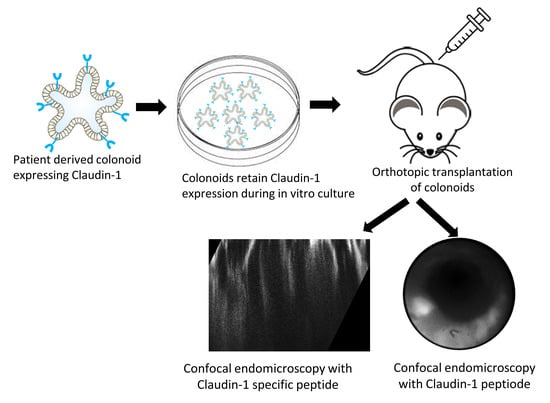
Near-Infrared In Vivo Imaging of Claudin-1 Expression by Orthotopically Implanted Patient-Derived Colonic Adenoma Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Specific for Claudin-1
2.2. Patient-Derived Colonoids in Culture
2.2.1. Colonoid Origins
2.2.2. Colonoid Cultures
2.2.3. Colonoid Preparation for Implantation
2.2.4. Pre-Clinical Colonoid Model
2.3. Claudin-1 Expression by Patient-Derived Colonoids
2.3.1. Preparation of Paraffin-Embedded Specimens
2.3.2. Immunohistochemistry (IHC)
2.4. Claudin-1 Expression in Patient Specimens
2.5. Wide-Field Imaging of Implanted Colonoids
2.6. Confocal Imaging of Implanted Colonoids
2.7. Toxicity Analysis
2.8. Statistics
3. Results
3.1. Peptide Specific for Claudin-1
3.2. Patient-Derived Colonoids in Culture
3.3. Claudin-1 Expression by Patient-Derived Colonoids
3.4. Claudin-1 Expression in Patient Specimens
3.5. Wide-Field Imaging of Implanted Colonoids
3.6. Confocal Imaging of Implanted Colonoids
3.7. Ex Vivo Validation of Implanted Colonoids
4. Discussion
4.1. Pre-Clinical Model of CRC
4.2. Patient-Derived Colonoids
4.3. Claudin-1-Targeting Peptide
4.4. In Vivo Imaging of Implanted Colonoids
4.5. Application of Patient-Derived Colonoid Transplant Model
5. Conclusions
- Patient-derived adenoma and normal colonoids implanted orthotopically in colonic mucosa of immunocompromised mice can be imaged.
- Implanted colonoids have a similar morphology to flat and subtle human colonic adenomas.
- Fluorescently labeled peptides can distinguish overexpressed cell surface targets, such as claudin-1, expressed by adenoma versus normal colonoids in vivo.
- Multi-model imaging with wide-field endoscopy and dual axes confocal endomicroscopy could be used to localize and optically section colonoids, respectively.
- In summary, a human pre-clinical model has demonstrated in vivo uptake of an NIR-labeled peptide specific for claudin-1 to localize pre-malignant colonic lesions in vivo using wide-field endoscopy and confocal endomicroscopy by generating high fluorescence contrast.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Li, Z.; Zhang, P.; Li, X.; Cao, X.; Du, H.; Zhang, J.; Zhang, L. Colonoscopic screening is associated with reduced colorectal cancer incidence and mortality: A systematic review and meta-analysis. J. Cancer 2020, 11, 5953. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W.; et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019, 156, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Bannert, C.; Dunkler, D.; Salzl, P.; Trauner, M.; Renner, F.; Knoflach, P.; Ferlitsch, A.; Weiss, W.; Ferlitsch, M. Prevalence of flat lesions in a large screening population and their role in colonoscopy quality improvement. Endoscopy 2013, 45, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Stibbe, J.A.; Hoogland, P.; Achterberg, F.B.; Holman, D.R.; Sojwal, R.S.; Burggraaf, J.; Vahrmeijer, A.L.; Nagengast, W.B.; Rogalla, S. Highlighting the undetectable-fluorescence molecular imaging in gastrointestinal endoscopy. Mol. Imaging Biol. 2023, 25, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Bürtin, F.; Mullins, C.S.; Linnebacher, M. Mouse models of colorectal cancer: Past, present and future perspectives. World J. Gastroenterol. 2020, 26, 1394. [Google Scholar] [CrossRef] [PubMed]
- Hinoi, T.; Akyol, A.; Theisen, B.K.; Ferguson, D.O.; Greenson, J.K.; Williams, B.O.; Cho, K.R.; Fearon, E.R. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007, 67, 9721–9730. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357. [Google Scholar] [CrossRef]
- Barbáchano, A.; Fernández-Barral, A.; Bustamante-Madrid, P.; Prieto, I.; Rodríguez-Salas, N.; Larriba, M.J.; Muñoz, A. Organoids and colorectal cancer. Cancers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Xue, X.; Ramakrishnan, S.K.; Weisz, K.; Triner, D.; Xie, L.; Attili, D.; Pant, A.; Győrffy, B.; Zhan, M.; Carter-Su, C.; et al. Iron Uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016, 24, 447–461. [Google Scholar] [CrossRef]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life 2015, 67, 29–35. [Google Scholar] [CrossRef]
- Mrsny, R.J.; Brown, G.T.; Gerner-Smidt, K.; Buret, A.G.; Meddings, J.B.; Quan, C.; Koval, M.; Nusrat, A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am. J. Path. 2008, 172, 905–915. [Google Scholar] [CrossRef]
- Miwa, N.; Furuse, M.; Tsukita, S.; Niikawa, N.; Nakamura, Y.; Furukawa, Y. Involvement of claudin-1 in the β-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol. Res. 2001, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Sharma, A.; Pope, J.; Krishnan, M.; Washington, M.K.; Singh, A.B.; Dhawan, P. Caudal homeobox protein Cdx-2 cooperates with Wnt pathway to regulate claudin-1 expression in colon cancer cells. PLoS ONE 2012, 7, e37174. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation. Int. J. Mol. Med. 2018, 42, 713–725. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin family participates in the pathogenesis of inflammatory bowel diseases and colitis-associated colorectal cancer. Front. Immunol. 2019, 10, 1441. [Google Scholar] [CrossRef]
- Caruso, M.; Fung, K.Y.; Moore, J.; Brierley, G.V.; Cosgrove, L.J.; Thomas, M.; Cheetham, G.; Brook, E.; Fraser, L.M.; Tin, T.; et al. Claudin-1 expression is elevated in colorectal cancer precursor lesions harboring the BRAF V600E mutation. Transl. Oncol. 2014, 7, 456–463. [Google Scholar] [CrossRef]
- Rabinsky, E.F.; Joshi, B.P.; Pant, A.; Zhou, J.; Duan, X.; Smith, A.; Kuick, R.; Fan, S.; Nusrat, A.; Owens, S.R.; et al. Overexpressed claudin-1 can be visualized endoscopically in colonic adenomas in vivo. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 222–237. [Google Scholar] [CrossRef]
- Wang, F.; Duan, X.; Chen, J.; Gao, Z.; Zhou, J.; Wu, X.; Chang, T.S.; Lee, M.; Li, G.; Nusrat, A.; et al. Integrated imaging methodology detects claudin-1 expression in premalignant nonpolypoid and polypoid colonic epithelium in mice. Clin. Transl. Gastroenterol. 2020, 11, e0089. [Google Scholar] [CrossRef]
- Shirazi, A.; Sahraeibelverdi, T.; Lee, M.; Li, H.; Yu, J.; Jaiswal, S.; Oldham, K.R.; Wang, T.D. Miniature side-view dual axes confocal endomicroscope for repetitive in vivo imaging. Biomed. Opt. Express 2023, 14, 4277–4295. [Google Scholar] [CrossRef]
- Wong, L.K.; Mandella, M.J.; Kino, G.S.; Wang, T.D. Improved rejection of multiply scattered photons in confocal microscopy using dual-axes architecture. Opt. Lett. 2007, 32, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Dame, M.K.; Attili, D.; McClintock, S.D.; Dedhia, P.H.; Ouillette, P.; Hardt, O.; Chin, A.M.; Xue, X.; Laliberte, J.; Katz, E.L.; et al. Identification, isolation and characterization of human LGR5-positive colon adenoma cells. Development 2018, 145, dev153049. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Czerwinski, M.; Wu, A.; Dame, M.K.; Attili, D.; Hill, E.; Colacino, J.A.; Nowacki, L.M.; Shroyer, N.F.; Higgins, P.D.R.; et al. A method for cryogenic preservation of human biopsy specimens and subsequent organoid culture. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 218–222.e217. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Prot. 2013, 8, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakamura, T.; Sato, T.; Nemoto, Y.; Mizutani, T.; Zheng, X.; Ichinose, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 2012, 18, 618–623. [Google Scholar] [CrossRef]
- Liu, Z.; Miller, S.J.; Joshi, B.P.; Wang, T.D. In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut 2013, 62, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.P.; Pant, A.; Duan, X.; Prabhu, A.; Wamsteker, E.J.; Kwon, R.S.; Elta, G.H.; Owens, S.R.; Appelman, H.D.; Wang, T.D.; et al. Multimodal video colonoscope for targeted wide-field detection of nonpolypoid colorectal neoplasia. Gastroenterology 2016, 150, 1084–1086. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.W.; Jaiswal, S.; Chang, T.S.; Zhang, R.; Dame, M.K.; Duan, Y.; Jiang, H.; Spence, J.R.; Hsieh, S.Y.; et al. Near-infrared imaging of colonic adenomas in vivo using orthotopic human organoids for early cancer detection. Cancers 2023, 15, 4795. [Google Scholar] [CrossRef] [PubMed]
- Sheffer, M.; Bacolod, M.D.; Zuk, O.; Giardina, S.F.; Pincas, H.; Barany, F.; Paty, P.B.; Gerald, W.L.; Notterman, D.A.; Domany, E. Association of survival and disease progression with chromosomal instability: A genomic exploration of colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7131–7136. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, S.; Liu, X.; Jacobs, J.L.; Cheng, M.; Niu, Y.; Jin, Q.; Wang, T.; Yang, W. A human claudin-1–derived peptide inhibits hepatitis C virus entry. Hepatology 2012, 56, 507–515. [Google Scholar] [CrossRef]
- Bony, B.A.; Tarudji, A.W.; Miller, H.A.; Gowrikumar, S.; Roy, S.; Curtis, E.T.; Gee, C.C.; Vecchio, A.; Dhawan, P.; Kievit, F.M. Claudin-1-targeted nanoparticles for delivery to aging-induced alterations in the blood–brain barrier. ACS Nano 2021, 15, 18520–18531. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, S.; Wang, F.; Wu, X.; Chang, T.-S.; Shirazi, A.; Lee, M.; Dame, M.K.; Spence, J.R.; Wang, T.D. Near-Infrared In Vivo Imaging of Claudin-1 Expression by Orthotopically Implanted Patient-Derived Colonic Adenoma Organoids. Diagnostics 2024, 14, 273. https://doi.org/10.3390/diagnostics14030273
Jaiswal S, Wang F, Wu X, Chang T-S, Shirazi A, Lee M, Dame MK, Spence JR, Wang TD. Near-Infrared In Vivo Imaging of Claudin-1 Expression by Orthotopically Implanted Patient-Derived Colonic Adenoma Organoids. Diagnostics. 2024; 14(3):273. https://doi.org/10.3390/diagnostics14030273
Chicago/Turabian StyleJaiswal, Sangeeta, Fa Wang, Xiaoli Wu, Tse-Shao Chang, Ahmad Shirazi, Miki Lee, Michael K. Dame, Jason R. Spence, and Thomas D. Wang. 2024. "Near-Infrared In Vivo Imaging of Claudin-1 Expression by Orthotopically Implanted Patient-Derived Colonic Adenoma Organoids" Diagnostics 14, no. 3: 273. https://doi.org/10.3390/diagnostics14030273