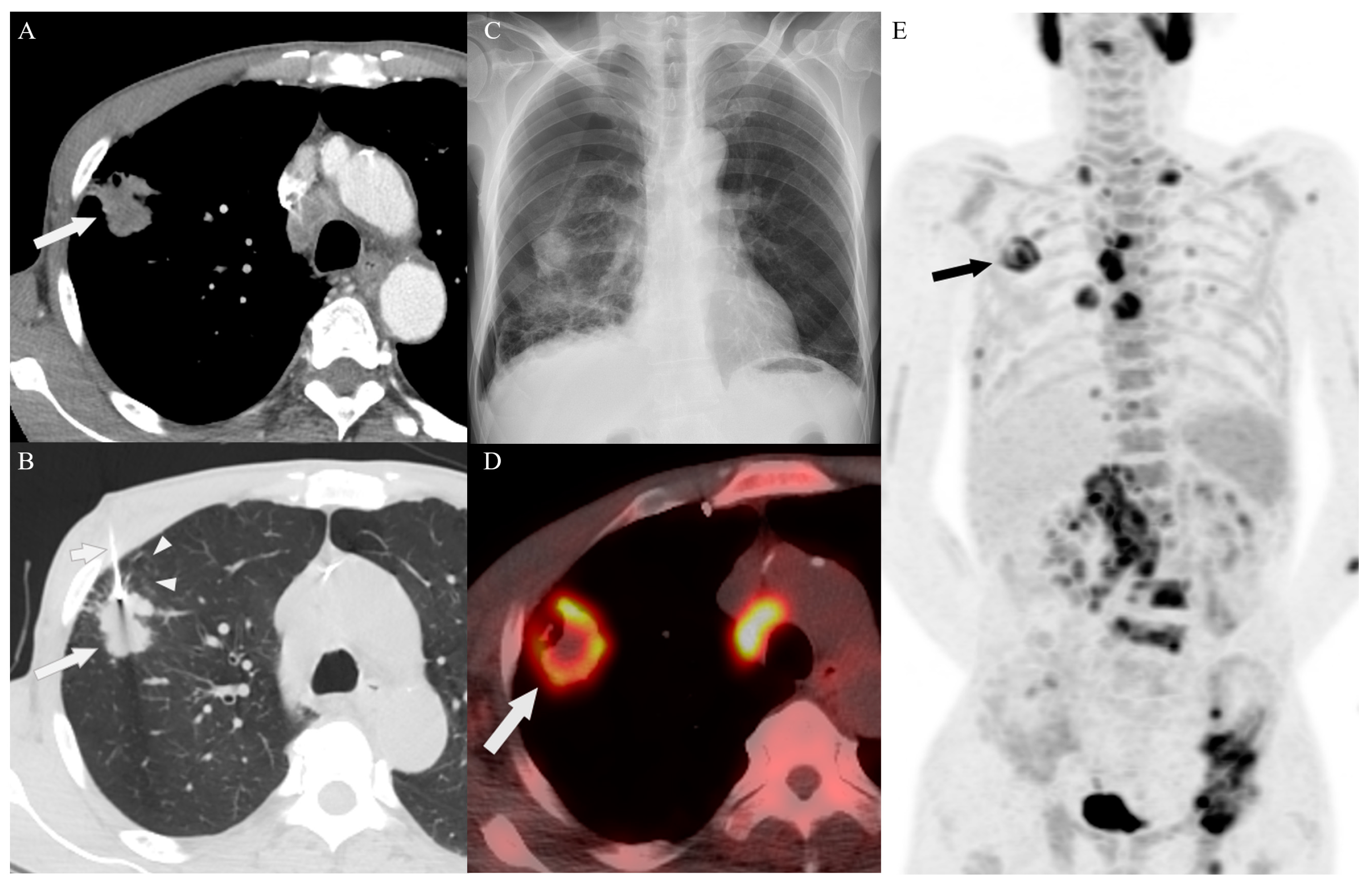
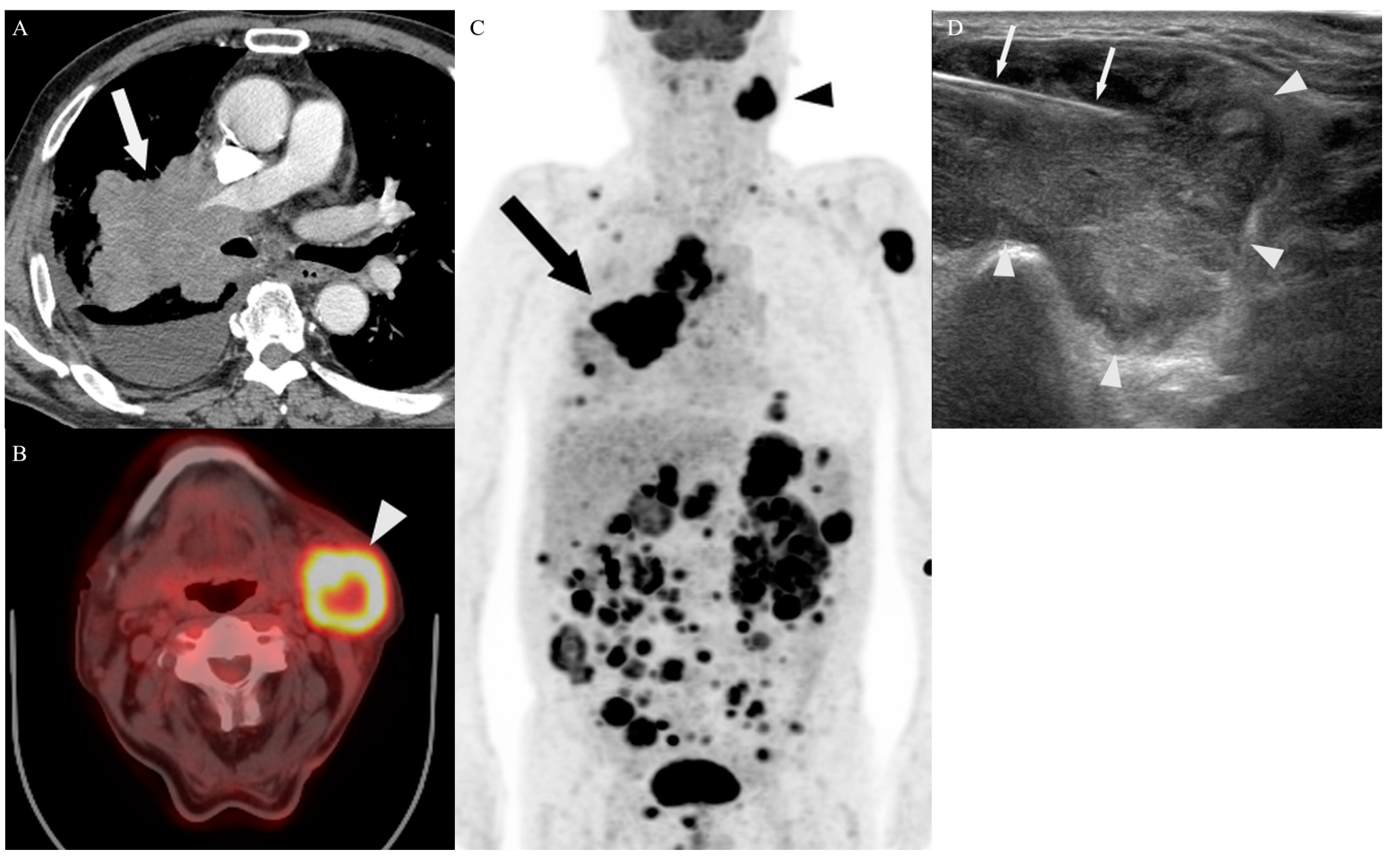
3.4. Target Site Selection, Complications, and Diagnostic Yield According to PET/CT Timing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PNB | percutaneous needle biopsy |
| CT | computed tomography |
| US | ultrasound |
| PET/CT | positron emission tomography computed tomography |
| F-18 FDG | fluorine-18 fluorodeoxyglucose |
References
- Balata, H.; Fong, K.M.; Hendriks, L.E.; Lam, S.; Ostroff, J.S.; Peled, N.; Wu, N.; Aggarwal, C. Prevention and Early Detection for NSCLC: Advances in Thoracic Oncology 2018. J. Thorac. Oncol. 2019, 14, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Laurent, F.; Montaudon, M.; Latrabe, V.; Bégueret, H. Percutaneous biopsy in lung cancer. Eur. J. Radiol. 2003, 45, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Al Ahmar, M.; Bonnet, B.; Delpla, A.; Kobe, A.; Madani, K.; Roux, C.; Deschamps, F.; de Baère, T.; Tselikas, L. The PEARL Approach for CT-guided Lung Biopsy: Assessment of Complication Rate. Radiology 2022, 302, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Mauri, G.; Grossi, F.; Truini, M.; Serafini, G.; Sardanelli, F.; Murolo, C. Pleural and peripheral lung lesions: Comparison of US- and CT-guided biopsy. Radiology 2013, 266, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.D.; Yoon, S.H.; Hong, H.; Hwang, J.H.; Goo, J.M.; Park, S. Tissue Adequacy and Safety of Percutaneous Transthoracic Needle Biopsy for Molecular Analysis in Non-Small Cell Lung Cancer: A Systematic Review and Meta-analysis. Korean J. Radiol. 2021, 22, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Park, C.M.; Goo, J.M.; Park, Y.K.; Sung, W.; Lee, H.J.; Lee, S.M.; Ko, J.Y.; Shim, M.S. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (</= 20 mm) lung nodules: Diagnostic accuracy and complications in 161 patients. AJR Am. J. Roentgenol. 2012, 199, W322–W330. [Google Scholar]
- Heerink, W.J.; de Bock, G.H.; de Jonge, G.J.; Groen, H.J.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.B.; Girard, N.; Pialat, J.-B.; Bringuier, P.-P.; Devouassoux-Shisheboran, M.; Rousseau, J.-C.; Isaac, S.; Thivolet-Bejui, F.; Clezardin, P.; Brevet, M. Mutational profiling of bone metastases from lung adenocarcinoma: Results of a prospective study (POUMOS-TEC). Bonekey Rep. 2014, 3, 580. [Google Scholar] [CrossRef]
- Livi, V.; Paioli, D.; Cancellieri, A.; Betti, S.; Natali, F.; Ferrari, M.; Fiorentino, M.; Trisolini, R. Diagnosis and Molecular Profiling of Lung Cancer by Percutaneous Ultrasound-Guided Biopsy of Superficial Metastatic Sites Is Safe and Highly Effective. Respiration 2021, 100, 515–522. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Lim, J.-K.; Shin, K.M.; Lee, J.; Kim, C.H.; Seo, H.; Lee, Y.H.; Heo, J.; Do, Y.W. Ultrasound-Guided Percutaneous Needle Biopsy for Small Pleural Lesions: Diagnostic Yield and Impact of CT and Ultrasound Characteristics. AJR Am. J. Roentgenol. 2021, 217, 699–706. [Google Scholar] [CrossRef]
- Toffart, A.-C.; Asfari, S.; Mc Leer, A.; Reymond, E.; Jankowski, A.; Moro-Sibilot, D.; Stephanov, O.; Ghelfi, J.; Lantuejoul, S.; Ferretti, G.R. Percutaneous CT-guided biopsy of lytic bone lesions in patients clinically suspected of lung cancer: Diagnostic performances for pathological diagnosis and molecular testing. Lung Cancer 2020, 140, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, J.; Lv, N.; Xuan, S.; Bai, L.; Ji, B.; Gao, S. Performance of Ultrasound-Guided Core Biopsy Driven by FDG-avid Supraclavicular Lymph Nodes in Patients with Suspected Lung Cancer. Front. Med. 2021, 8, 803500. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Chowdhury, M.S.; Jeon, S.; Kang, S.R.; Lee, C.; Jabin, Z.; Kim, J.; Cho, S.G.; Song, H.C.; Bom, H.S.; et al. Clinical Impact of F-18 FDG PET-CT on Biopsy Site Selection in Patients with Suspected Bone Metastasis of Unknown Primary Site. Nucl. Med. Mol. Imaging 2020, 54, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Hao, B.; Chen, H.-J.; Zhao, L.; Luo, Z.-M.; Wu, H.; Sun, L. PET/CT-guided percutaneous biopsy of FDG-avid metastatic bone lesions in patients with advanced lung cancer: A safe and effective technique. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Schuster, D.M. PET Molecular Imaging-Directed Biopsy: A Review. AJR Am. J. Roentgenol. 2017, 209, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Stigt, J.A.; Oostdijk, A.H.; Boers, J.E.; van den Berg, J.W.; Groen, H.J. Percutaneous ultrasound-guided biopsies in the evaluation of thoracic tumours after PET-CT: A prospective diagnostic study. Respiration 2012, 83, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Intepe, Y.S.; Metin, B.; Sahin, S.; Kaya, B.; Okur, A. Our transthoracic biopsy practices accompanied by the imaging process: The contribution of positron emission tomography usage to accurate diagnosis. Acta Clin. Belg. 2016, 71, 214–220. [Google Scholar] [CrossRef]
- Maconachie, R.; Mercer, T.; Navani, N.; McVeigh, G.; Guideline, C. Lung cancer: Diagnosis and management: Summary of updated NICE guidance. BMJ 2019, 364, l1049. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e211S–e250S. [Google Scholar] [CrossRef]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer (Version 2.2023); Network NCC: Canton, OH, USA, 2023. [Google Scholar]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Cell Lung Cancer (Version 3.2023); Network NCC: Canton, OH, USA, 2022. [Google Scholar]
- Volpi, S.; Ali, J.M.; Tasker, A.; Peryt, A.; Aresu, G.; Coonar, A.S. The role of positron emission tomography in the diagnosis, staging and response assessment of non-small cell lung cancer. Ann. Transl. Med. 2018, 6, 95. [Google Scholar] [CrossRef]
- Baldwin, D.R.; Callister, M.E.; Guideline Development, G. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015, 70, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Norman, R.; Piccolo, F.; Manners, D. The optimal timing of FDG-PET/CT in non-small cell lung cancer diagnosis and staging in an Australian centre. BMC Pulm. Med. 2021, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lim, K.Y.; Suh, Y.J.; Hur, J.; Han, D.H.; Kang, M.-J.; Choo, J.Y.; Kim, C.; Kim, J.I.; Yoon, S.H.; et al. Nondiagnostic Percutaneous Transthoracic Needle Biopsy of Lung Lesions: A Multicenter Study of Malignancy Risk. Radiology 2019, 290, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology clinical practice guidelines. J. Vasc. Interv. Radiol. 2003, 14 Pt 2, S199–S202. [Google Scholar] [CrossRef]
- Han, F.; Xu, M.; Xie, T.; Wang, J.W.; Lin, Q.G.; Guo, Z.X.; Zheng, W.; Han, J.; Lin, X.; Zou, R.H.; et al. Efficacy of ultrasound-guided core needle biopsy in cervical lymphadenopathy: A retrospective study of 6695 cases. Eur. Radiol. 2018, 28, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Hasebe, T.; Baba, Y.; Chosa, K.; Kondo, S.; Yamada, S.; Yoshimatsu, R.; Kubota, T.; Fujitaka, K.; Awai, K.; et al. Feasibility and Safety of CT-guided Intrathoracic and Bone Re-biopsy for Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 3587–3592. [Google Scholar] [CrossRef] [PubMed]
- Celik, B.; Sahin, E.; Nadir, A.; Kaptanoglu, M. Iatrogenic pneumothorax: Etiology, incidence and risk factors. Thorac. Cardiovasc. Surg. 2009, 57, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Watanabe, T.; Yamada, K.; Nakai, T.; Suzumura, T.; Sakagami, K.; Yoshimoto, N.; Sato, K.; Tanaka, H.; Mitsuoka, S.; et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: Comparison with computed tomography (CT) guided needle biopsy. J. Thorac. Dis. 2019, 11, 936–943. [Google Scholar] [CrossRef]
- Portela-Oliveira, E.; Souza, C.A.; Gupta, A.; Bayanati, H.; Inacio, J.; Rakhra, K. Ultrasound-guided percutaneous biopsy of thoracic lesions: High diagnostic yield and low complication rate. Clin. Radiol. 2021, 76, 281–286. [Google Scholar] [CrossRef]
- Mychajlowycz, M.; Alabousi, A.; Mironov, O. Ultrasound- Versus CT-Guided Subpleural Lung and Pleural Biopsy: An Analysis of Wait Times, Procedure Time, Safety, and Diagnostic Adequacy. Can. Assoc. Radiol. J. 2021, 72, 883–889. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Ellis, J.H.; Elkhouly, T.; Ream, J.M.; Bowerson, M.; Khan, A.; Caoili, E.M. Diagnostic yield of percutaneous image-guided tissue biopsy of focal hepatic lesions in cancer patients: Ten percent are not metastases from the primary malignancy. Cancer 2011, 117, 4041–4048. [Google Scholar] [CrossRef]
- Broccoli, A.; Nanni, C.; Cappelli, A.; Bacci, F.; Gasbarrini, A.; Tabacchi, E.; Piovani, C.; Argnani, L.; Ghermandi, R.; Sabattini, E.; et al. Diagnostic accuracy of positron emission tomography/computed tomography-driven biopsy for the diagnosis of lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3058–3065. [Google Scholar] [CrossRef]
- Cornelis, F.; Silk, M.; Schoder, H.; Takaki, H.; Durack, J.C.; Erinjeri, J.P.; Sofocleous, C.T.; Siegelbaum, R.H.; Maybody, M.; Solomon, S.B. Performance of intra-procedural 18-fluorodeoxyglucose PET/CT-guided biopsies for lesions suspected of malignancy but poorly visualized with other modalities. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2265–2272. [Google Scholar] [CrossRef]
- Lee, M.H.; Lubner, M.G.; Hinshaw, J.L.; Pickhardt, P.J. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am. J. Roentgenol. 2018, 210, W110–W117. [Google Scholar] [CrossRef]
- Lee, K.H.; Lim, K.Y.; Suh, Y.J.; Hur, J.; Han, D.H.; Kang, M.-J.; Choo, J.Y.; Kim, C.; Kim, J.I.; Yoon, S.H.; et al. Diagnostic Accuracy of Percutaneous Transthoracic Needle Lung Biopsies: A Multicenter Study. Korean J. Radiol. 2019, 20, 1300–1310. [Google Scholar] [CrossRef] [PubMed]

| Variables | Number of Patients | % |
|---|---|---|
| (n = 1297) | ||
| Sex | ||
| Male | 924 | 71.2 |
| Female | 373 | 28.8 |
| Age † (in years) | 71.4 ± 10.2 | |
| PET/CT timing | ||
| Upfront PET/CT group | 510 | 39.3 |
| Delayed PET/CT group | 787 | 60.7 |
| Biopsy technique | ||
| Multiple passes | 1261 | 97.2 |
| Coaxial system | 36 | 2.8 |
| Location of lesions biopsied | ||
| Lung | 803 | 61.9 |
| Non-lung | 494 | 38.1 |
| Lymph node | 133 | 10.3 |
| Pleura | 205 | 15.8 |
| Bone | 88 | 6.8 |
| Soft tissue | 23 | 1.8 |
| Kidney | 2 | 0.2 |
| Liver | 43 | 3.3 |
| Histological diagnosis | ||
| Non-small cell lung cancer, not otherwise specified | 181 | 14.0 |
| Adenocarcinoma | 692 | 53.4 |
| Squamous cell carcinoma | 237 | 18.3 |
| Large cell carcinoma | 13 | 1.0 |
| Sarcomatoid carcinoma | 10 | 0.8 |
| Small cell lung cancer | 164 | 12.6 |
| Non-Lung Target | Lung Target | Total | p-Value | |
|---|---|---|---|---|
| (n = 494) | (n = 803) | (n = 1297) | ||
| Sex | 0.856 | |||
| Male | 350 (70.9) | 574 (71.5) | 924 (71.2) | |
| Female | 144 (29.1) | 229 (28.5) | 373 (28.8) | |
| Age † (in years) | 71.4 ± 10.8 | 71.4 ± 9.9 | 71.4 ± 10.2 | 0.970 |
| Guidance modality | <0.001 * | |||
| CT | 31 (6.3) | 346 (43.1) | 377 (29.1) | |
| Cone-beam CT | 0 (0) | 117 (14.6) | 117 (9.0) | |
| Ultrasound | 463 (93.7) | 340 (42.3) | 803 (61.9) | |
| Number of cores obtained † | 2.4 ± 1.0 | 1.6 ± 0.8 | 1.9 ± 1.0 | <0.001 * |
| Procedure time, second † | 643.7 ± 309.6 | 781.6 ± 374.3 | 729.1 ± 357.3 | <0.001 * |
| Hospital stay, days † | 9.8 ± 4.7 | 9.9 ± 7.5 | 9.9 ± 6.6 | 0.848 |
| Variation in biopsy target selection | <0.001 * | |||
| Radiologist A | 223 (45.1) | 451 (56.2) | 674 (52.0) | |
| Radiologist B | 271 (54.9) | 352 (43.8) | 623 (48.0) | |
| Sample adequacy | 0.629 | |||
| Adequate | 480 (97.2) | 785 (97.8) | 1265 (97.5) | |
| Inadequate | 14 (2.8) | 18 (2.2) | 32 (2.5) | |
| Success rate | 1.000 | |||
| Diagnostic success | 461 (93.3) | 750 (93.4) | 1211 (93.4) | |
| Diagnostic failure | 33 (6.7) | 53 (6.6) | 86 (6.6) | |
| All complications | 41 (8.3) | 157 (19.6) | 198 (15.3) | <0.001 * |
| Minor complications | 40 (8.1) | 130 (16.2) | 170 (13.1) | <0.001 * |
| Major complications | 1 (0.2) | 27 (3.4) | 28 (2.2) | <0.001 * |
| Location of Lesions Biopsied | Results |
|---|---|
| Lung | |
| Minor complications | 130 patients |
| Pneumothorax | 83 cases |
| Hemoptysis | 15 cases |
| Parenchymal hemorrhage | 33 cases |
| Hematoma | 4 cases |
| Hemothorax | 1 case |
| Major complications | 27 patients |
| Pneumothorax | 26 cases |
| Hemothorax | 1 case |
| Non-lung | |
| Lymph node | |
| Minor complications | 25 patients |
| Hematoma | 25 cases |
| Major complications | 0 patient |
| Pleura | |
| Minor complications | 5 patients |
| Pneumothorax | 2 cases |
| Hemorrhage | 2 cases |
| Hematoma | 1 case |
| Major complications | 1 patient |
| Pneumothorax | 1 case |
| Bone | |
| Minor complications | 10 patients |
| Hematoma | 10 cases |
| Major complications | 0 patient |
| Soft tissue, kidney, liver | |
| Minor and major complications | 0 patient |
| Variables | Upfront PET/CT Group | Delayed PET/CT Group | Total | p-Value |
|---|---|---|---|---|
| (n = 510) | (n = 787) | (n = 1297) | ||
| Target site performed by radiologists A and B | <0.001 * | |||
| Lung | 275 (53.9) | 528 (67.1) | 803 (61.9) | |
| Non-lung | 235 (46.1) | 259 (32.9) | 494 (38.1) | |
| Target site performed by radiologist A | 0.007 | |||
| Lung | 143 (60.1) | 308 (70.6) | 451 (66.9) | |
| Non-lung | 95 (39.9) | 128 (29.4) | 223 (33.1) | |
| Target site performed by radiologist B | 0.001 | |||
| Lung | 132 (48.5) | 220 (62.7) | 352 (56.5) | |
| Non-lung | 140 (51.5) | 131 (37.3) | 271 (43.5) | |
| All complications | 73 (14.3) | 125(15.9) | 198 (15.3) | 0.491 |
| Minor complications | 68 (13.3) | 102 (13.0) | 170 (13.1) | 0.912 |
| Major complications | 5 (1.0) | 23 (2.9) | 28 (2.2) | 0.031 * |
| Sample adequacy | 1.000 | |||
| Adequate | 497 (97.5) | 768 (97.6) | 1265(97.5) | |
| Inadequate | 13 (2.5) | 19 (2.4) | 32 (2.5) | |
| Success rate | 0.540 | |||
| Diagnostic success | 473 (92.7) | 738 (93.8) | 1211(93.4) | |
| Diagnostic failure | 37 (7.3) | 49 (6.2) | 86 (6.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Lim, J.-K.; Shin, K.M.; Hong, J.; Cha, J.G.; Cho, S.H.; Park, S.Y.; Ryeom, H.K.; Kim, S.H.; Seo, A.N.; et al. Clinical Role of Upfront F-18 FDG PET/CT in Determining Biopsy Sites for Lung Cancer Diagnosis. Diagnostics 2024, 14, 153. https://doi.org/10.3390/diagnostics14020153
Park B, Lim J-K, Shin KM, Hong J, Cha JG, Cho SH, Park SY, Ryeom HK, Kim SH, Seo AN, et al. Clinical Role of Upfront F-18 FDG PET/CT in Determining Biopsy Sites for Lung Cancer Diagnosis. Diagnostics. 2024; 14(2):153. https://doi.org/10.3390/diagnostics14020153
Chicago/Turabian StylePark, Byunggeon, Jae-Kwang Lim, Kyung Min Shin, Jihoon Hong, Jung Guen Cha, Seung Hyun Cho, Seo Young Park, Hun Kyu Ryeom, See Hyung Kim, An Na Seo, and et al. 2024. "Clinical Role of Upfront F-18 FDG PET/CT in Determining Biopsy Sites for Lung Cancer Diagnosis" Diagnostics 14, no. 2: 153. https://doi.org/10.3390/diagnostics14020153





