Analysis of Epicardial Adipose Tissue Texture in Relation to Coronary Artery Calcification in PCCT: The EAT Signature!
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Chest CT Imaging Protocol
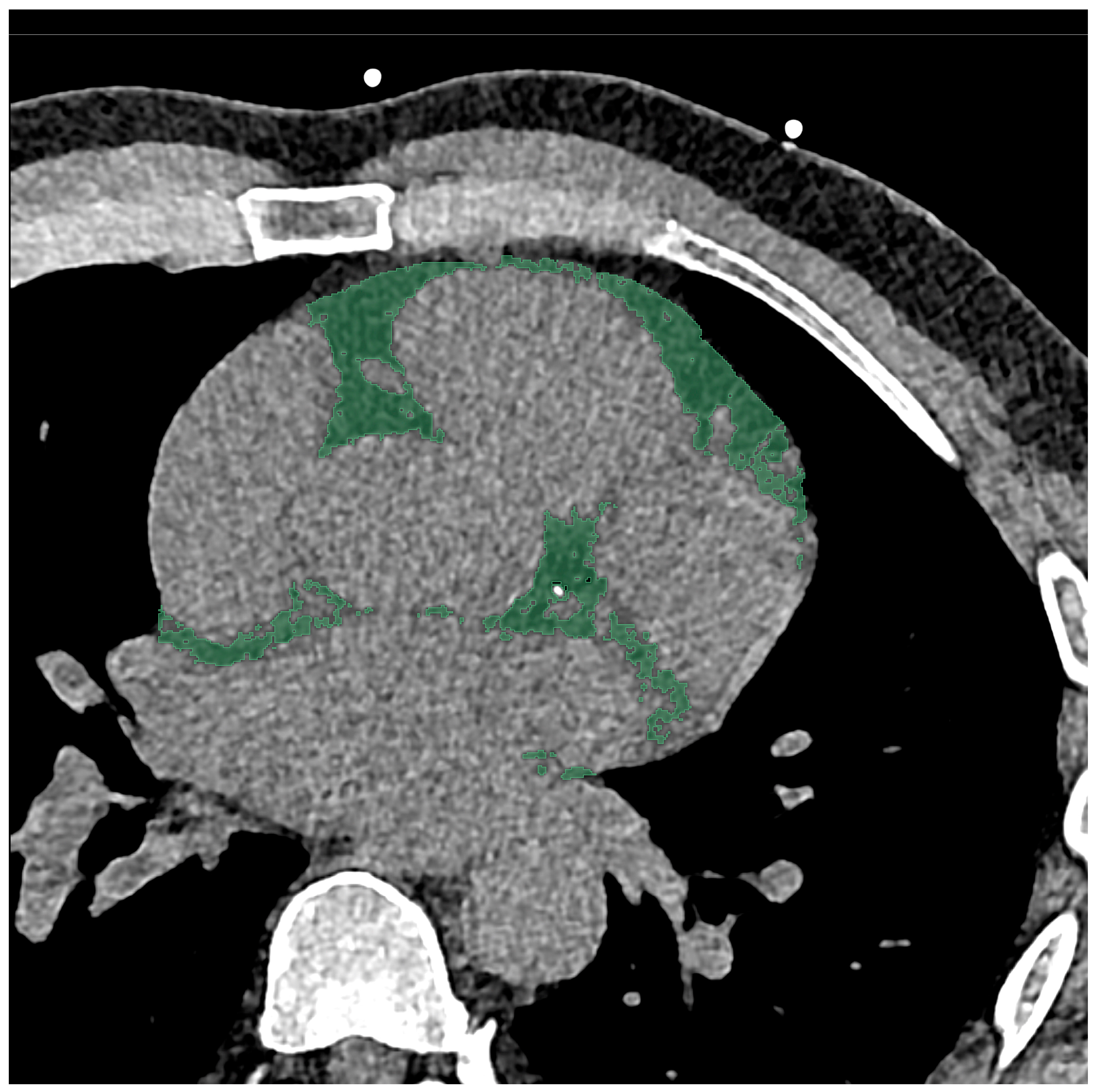
2.3. Chest CT Imaging Analysis
2.4. Radiomics Feature Extraction and Statistical Analysis
3. Results
3.1. Patient Collective
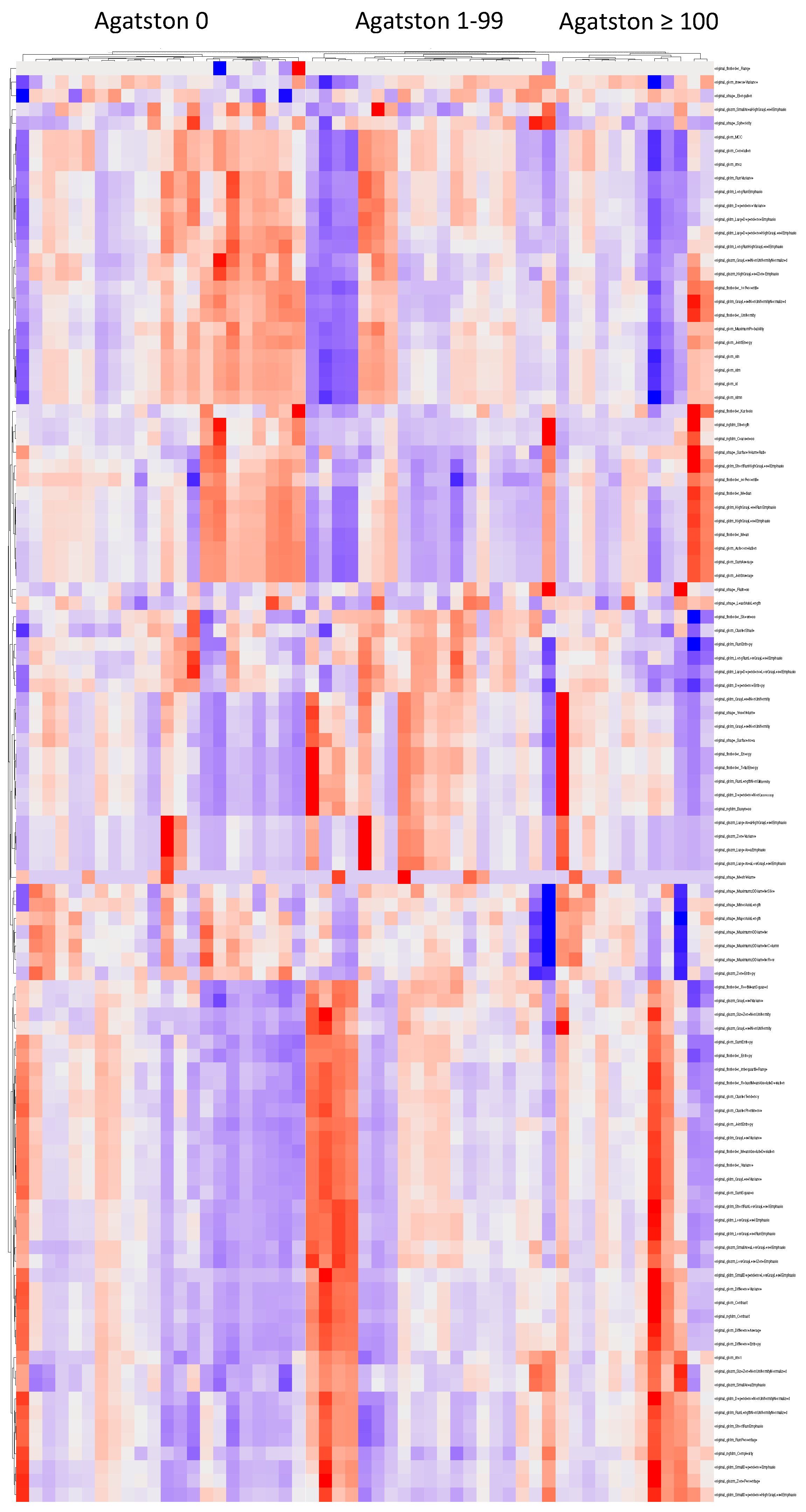
3.2. Cluster Analysis
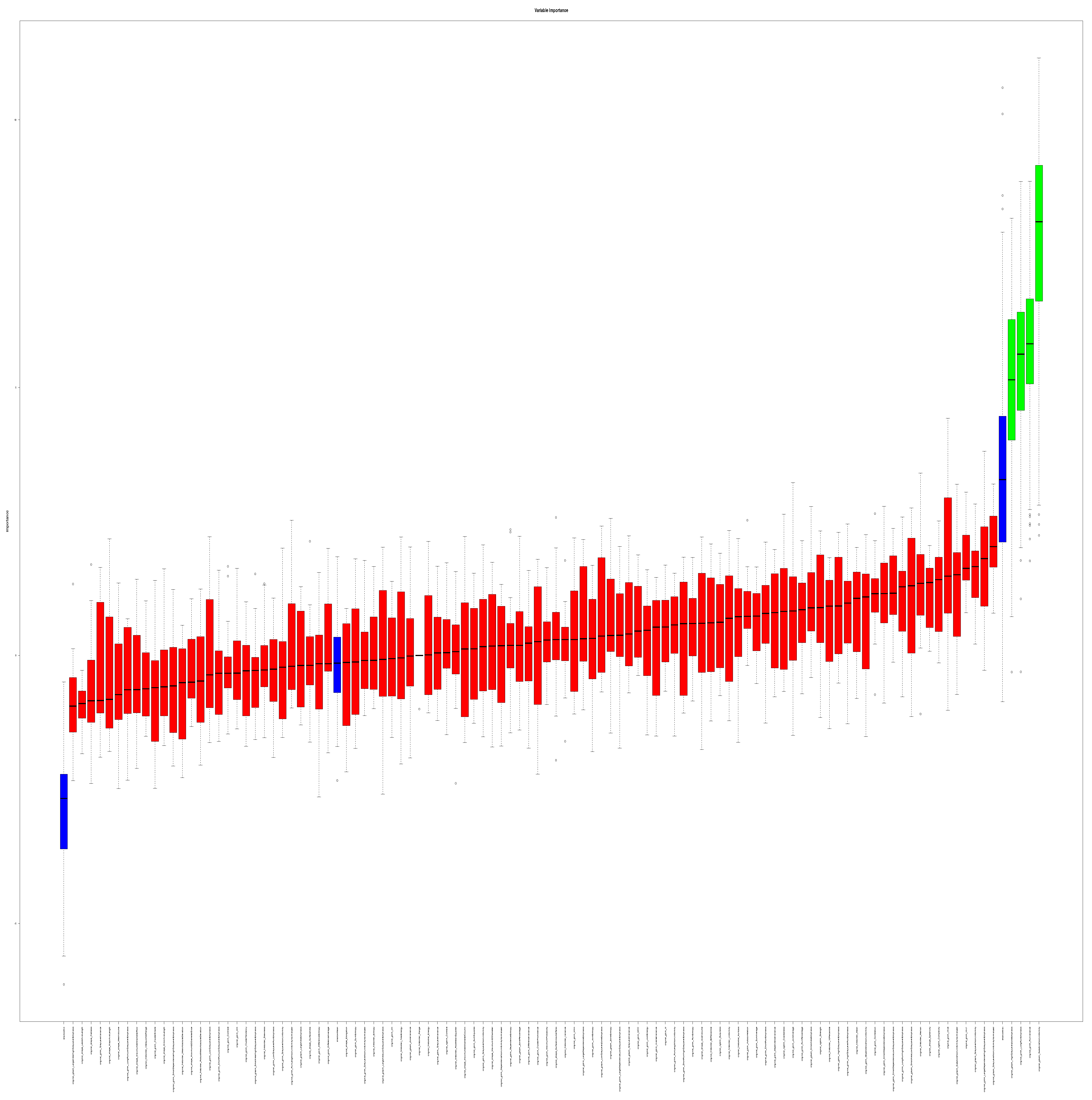
3.3. Feature Selection
3.4. Internal Validation
3.5. Additional Imaging Features
3.6. Feature Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonopoulos, A.S.; Antoniades, C. The Role of Epicardial Adipose Tissue in Cardiac Biology: Classic Concepts and Emerging Roles: Epicardial Fat and Cardiac Biology. J. Physiol. 2017, 595, 3907–3917. [Google Scholar] [CrossRef]
- Ansaldo, A.M.; Montecucco, F.; Sahebkar, A.; Dallegri, F.; Carbone, F. Epicardial Adipose Tissue and Cardiovascular Diseases. Int. J. Cardiol. 2019, 278, 254–260. [Google Scholar] [CrossRef]
- Cosson, E.; Nguyen, M.T.; Rezgani, I.; Tatulashvili, S.; Sal, M.; Berkane, N.; Allard, L.; Brillet, P.-Y.; Bihan, H. Epicardial Adipose Tissue Volume and Coronary Calcification among People Living with Diabetes: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 35. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef]
- Doesch, C.; Streitner, F.; Bellm, S.; Suselbeck, T.; Haghi, D.; Heggemann, F.; Schoenberg, S.O.; Michaely, H.; Borggrefe, M.; Papavassiliu, T. Epicardial Adipose Tissue Assessed by Cardiac Magnetic Resonance Imaging in Patients with Heart Failure Due to Dilated Cardiomyopathy. Obesity 2013, 21, E253–E261. [Google Scholar] [CrossRef]
- The Top 10 Causes of Death, WHO. 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 December 2023).
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- DISCHARGE Trial Group; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Franssens, B.T.; Nathoe, H.M.; Visseren, F.L.J.; Van Der Graaf, Y.; Leiner, T.; Algra, A.; Van Der Graaf, Y.; Grobbee, D.E.; Rutten, G.E.H.M.; Visseren, F.L.J.; et al. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. Am. J. Cardiol. 2017, 119, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Nabi, F.; Xu, J.; Pratt, C.M.; Mahmarian, A.C.; Frias, M.E.; Mahmarian, J.J. Value of CACS Compared with ETT and Myocardial Perfusion Imaging for Predicting Long-Term Cardiac Outcome in Asymptomatic and Symptomatic Patients at Low Risk for Coronary Disease: Clinical Implications in a Multimodality Imaging World. JACC Cardiovasc. Imaging 2015, 8, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.; Kahn, R.; Robertson, R.M.; Clark, N.G.; Doyle, C.; Hong, Y.; Gansler, T.; Glynn, T.; Smith, R.A.; Taubert, K.; et al. Preventing Cancer, Cardiovascular Disease, and Diabetes: A Common Agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation 2004, 109, 3244–3255. [Google Scholar] [CrossRef]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors. J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [CrossRef]
- Hwang, I.-C.; Park, H.E.; Choi, S.-Y. Epicardial Adipose Tissue Contributes to the Development of Non-Calcified Coronary Plaque: A 5-Year Computed Tomography Follow-up Study. J. Atheroscler. Thromb. 2017, 24, 262–274. [Google Scholar] [CrossRef]
- Liu, J.; Fan, W.; Liu, Y.; Bu, H.; Song, J.; Sun, L. Association of Epicardial and Pericardial Adipose Tissue Volumes with Coronary Artery Calcification. Int. Heart J. 2022, 63, 1019–1025. [Google Scholar] [CrossRef]
- Zheng, R.L.; Gu, L.Y.; Duan, S.F.; Jiang, W.L.; Meng, X.P. Value of Pericoronary Adipse Tissue Texture Analysis in Diagnosis of Coronary Artery Disease. Austin Intern. Med. 2021, 5, 1052. [Google Scholar]
- Mundt, P.; Tharmaseelan, H.; Hertel, A.; Rotkopf, L.T.; Nörenberg, D.; Riffel, P.; Schoenberg, S.O.; Froelich, M.F.; Ayx, I. Periaortic Adipose Radiomics Texture Features Associated with Increased Coronary Calcium Score—First Results on a Photon-Counting-CT. BMC Med. Imaging 2023, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non—Small Cell Lung Cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Mithun, S.; Purandare, N.C.; Kumar, R.; Rangarajan, V.; Wee, L.; Dekker, A. Radiomics: A Quantitative Imaging Biomarker in Precision Oncology. Nucl. Med. Commun. 2022, 43, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Taha, B.; Boley, D.; Sun, J.; Chen, C.C. State of Radiomics in Glioblastoma. Neurosurgery 2021, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ayx, I.; Froelich, M.F.; Baumann, S.; Papavassiliu, T.; Schoenberg, S.O. Radiomics in Cardiac Computed Tomography. Diagnostics 2023, 13, 307. [Google Scholar] [CrossRef]
- Eslami, P.; Parmar, C.; Foldyna, B.; Scholtz, J.-E.; Ivanov, A.; Zeleznik, R.; Lu, M.T.; Ferencik, M.; Vasan, R.S.; Baltrusaitis, K.; et al. Radiomics of Coronary Artery Calcium in the Framingham Heart Study. Radiol. Cardiothorac. Imaging 2020, 2, e190119. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Kolossváry, M.; Yuvaraj, J.; Cadet, S.; McElhinney, P.A.; Jiang, C.; Nerlekar, N.; Nicholls, S.J.; Slomka, P.J.; Maurovich-Horvat, P.; et al. Myocardial Infarction Associates with a Distinct Pericoronary Adipose Tissue Radiomic Phenotype. JACC Cardiovasc. Imaging 2020, 13, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, S.Y.; Kim, H.J.; Kim, H.S. Reproducibility and Generalizability in Radiomics Modeling: Possible Strategies in Radiologic and Statistical Perspectives. Korean J. Radiol. 2019, 20, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pal, D.; Abadi, E.; Sauer, T.; Segars, P.; Hsieh, J.; Samei, E. Can Photon-Counting CT Improve Estimation Accuracy of Morphological Radiomics Features? A Simulation Study for Assessing the Quantitative Benefits from Improved Spatial Resolution in Deep Silicon-Based Photon-Counting CT. Acad. Radiol. 2023, 30, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-Counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Hertel, A.; Tharmaseelan, H.; Rotkopf, L.T.; Nörenberg, D.; Riffel, P.; Nikolaou, K.; Weiss, J.; Bamberg, F.; Schoenberg, S.O.; Froelich, M.F.; et al. Phantom-Based Radiomics Feature Test–Retest Stability Analysis on Photon-Counting Detector CT. Eur. Radiol. 2023, 33, 4905–4914. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hrybouski, S.; Paterson, D.I.; Li, Z.; Lan, Y.; Luo, L.; Shen, X.; Xu, L. Comparison of Epicardial Adipose Tissue Volume Quantification between ECG-Gated Cardiac and Non-ECG-Gated Chest Computed Tomography Scans. BMC Cardiovasc. Disord. 2022, 22, 545. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 April 2022).
- R Studio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: https://www.rstudio.com/ (accessed on 2 April 2022).
- IBM Corp. IBM SPSS Statistics for Windows; Version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Thibault, G.; Fertil, B.; Navarro, C.; Pereira, S.; Cau, P.; Levy, N.; Sequeira, J.; Mari, J.-L. Texture Indexes and Gray Level Size Zone Matrix. Application to Cell Nuclei Classification. In Proceedings of the Pattern Recognition and Information Processing (PRIP), Minsk, Belarus, 19–21 May 2009; pp. 140–145. [Google Scholar]
- Xu, D.-H.; Kurani, A.; Furst, J.; Raicu, D. Run-length encoding for volumetric texture. In Proceedings of the 4th IASTED International Conference on Visualization, Imaging, and Image Processing, Marbella, Spain, 6–8 September 2004. [Google Scholar]
- Pandey, N.N.; Sharma, S.; Jagia, P.; Kumar, S. Epicardial Fat Attenuation, Not Volume, Predicts Obstructive Coronary Artery Disease and High Risk Plaque Features in Patients with Atypical Chest Pain. Br. J. Radiol. 2020, 93, 20200540. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Wang, Y.; Zhou, N.; Shu, J.; Stamm, C.; Jiang, M.; Luo, F. Association of Epicardial Adipose Tissue Attenuation with Coronary Atherosclerosis in Patients with a High Risk of Coronary Artery Disease. Atherosclerosis 2019, 284, 230–236. [Google Scholar] [CrossRef]
- Gao, Z.; Zuo, Y.; Jia, L.; Yin, Y.; Yang, X.; Fan, Y.; Liu, H. Association between Epicardial Adipose Tissue Density and Characteristics of Coronary Plaques Assessed by Coronary Computed Tomographic Angiography. Int. J. Cardiovasc. Imaging 2022, 38, 673–681. [Google Scholar] [CrossRef]
- Kahmann, J.; Tharmaseelan, H.; Riffel, P.; Overhoff, D.; Papavassiliu, T.; Schoenberg, S.O.; Froelich, M.F.; Ayx, I. Pericoronary Radiomics Texture Features Associated with Hypercholesterolemia on a Photon-Counting-CT. Front. Cardiovasc. Med. 2023, 10, 1223035. [Google Scholar] [CrossRef] [PubMed]
- Ayx, I.; Tharmaseelan, H.; Hertel, A.; Nörenberg, D.; Overhoff, D.; Rotkopf, L.T.; Riffel, P.; Schoenberg, S.O.; Froelich, M.F. Comparison Study of Myocardial Radiomics Feature Properties on Energy-Integrating and Photon-Counting Detector CT. Diagnostics 2022, 12, 1294. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Maurice, J.-B.; Devos, L.; Remy, M.; Dubus, F. Image Quality Analysis of Photon-Counting CT Compared with Dual-Source CT: A Phantom Study for Chest CT Examinations. Diagnostics 2023, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
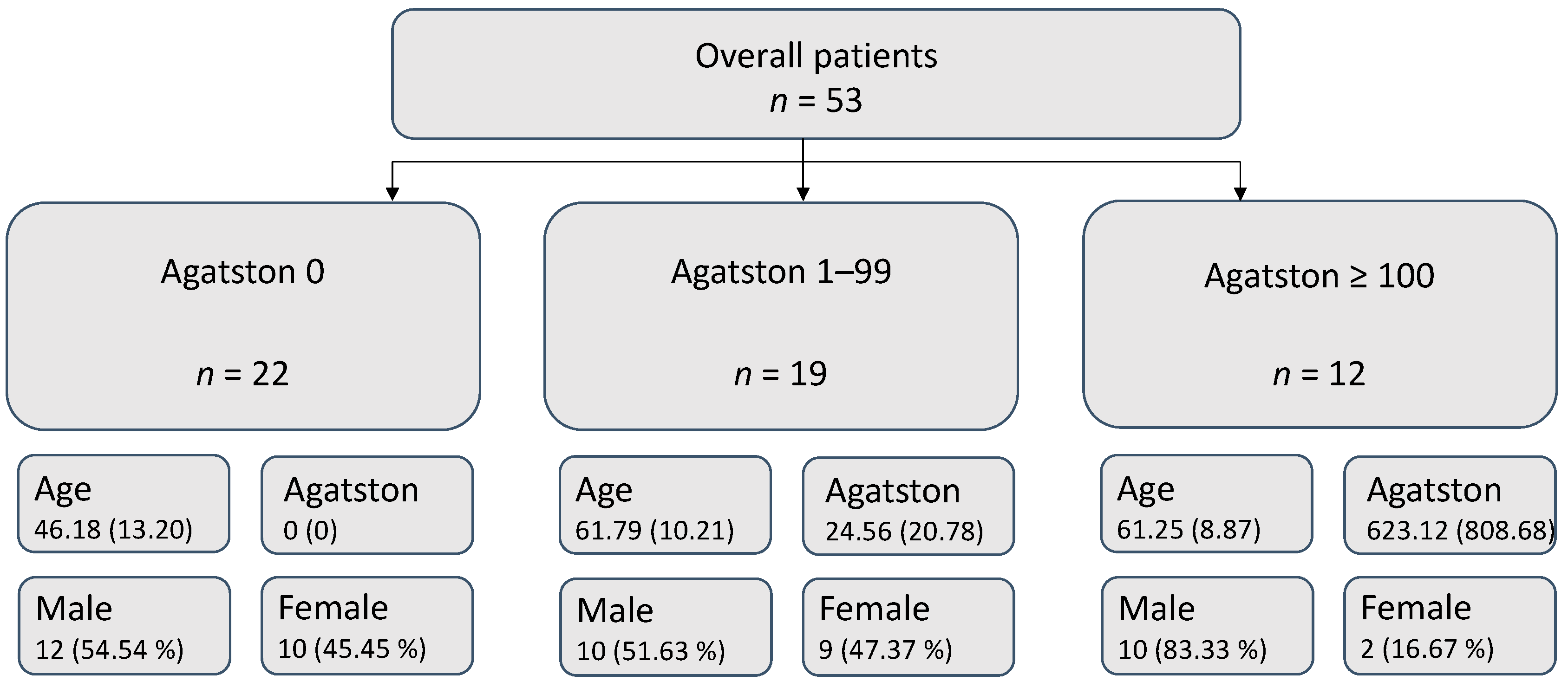
| Overall Patients | Agatston 0 | Agatston 1–99 | Agatston ≥ 100 | |
|---|---|---|---|---|
| n | 53 | 22 | 19 | 12 |
| Patients with known cardiovascular risk factors | 24 | 7 | 10 | 7 |
| Diabetes | 1 | 1 | 0 | 0 |
| Hypertension | 19 | 5 | 9 | 5 |
| Dislipidemia | 4 | 3 | 1 | 0 |
| Nicotine abuse | 5 | 1 | 2 | 2 |
| Feature | Agatston 0 | Agatston 1–99 | Agatston ≥ 100 | p-Value |
|---|---|---|---|---|
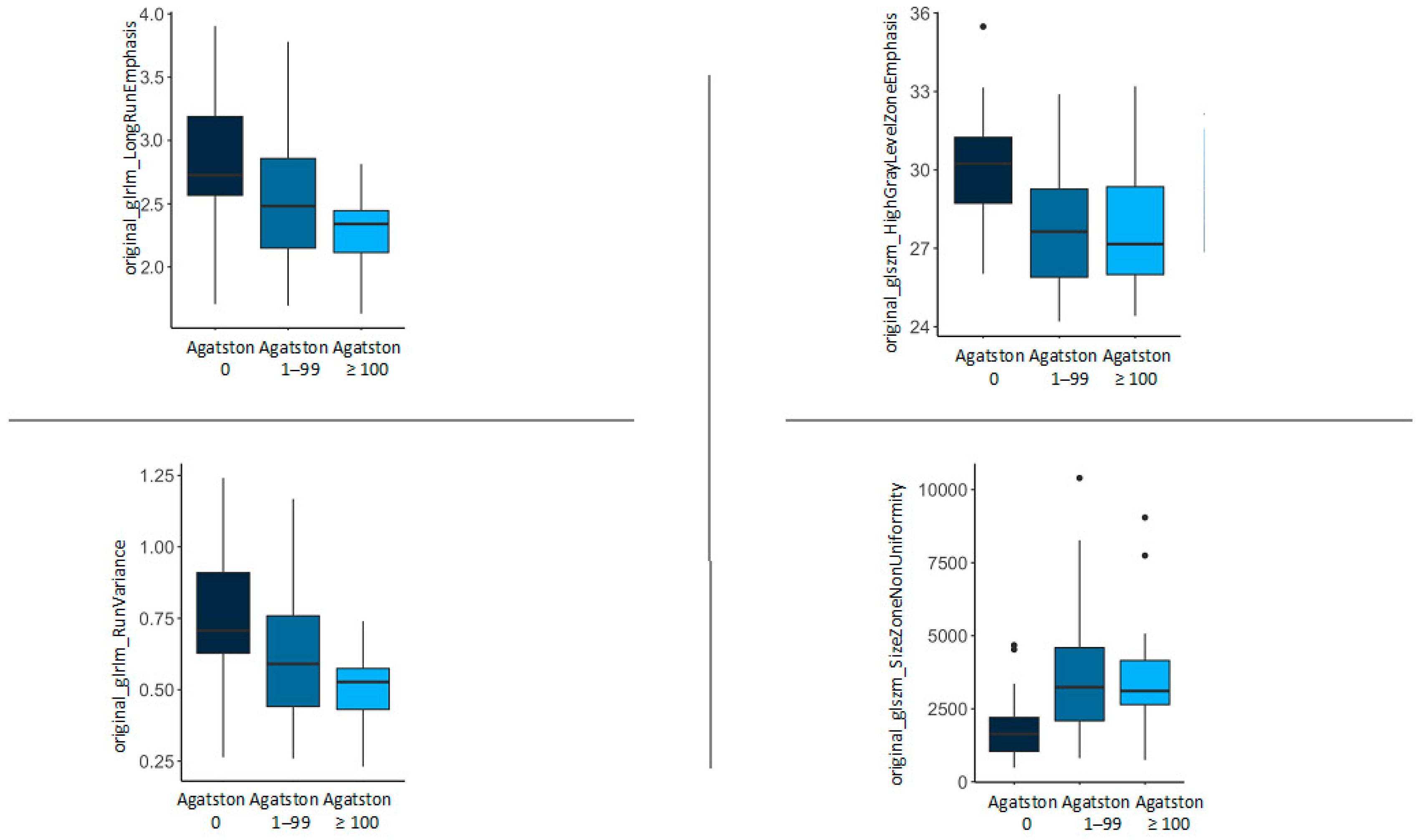
| original_glrlm_LongRunEmphasis | 2.82 (0.51) | 2.55 (0.56) | 2.27 (0.34) | 0.013 |
| original_glrlm_RunVariance | 0.74 (0.23) | 0.62 (0.25) | 0.50 (0.15) | 0.013 |
| original_glszm_HighGrayLevelZoneEmphasis | 30.16 (2.32) | 27.68 (2.42) | 27.83 (2.43) | 0.003 |
| original_glszm_SizeZoneNonUniformity | 1893.05 (1156.82) | 3815.24 (2487.78) | 3817.49 (2390.38) | 0.005 |
| Density (HU) | −79.11 (5.81) | −86.38 (6.14) | −81.73 (8.06) | 0.003 |
| Volume (mm3) | 257,339.30 (109,963.91) | 386,997.04 (162,348.54) | 333,642.30 (203,406.17) | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundt, P.; Hertel, A.; Tharmaseelan, H.; Nörenberg, D.; Papavassiliu, T.; Schoenberg, S.O.; Froelich, M.F.; Ayx, I. Analysis of Epicardial Adipose Tissue Texture in Relation to Coronary Artery Calcification in PCCT: The EAT Signature! Diagnostics 2024, 14, 277. https://doi.org/10.3390/diagnostics14030277
Mundt P, Hertel A, Tharmaseelan H, Nörenberg D, Papavassiliu T, Schoenberg SO, Froelich MF, Ayx I. Analysis of Epicardial Adipose Tissue Texture in Relation to Coronary Artery Calcification in PCCT: The EAT Signature! Diagnostics. 2024; 14(3):277. https://doi.org/10.3390/diagnostics14030277
Chicago/Turabian StyleMundt, Peter, Alexander Hertel, Hishan Tharmaseelan, Dominik Nörenberg, Theano Papavassiliu, Stefan O. Schoenberg, Matthias F. Froelich, and Isabelle Ayx. 2024. "Analysis of Epicardial Adipose Tissue Texture in Relation to Coronary Artery Calcification in PCCT: The EAT Signature!" Diagnostics 14, no. 3: 277. https://doi.org/10.3390/diagnostics14030277





