Recent Advances in Endoscopic Ultrasound for Gallbladder Disease Diagnosis
Abstract
:1. Introduction
2. Differential Diagnosis of GB Disease
2.1. GB Wall-Thickening Lesions (Table 1)
- (i)
- Adenomyomatosis
- (ii)
- Chronic cholecystitis, Xanthogranulomatous cholecystitis
- (iii)
- Immunoglobulin G4-related sclerosing cholecystitis (IgG4-CC) (Figure 3)
- (iv)
- GB mucosal hyperplasia associated with pancreaticobiliary maljunction (PBM) (Figure 4)
- GB carcinoma
2.2. Protuberant Lesions (PLs) (Table 2)
- (i)
- Nonneoplastic lesions (GB polyps)
- (ii)
- Neoplastic lesions
3. Comparison of EUS and Other Modalities in the Detection of GB Lesions
4. EUS Scope
- (i)
- RS-EUS
- (ii)
- CL-EUS
5. Contrast Harmonic Imaging (CH-Imaging)
- (i)
- CH-TUS
- (ii)
- CH-EUS (Table 4)
| Author | Year | Patient | Study Design | Contrast Agent | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Park, C.H et al. [64] | 2013 | 20 | retrospective | SonoVue | 0.75 | 0.67 |
| Choi et al. [65] | 2013 | 90 | retrospective | SonoVue | 0.94 | 0.93 |
| Imazu et al. [66] | 2014 | 36 | retrospective | Sonazoid | 0.90 | 0.98 |
| Sugimoto et al. [67] | 2016 | 24 | retrospective | Sonazoid | 1.00 | 0.94 |
| Kamata et al. [3] | 2017 | 125 | retrospective | Sonazoid | 0.90 | 0.98 |
| Leem et al. [68] | 2018 | 145 | retrospective | SonoVue | 0.97 | 0.55 |
- (iii)
- CH-imaging phase
6. Cytology and Biopsy for GB Lesion
- (i)
- Endoscopic Transpupillary Approach
- (ii)
| Author | Year | Patient | Study Design | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|
| Ogura et al. [89] | 2014 | 16 | retrospective | 1.00 | 1.00 | 1.00 |
| Singla et al. [86] | 2018 | 101 | retrospective | 0.91 | 1.00 | 0.91 |
| Goyal, S et al. [84] | 2023 | 489 | retrospective | - | - | 0.95 |
| Tong, T et al. [85] | 2023 | 27 | retrospective | 0.95 | 1.00 | 0.96 |
| Kuraishi et al. [94] | 2023 | 187 | retrospective | 0.97 | 1.00 | 0.97 |
- When it is difficult to categorize a lesion as benign or malignant or when surgery is extremely invasive, EUS-FNA should be considered.
- In patients with GB tumors accompanied by either liver invasion or metastasis or both, including lymph nodes, they should be first punctured.
- For GB masses or wall thickening, the lesion should be punctured without the puncture needle passing through the lumen of the GB [2].
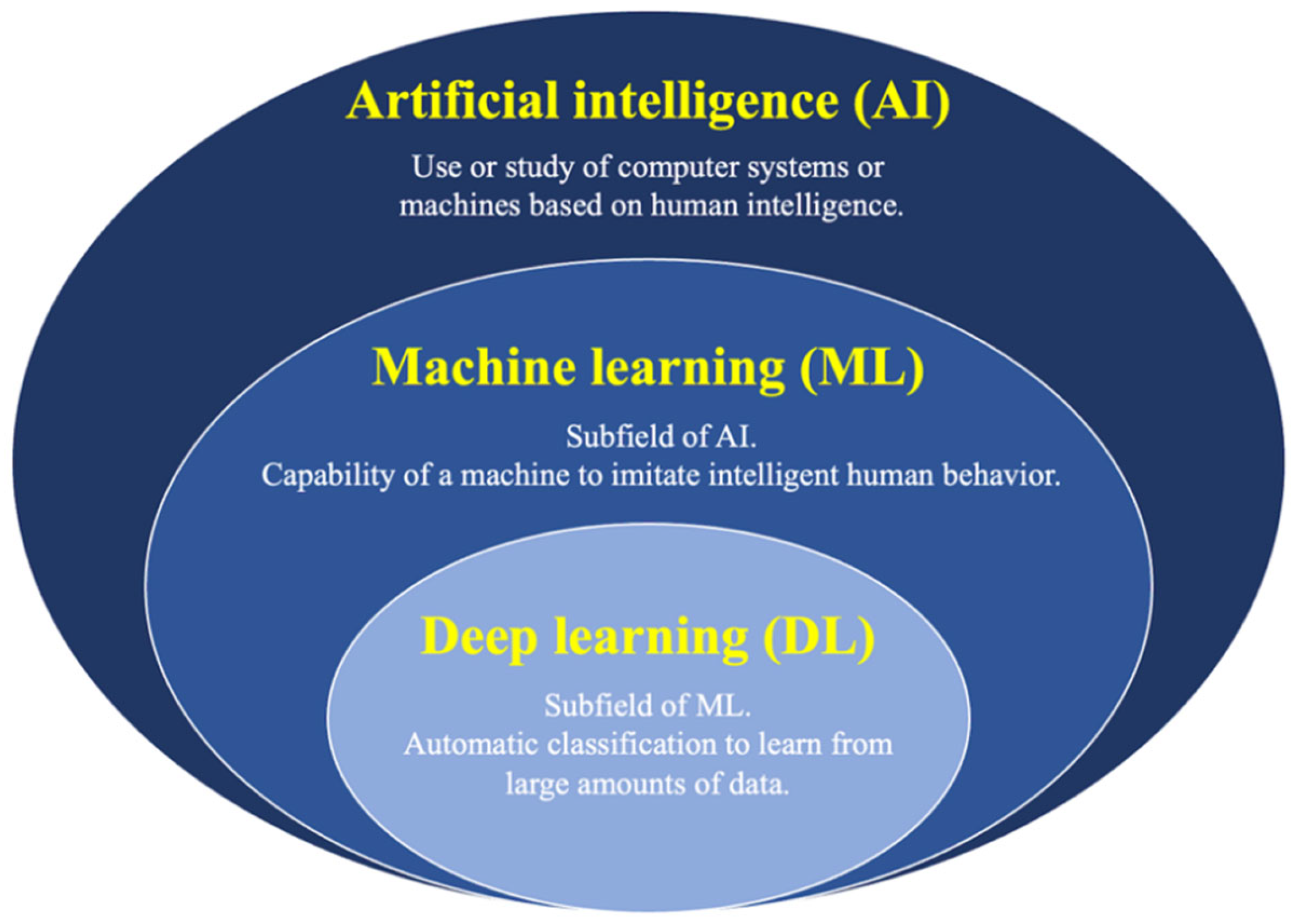
7. AI Approach to the Diagnosis of GB Lesions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihara, S.; Yoshioka, R.; Tamada, M. Update of ultrasonographic screening for gallbladder cancer in mass survey (in Japanese). Tanto Sui 2005, 26, 801–804. [Google Scholar]
- Hijioka, S.; Nagashio, Y.; Ohba, A.; Maruki, Y.; Okusaka, T. The Role of EUS and EUS-FNA in Differentiating Benign and Malignant Gallbladder Lesions. Diagnostics 2021, 11, 1586. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Nishida, N.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig. Endosc. 2018, 30, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Ogura, T.; Haba, S.; Mekky, M.A.; Bhatia, V.; Hosoda, W.; Yatabe, Y.; et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J. Hepatobiliary Pancreat. Sci. 2012, 19, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Mekky, M.A.; Bhatia, V.; Sawaki, A.; Mizuno, N.; Hara, K.; Hosoda, W.; Shimizu, Y.; Tamada, K.; Niwa, Y.; et al. Can EUS-guided FNA distinguish between gallbladder cancer and xanthogranulomatous cholecystitis? Gastrointest. Endosc. 2010, 72, 622–627. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S.E.; East, J.E.; Rastogi, A.; Bretthauer, M.; Misawa, M.; Sekiguchi, M.; Matsuda, T.; Saito, Y.; Ikematsu, H.; et al. Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: An add-on analysis of a clinical trial (with video). Gastrointest. Endosc. 2020, 92, 905–911.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, W.; Wan, X.; Zhang, J.; Shen, L.; Hu, S.; Ding, Q.; Mu, G.; Yin, A.; Huang, X.; et al. A deep neural network improves endoscopic detection of early gastric cancer without blind spots. Endoscopy 2019, 51, 522–531. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nakaoka, K.; Kawabe, N.; Kuzuya, T.; Funasaka, K.; Nagasaka, M.; Nakagawa, Y.; Miyahara, R.; Shibata, T.; Hirooka, Y. The Role of Endoscopic Ultrasound in the Diagnosis of Gallbladder Lesions. Diagnostics 2021, 11, 1789. [Google Scholar] [CrossRef]
- Okaniwa, S. Everything you need to know about ultrasound for diagnosis of gallbladder diseases. J. Med. Ultrason 2001 2021, 48, 145–147. [Google Scholar] [CrossRef]
- Okaniwa, S. How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics 2021, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Kim, Y.J.; Park, H.S.; Jung, S.I. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J. Gastroenterol. 2020, 26, 2967–2986. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kamisawa, T.; Chiba, K.; Kikuyama, M.; Nakahodo, J.; Igarashi, Y. Gallbladder wall thickening in patients with IgG4-related diseases, with special emphasis on IgG4-related cholecystitis. Scand. J. Gastroenterol. 2021, 56, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Mihara, K.; Amemiya, R.; Nakagawa, M.; Hanada, R.; Inoue, K.; Shito, M.; Orikasa, H.; Aiura, K. Isolated IgG4-related cholecystitis with localized gallbladder wall thickening mimicking gallbladder cancer: A case report and literature review. BMC Gastroenterol. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Kambadakone, A.; Nikolaidis, P.; Subbiah, V.; Subbiah, I.M.; Devine, C. Current update on gallbladder carcinoma. Abdom. Radiol. 2021, 46, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Inui, K.; Katano, Y.; Tachi, Y.; Yamamoto, S. B-mode ultrasonographic diagnosis in gallbladder wall thickening. J. Med. Ultrason. 2001 2021, 48, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Katanuma, A.; Hayashi, T.; Kin, T.; Takahashi, K. Role of endoscopic ultrasound for gallbladder disease. J. Med. Ultrason. 2001 2021, 48, 187–198. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Winsberg, F. Comet-tail artifact from cholesterol crystals: Observations in the postlithotripsy gallbladder and an in vitro model. Radiology 1990, 177, 153–156. [Google Scholar] [CrossRef]
- Nabatame, N.; Shirai, Y.; Nishimura, A.; Yokoyama, N.; Wakai, T.; Hatakeyama, K. High risk of gallbladder carcinoma in elderly patients with segmental adenomyomatosis of the gallbladder. J. Exp. Clin. Cancer Res. 2004, 23, 593–598. [Google Scholar]
- Päivänsalo, M.; Siniluoto, T.; Myllylä, V.; Kairaluoma, M.I.; Kallioinen, M. Ultrasound in acute and chronic cholecystitis. RoFo 1987, 147, 84–87. [Google Scholar] [CrossRef]
- Kim, P.N.; Ha, H.K.; Kim, Y.H.; Lee, M.G.; Kim, M.H.; Auh, Y.H. US findings of xanthogranulomatous cholecystitis. Clin. Radiol. 1998, 53, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chon, H.K.; Choi, K.H. IgG4-related sclerosing cholangitis involving the gallbladder mimicking a hilar cholangiocarcinoma. Endoscopy 2022, 54, E739–E740. [Google Scholar] [CrossRef] [PubMed]
- Ichinokawa, M.; Matsumoto, J.; Kuraya, T.; Kuwabara, S.; Wada, H.; Kato, K.; Ikeda, A.; Murakawa, K.; Ono, K. A rare case of localized IgG4-related sclerosing cholecystitis mimicking gallbladder cancer. J. Rural Med. 2019, 14, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lin, H.M.; Cai, Z.X.; Du, S.J.; Zeng, H.; Xu, L.B.; Wang, J.; Liu, C. Clinical strategies for differentiating IgG4-related cholecystitis from gallbladder carcinoma to avoid unnecessary surgical resection. Sci. China Life Sci. 2020, 63, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Kuwatani, M.; Sakamoto, N. Clinical and Image Characteristics of IgG4-Related Sclerosing Cholecystitis. Diagnostics 2021, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, A.; Itoi, T.; Endo, M.; Kitamura, K.; Mukaide, M.; Itokawa, F.; Ozawa, T.; Aoki, T. Pathological features and surgical outcome of pancreaticobiliary maljunction without dilatation of the extrahepatic bile duct. Oncol. Rep. 2004, 11, 269–276. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Kudo, S.; Fukahori, T.; Matsuo, Y.; Miyazaki, K.; Tokunaga, O.; Koyama, T.; Fujimoto, K. Endoscopic ultrasonography for demonstrating loss of multiple-layer pattern of the thickened gallbladder wall in the preoperative diagnosis of gallbladder cancer. Eur. Radiol. 1997, 7, 1323–1327. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.H.; Park, D.I.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Choi, S.H. Clinical usefulness of endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig. Dis. Sci. 2012, 57, 508–515. [Google Scholar] [CrossRef]
- Terzi, C.; Sökmen, S.; Seçkin, S.; Albayrak, L.; Uğurlu, M. Polypoid lesions of the gallbladder: Report of 100 cases with special reference to operative indications. Surgery 2000, 127, 622–627. [Google Scholar] [CrossRef]
- Mellnick, V.M.; Menias, C.O.; Sandrasegaran, K.; Hara, A.K.; Kielar, A.Z.; Brunt, E.M.; Doyle, M.B.; Dahiya, N.; Elsayes, K.M. Polypoid lesions of the gallbladder: Disease spectrum with pathologic correlation. Radiographics 2015, 35, 387–399. [Google Scholar] [CrossRef]
- Kimura, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K. Differential diagnosis of large-sized pedunculated polypoid lesions of the gallbladder by endoscopic ultrasonography: A prospective study. J. Gastroenterol. 2001, 36, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Park, J.Y.; Kim, Y.J.; Kim, H.M.; Kim, H.J.; Hong, S.P.; Park, S.W.; Chung, J.B.; Song, S.Y.; Bang, S. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest. Endosc. 2009, 69, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Atomi, Y.; Yamato, T. Endoscopic ultrasonography for differential diagnosis of polypoid gall bladder lesions: Analysis in surgical and follow up series. Gut 2000, 46, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Yoshikawa, T.; Araida, T.; Takasaki, K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am. J. Surg. 2001, 181, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cocco, G.; Basilico, R.; Delli Pizzi, A.; Cocco, N.; Boccatonda, A.; D’Ardes, D.; Fabiani, S.; Anzoletti, N.; D’Alessandro, P.; Vallone, G.; et al. Gallbladder polyps ultrasound: What the sonographer needs to know. J. Ultrasound 2021, 24, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.H.; Kim, Y.J.; Ryu, J.K.; Kim, Y.T.; Lee, J.Y.; Han, J.K. Diagnostic accuracy of transabdominal high-resolution US for staging gallbladder cancer and differential diagnosis of neoplastic polyps compared with EUS. Eur. Radiol. 2017, 27, 3097–3103. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, S.W.; Lee, S.E.; Hwang, D.W.; Kim, E.J.; Lee, J.Y.; Kim, S.J.; Ryu, J.K.; Kim, Y.T. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann. Surg. 2009, 250, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Katanuma, A.; Maguchi, H.; Takahashi, K.; Osanai, M.; Yane, K.; Hashigo, S.; Harada, R.; Kato, S.; Kato, R.; et al. Prospective, randomized, comparative study of delineation capability of radial scanning and curved linear array endoscopic ultrasound for the pancreaticobiliary region. Endosc. Int. Open 2014, 2, E160–E170. [Google Scholar] [CrossRef]
- Harvey, C.J.; Blomley, M.J.; Eckersley, R.J.; Cosgrove, D.O. Developments in ultrasound contrast media. Eur. Radiol. 2001, 11, 675–689. [Google Scholar] [CrossRef]
- Numata, K.; Tanaka, K.; Kiba, T.; Saito, S.; Ikeda, M.; Hara, K.; Tanaka, N.; Morimoto, M.; Iwase, S.; Sekihara, H. Contrast-enhanced, wide-band harmonic gray scale imaging of hepatocellular carcinoma: Correlation with helical computed tomographic findings. J. Ultrasound Med. 2001, 20, 89–98. [Google Scholar] [CrossRef]
- Xu, J.M.; Guo, L.H.; Xu, H.X.; Zheng, S.G.; Liu, L.N.; Sun, L.P.; Lu, M.D.; Wang, W.P.; Hu, B.; Yan, K.; et al. Differential diagnosis of gallbladder wall thickening: The usefulness of contrast-enhanced ultrasound. Ultrasound Med. Biol. 2014, 40, 2794–2804. [Google Scholar] [CrossRef]
- Saftoiu, A.; Napoleon, B.; Arcidiacono, P.G.; Braden, B.; Burmeister, S.; Carrara, S.; Cui, X.W.; Fusaroli, P.; Gottschalk, U.; Hocke, M.; et al. Do we need contrast agents for EUS? Endosc. Ultrasound 2020, 9, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Lariño-Noia, J.; de la Iglesia-García, D.; Dominguez-Muñoz, J.E. Endoscopic ultrasonography: Enhancing diagnostic accuracy. Best Pract. Res. Clin. Gastroenterol. 2022, 60–61, 101808. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tobari, M.; Nagamatsu, H.; Shiina, S.; Yamaguchi, T. Contrast-enhanced ultrasonography for the management of portal hypertension in cirrhosis. Front. Med. 2022, 9, 1057045. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.K.; Kang, H.J.; Choi, S.H.; Park, M.S.; Yu, M.H.; Kim, B.; You, M.W.; Lim, S.; Cho, Y.S.; Lee, M.W.; et al. Diagnosing Hepatocellular Carcinoma Using Sonazoid Contrast-Enhanced Ultrasonography: 2023 Guidelines From the Korean Society of Radiology and the Korean Society of Abdominal Radiology. Korean J. Radiol. 2023, 24, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.H.; Xu, H.X.; Xie, X.Y.; Lu, M.D.; Kuang, M.; Xu, Z.F.; Liu, G.J.; Wang, Z.; Liang, J.Y.; Chen, L.D.; et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur. Radiol. 2010, 20, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Bai, M.; Gu, J.Y.; He, Y.Q.; Qiao, X.H.; Du, L.F. Value of contrast-enhanced ultrasound in the differential diagnosis of gallbladder lesion. World J. Gastroenterol. 2018, 24, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Zengel, P.; Armbruster, M.; Rübenthaler, J.; Clevert, D.A. Contrast-enhanced ultrasound (CEUS) and gallbladder diseases—A retrospective mono-center analysis of imaging findings with histopathological correlation. Clin. Hemorheol. Microcirc. 2019, 71, 151–158. [Google Scholar] [CrossRef]
- Miwa, H.; Numata, K.; Sugimori, K.; Sanga, K.; Hirotani, A.; Tezuka, S.; Goda, Y.; Irie, K.; Ishii, T.; Kaneko, T.; et al. Differential diagnosis of gallbladder polypoid lesions using contrast-enhanced ultrasound. Abdom. Radiol. 2019, 44, 1367–1378. [Google Scholar] [CrossRef]
- Miwa, H.; Numata, K.; Sugimori, K.; Kaneko, T.; Maeda, S. Vascular evaluation using transabdominal ultrasound for gallbladder polyps. J. Med. Ultrason. 2001 2021, 48, 159–173. [Google Scholar] [CrossRef]
- Zhu, L.; Han, P.; Lee, R.; Jiang, B.; Jiao, Z.; Li, N.; Tang, W.; Fei, X. Contrast-enhanced ultrasound to assess gallbladder polyps. Clin. Imaging 2021, 78, 8–13. [Google Scholar] [CrossRef]
- Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Armbruster, M.; Rübenthaler, J.; Clevert, D.A. Contrast-enhanced ultrasound (CEUS) for the evaluation of gallbladder diseases in comparison to cross-sectional imaging modalities and histopathological results. Clin. Hemorheol. Microcirc. 2019, 71, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ashida, R.; Kitano, M. The usefulness of endoscopic ultrasound in the diagnosis of gallbladder lesions. Front. Med. 2022, 9, 957557. [Google Scholar] [CrossRef]
- Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Zengel, P.; Armbruster, M.; Rübenthaler, J.; Clevert, D.A. Diagnostic performance of contrast-enhanced ultrasound (CEUS) for the evaluation of gallbladder diseases1. Clin. Hemorheol. Microcirc. 2018, 69, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Negrão de Figueiredo, G.; Mueller-Peltzer, K.; Schwarze, V.; Zhang, L.; Rübenthaler, J.; Clevert, D.A. Performance of contrast-enhanced ultrasound (CEUS) compared to MRI in the diagnostic of gallbladder diseases. Clin. Hemorheol. Microcirc. 2019, 73, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Franchellucci, G.; Andreozzi, M.; Carrara, S.; De Luca, L.; Auriemma, F.; Paduano, D.; Calabrese, F.; Facciorusso, A.; Poletti, V.; Zerbi, A.; et al. Contrast Enhanced EUS for Predicting Solid Pancreatic Neuroendocrine Tumor Grade and Aggressiveness. Diagnostics 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, Y.; Kitano, M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 81. [Google Scholar] [CrossRef]
- Ishii, T.; Katanuma, A.; Toyonaga, H.; Chikugo, K.; Nasuno, H.; Kin, T.; Hayashi, T.; Takahashi, K. Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 316. [Google Scholar] [CrossRef]
- Hussain, A.; Weimer, D.S.; Mani, N. Diagnosing Pancreatic Adenocarcinoma With Contrast-Enhanced Ultrasonography: A Literature Review of Research in Europe and Asia. Cureus 2022, 14, e22080. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ohno, E.; Mizutani, Y.; Iida, T.; Koya, T.; Sasaki, Y.; Ogawa, H.; Kinoshita, F.; Hirooka, Y.; Kawashima, H. Comparison of contrast-enhanced transabdominal ultrasonography following endoscopic ultrasonography with GD-EOB-DTPA-enhanced MRI for the sequential diagnosis of liver metastasis in patients with pancreatic cancer. J. Hepatobiliary Pancreat. Sci. 2022, 29, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Coluccio, C.; Marocchi, G.; Sbrancia, M.; Fabbri, C. The Role of Contrast-Enhanced Harmonic Endoscopic Ultrasound in Interventional Endoscopic Ultrasound. Medicina 2021, 57, 1085. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, T.; Ziogas, D.; Tziatzios, G.; Gkolfakis, P.; Koukoulioti, E.; Kapizioni, C.; Triantafyllou, K.; Facciorusso, A.; Papanikolaou, I.S. EUS-Guided Diagnosis of Gastric Subepithelial Lesions, What Is New? Diagnostics 2023, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chung, M.J.; Oh, T.G.; Park, J.Y.; Bang, S.; Park, S.W.; Kim, H.; Hwang, H.K.; Lee, W.J.; Song, S.Y. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg. Endosc. 2013, 27, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Seo, D.W.; Choi, J.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest. Endosc. 2013, 78, 484–493. [Google Scholar] [CrossRef]
- Imazu, H.; Mori, N.; Kanazawa, K.; Chiba, M.; Toyoizumi, H.; Torisu, Y.; Koyama, S.; Hino, S.; Ang, T.L.; Tajiri, H. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig. Dis. Sci. 2014, 59, 1909–1916. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Konno, N.; Suzuki, R.; Asama, H.; Hikichi, T.; Watanabe, K.; Waragai, Y.; Kikuchi, H.; Takasumi, M.; et al. The efficacy of contrast-enhanced harmonic endoscopic ultrasonography in diagnosing gallbladder cancer. Sci. Rep. 2016, 6, 25848. [Google Scholar] [CrossRef]
- Leem, G.; Chung, M.J.; Park, J.Y.; Bang, S.; Song, S.Y.; Chung, J.B.; Park, S.W. Clinical Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography in the Differential Diagnosis of Pancreatic and Gallbladder Masses. Clin. Endosc. 2018, 51, 80–88. [Google Scholar] [CrossRef]
- Numata, K. Advances in ultrasound systems for hepatic lesions in Japan. J. Med. Ultrason. 2001 2015, 42, 297–301. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med. 2018, 39, 154–180. [Google Scholar] [CrossRef]
- Kawahara, S.; Tomoda, T.; Kato, H.; Ueki, T.; Akimoto, Y.; Harada, R.; Toji, T.; Okada, H. Accuracy of Endoscopic Transpapillary Gallbladder Drainage with Liquid-Based Cytology for Gallbladder Disease. Digestion 2022, 103, 116–125. [Google Scholar] [CrossRef]
- Inui, K.; Yoshino, J.; Miyoshi, H. Diagnosis of gallbladder tumors. Intern. Med. 2011, 50, 1133–1136. [Google Scholar] [CrossRef]
- Matsumori, T.; Uza, N.; Okada, H.; Maruno, T.; Shiokawa, M.; Kodama, Y.; Seno, H. A novel technique for performing gallbladder tumor biopsy using a stent delivery system and biopsy forceps. Endoscopy 2020, 52, E415–E417. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobara, H.; Masaki, T. Novel cholangioscopy-guided targeted biopsy for diagnosing gallbladder carcinoma. Dig. Endosc. 2021, 33, e51–e53. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, T.; Uza, N.; Shiokawa, M.; Maruno, T.; Nishikawa, Y.; Morita, T.; Kuwada, T.; Marui, S.; Okada, H.; Taura, K.; et al. Clinical impact of a novel device delivery system in the diagnosis of bile duct lesions: A single-center experience. J. Gastroenterol. Hepatol. 2022, 37, 1360–1366. [Google Scholar] [CrossRef]
- Mandai, K.; Inoue, T.; Uno, K.; Yasuda, K. Transferring a naso-gallbladder drainage tube to the mouth for re-examination of a gallbladder lesion. J. Hepatobiliary Pancreat. Sci. 2022, 29, e77–e78. [Google Scholar] [CrossRef]
- Yane, K.; Sumiyoshi, T.; Kondo, H. Transpapillary gallbladder biopsy using newly designed endoscopic sheath. Dig. Endosc. 2021, 33, e146–e147. [Google Scholar] [CrossRef] [PubMed]
- Tacelli, M.; Bina, N.; Crinò, S.F.; Facciorusso, A.; Celsa, C.; Vanni, A.S.; Fantin, A.; Antonini, F.; Falconi, M.; Monica, F.; et al. Reliability of grading preoperative pancreatic neuroendocrine tumors on EUS specimens: A systematic review with meta-analysis of aggregate and individual data. Gastrointest. Endosc. 2022, 96, 898–908.e23. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Irie, H.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Nakamura, J.; Takasumi, M.; Hashimoto, M.; et al. Efficacy of EUS-guided FNB using a Franseen needle for tissue acquisition and microsatellite instability evaluation in unresectable pancreatic lesions. BMC Cancer 2020, 20, 1094. [Google Scholar] [CrossRef] [PubMed]
- Levine, I.; Trindade, A.J. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J. Gastroenterol. 2021, 27, 4194–4207. [Google Scholar] [CrossRef] [PubMed]
- Rangwani, S.; Ardeshna, D.R.; Mumtaz, K.; Kelly, S.G.; Han, S.Y.; Krishna, S.G. Update on endoscopic ultrasound-guided liver biopsy. World J. Gastroenterol. 2022, 28, 3586–3594. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Eloubeidi, M.A. Endoscopic ultrasound-guided fine-needle aspiration in the evaluation of gallbladder masses. Endoscopy 2005, 37, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Meara, R.S.; Jhala, D.; Eloubeidi, M.A.; Eltoum, I.; Chhieng, D.C.; Crowe, D.R.; Varadarajulu, S.; Jhala, N. Endoscopic ultrasound-guided FNA biopsy of bile duct and gallbladder: Analysis of 53 cases. Cytopathology 2006, 17, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Prasad, G.; Chaudhary, D.; Sakhuja, P.; Srivastava, S.; Aggarwal, A.K. Role of Guided FNA in Gallbladder Cancer: A Retrospective 3-Year Study. J. Cytol. 2023, 40, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Tian, L.; Deng, M.Z.; Chen, X.J.; Fu, T.; Ma, K.J.; Xu, J.H.; Wang, X.Y. The efficacy and safety of endoscopic ultrasound-guided fine-needle biopsy in gallbladder masses. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Agarwal, R.; Anikhindi, S.A.; Puri, P.; Kumar, M.; Ranjan, P.; Kumar, A.; Sharma, P.; Bansal, N.; Bakshi, P.; et al. Role of EUS-FNA for gallbladder mass lesions with biliary obstruction: A large single-center experience. Endosc. Int. Open 2019, 7, E1403–E1409. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.C.; Pitman, M.B.; Brugge, W.R. EUS-guided FNA for the diagnosis of gallbladder masses. Gastrointest. Endosc. 2003, 57, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.K.; Jang, J.W.; Kim, T.G.; Ryu, C.H.; Park, D.H.; Lee, S.S.; Seo, D.W.; Kim, M.H. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology 2012, 59, 1691–1695. [Google Scholar] [CrossRef]
- Ogura, T.; Kurisu, Y.; Masuda, D.; Imoto, A.; Onda, S.; Kamiyama, R.; Hayashi, M.; Mohamed, M.; Uchiyama, K.; Higuchi, K. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig. Dis. Sci. 2014, 59, 1917–1924. [Google Scholar] [CrossRef]
- Webber, C.; Gospodarowicz, M.; Sobin, L.H.; Wittekind, C.; Greene, F.L.; Mason, M.D.; Compton, C.; Brierley, J.; Groome, P.A. Improving the TNM classification: Findings from a 10-year continuous literature review. Int. J. Cancer 2014, 135, 371–378. [Google Scholar] [CrossRef]
- Seo, S.; Hatano, E.; Higashi, T.; Nakajima, A.; Nakamoto, Y.; Tada, M.; Tamaki, N.; Iwaisako, K.; Mori, A.; Doi, R.; et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery 2008, 143, 769–777. [Google Scholar] [CrossRef]
- Kurita, A.; Kodama, Y.; Nakamoto, Y.; Isoda, H.; Minamiguchi, S.; Yoshimura, K.; Kuriyama, K.; Sawai, Y.; Uza, N.; Hatano, E.; et al. Impact of EUS-FNA for preoperative para-aortic lymph node staging in patients with pancreatobiliary cancer. Gastrointest. Endosc. 2016, 84, 467–475.e1. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepatobiliary Pancreat. Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, Y.; Hara, K.; Haba, S.; Kuwahara, T.; Okuno, N.; Yanaidani, T.; Ishikawa, S.; Yasuda, T.; Yamada, M.; Fukui, T.; et al. Diagnostic performance and safety of endoscopic ultrasound-guided fine-needle aspiration/biopsy for gallbladder lesions. Dig. Endosc. 2024, 36, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yamashita, Y.; Kawaji, Y.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Okada, K.I.; Kawai, M.; Yamaue, H.; Kitano, M. Endoscopic ultrasound-guided fine needle aspiration with contrast-enhanced harmonic imaging for diagnosis of gallbladder tumor (with video). J. Hepatobiliary Pancreat. Sci. 2021, 28, e1–e3. [Google Scholar] [CrossRef]
- Tamura, T.; Yamashita, Y.; Itonaga, M.; Ashida, R.; Kitano, M. Usefulness of EUS-FNA with contrast-enhanced harmonic imaging for diagnosis of gallbladder tumor. Endosc. Ultrasound 2021, 10, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, M.; Brignola, S.; Munari, G.; Gatti, M.; Dadduzio, V.; Borga, C.; Bergamo, F.; Pellino, A.; Angerilli, V.; Mescoli, C.; et al. Prognostic impact of FGFR2/3 alterations in patients with biliary tract cancers receiving systemic chemotherapy: The BITCOIN study. Eur. J. Cancer 2022, 166, 165–175. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Hirata, K.; Kuwatani, M.; Suda, G.; Ishikawa, M.; Sugiura, R.; Kato, S.; Kawakubo, K.; Sakamoto, N. A Novel Approach for the Genetic Analysis of Biliary Tract Cancer Specimens Obtained Through Endoscopic Ultrasound-Guided Fine Needle Aspiration Using Targeted Amplicon Sequencing. Clin. Transl. Gastroenterol. 2019, 10, e00022. [Google Scholar] [CrossRef]
- Chan, H.P.; Samala, R.K.; Hadjiiski, L.M.; Zhou, C. Deep Learning in Medical Image Analysis. Adv. Exp. Med. Biol. 2020, 1213, 3–21. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zhang, K.; Fung, K.M.; Thai, T.C.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Recent advances and clinical applications of deep learning in medical image analysis. Med. Image Anal. 2022, 79, 102444. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Gao, B.; Song, Y.; Qin, J. A review of deep learning-based deformable medical image registration. Front. Oncol. 2022, 12, 1047215. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, M.; de Bel, T.; den Boer, M.; Steenbergen, E.J.; Kers, J.; Florquin, S.; Roelofs, J.; Stegall, M.D.; Alexander, M.P.; Smith, B.H.; et al. Deep Learning-Based Histopathologic Assessment of Kidney Tissue. J. Am. Soc. Nephrol. 2019, 30, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Jungo, A.; Scheidegger, O.; Reyes, M.; Balsiger, F. pymia: A Python package for data handling and evaluation in deep learning-based medical image analysis. Comput. Methods Programs Biomed. 2021, 198, 105796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Diao, Z.; Shi, T.; Zhou, Y.; Wang, F.; Hu, W.; Zhu, X.; Luo, S.; Tong, G.; Yao, Y.D. A review of deep learning-based multiple-lesion recognition from medical images: Classification, detection and segmentation. Comput. Biol. Med. 2023, 157, 106726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zeng, G.; Wang, S.; Wu, X.; Xu, C. Application of Deep Learning in Lung Cancer Imaging Diagnosis. J. Healthc. Eng. 2022, 2022, 6107940. [Google Scholar] [CrossRef] [PubMed]
- Akkus, Z.; Cai, J.; Boonrod, A.; Zeinoddini, A.; Weston, A.D.; Philbrick, K.A.; Erickson, B.J. A Survey of Deep-Learning Applications in Ultrasound: Artificial Intelligence-Powered Ultrasound for Improving Clinical Workflow. J. Am. Coll. Radiol. 2019, 16, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Le, E.P.V.; Wang, Y.; Huang, Y.; Hickman, S.; Gilbert, F.J. Artificial intelligence in breast imaging. Clin. Radiol. 2019, 74, 357–366. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef]
- Anwar, S.M.; Majid, M.; Qayyum, A.; Awais, M.; Alnowami, M.; Khan, M.K. Medical Image Analysis using Convolutional Neural Networks: A Review. J. Med. Syst. 2018, 42, 226. [Google Scholar] [CrossRef]
- Zhou, S.K.; Greenspan, H.; Davatzikos, C.; Duncan, J.S.; van Ginneken, B.; Madabhushi, A.; Prince, J.L.; Rueckert, D.; Summers, R.M. A review of deep learning in medical imaging: Imaging traits, technology trends, case studies with progress highlights, and future promises. Proc. IEEE Inst. Electr. Electron. Eng. 2021, 109, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.M.; Turki, A.; Bellaaj, H.; Ksantini, M.; AlTaee, A.; Alaerjan, A. Detection of Gallbladder Disease Types Using Deep Learning: An Informative Medical Method. Diagnostics 2023, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Kim, Y.J.; Kim, E.J.; Kang, H.; Shon, S.J.; Seol, Y.J.; Lee, D.K.; Kim, K.G.; Cho, J.H. Diagnostic performance of endoscopic ultrasound-artificial intelligence using deep learning analysis of gallbladder polypoid lesions. J. Gastroenterol. Hepatol. 2021, 36, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, J.H.; Chae, H.D.; Park, S.J.; Bae, J.S.; Joo, I.; Han, J.K. Deep learning-based decision support system for the diagnosis of neoplastic gallbladder polyps on ultrasonography: Preliminary results. Sci. Rep. 2020, 10, 7700. [Google Scholar] [CrossRef] [PubMed]
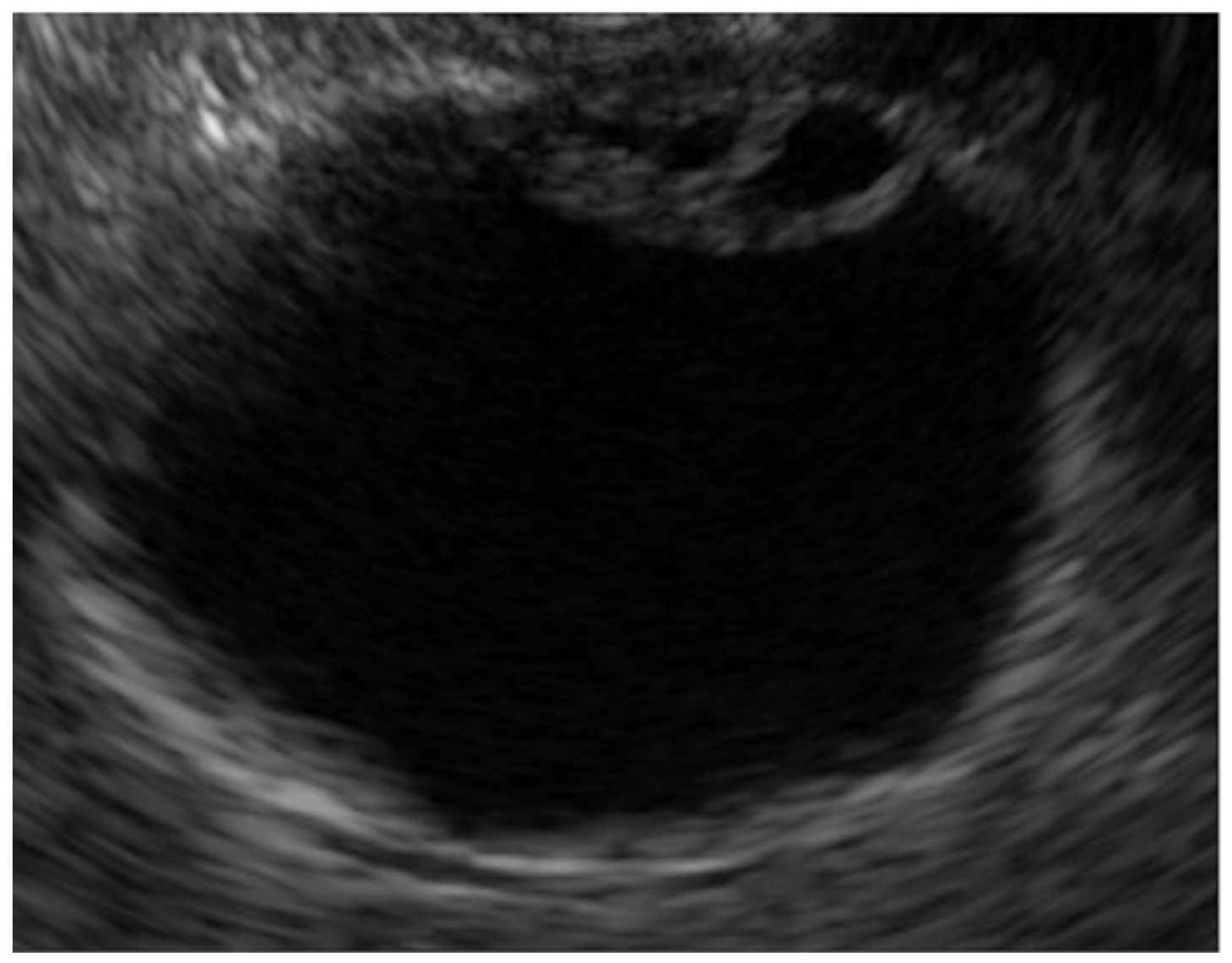


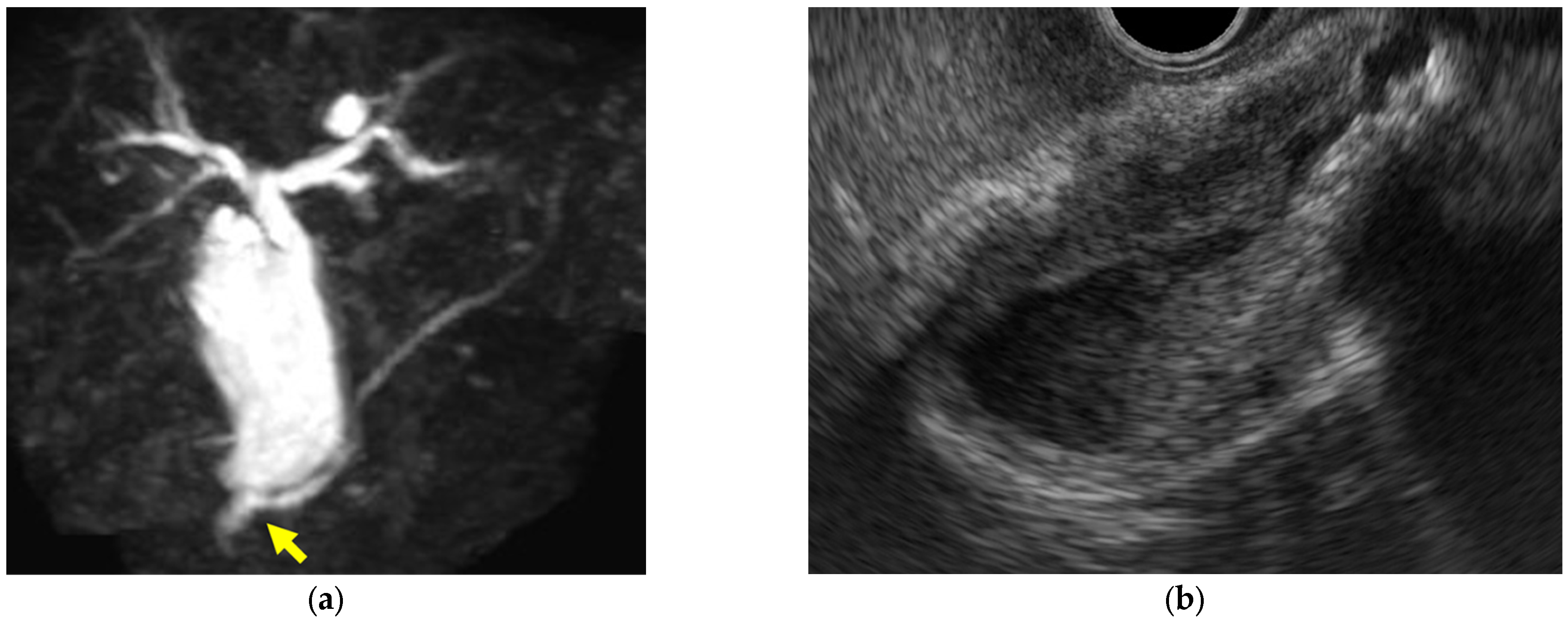
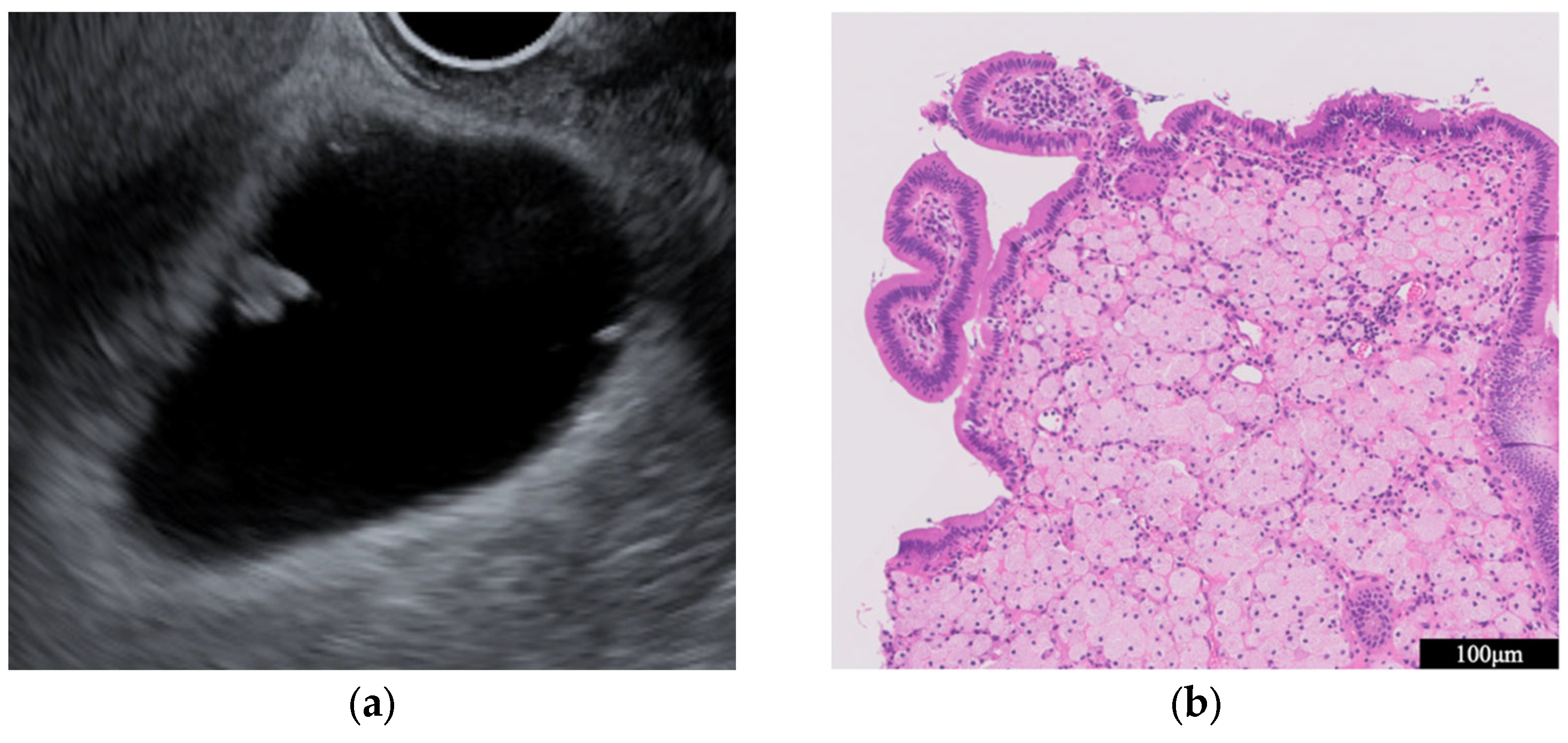
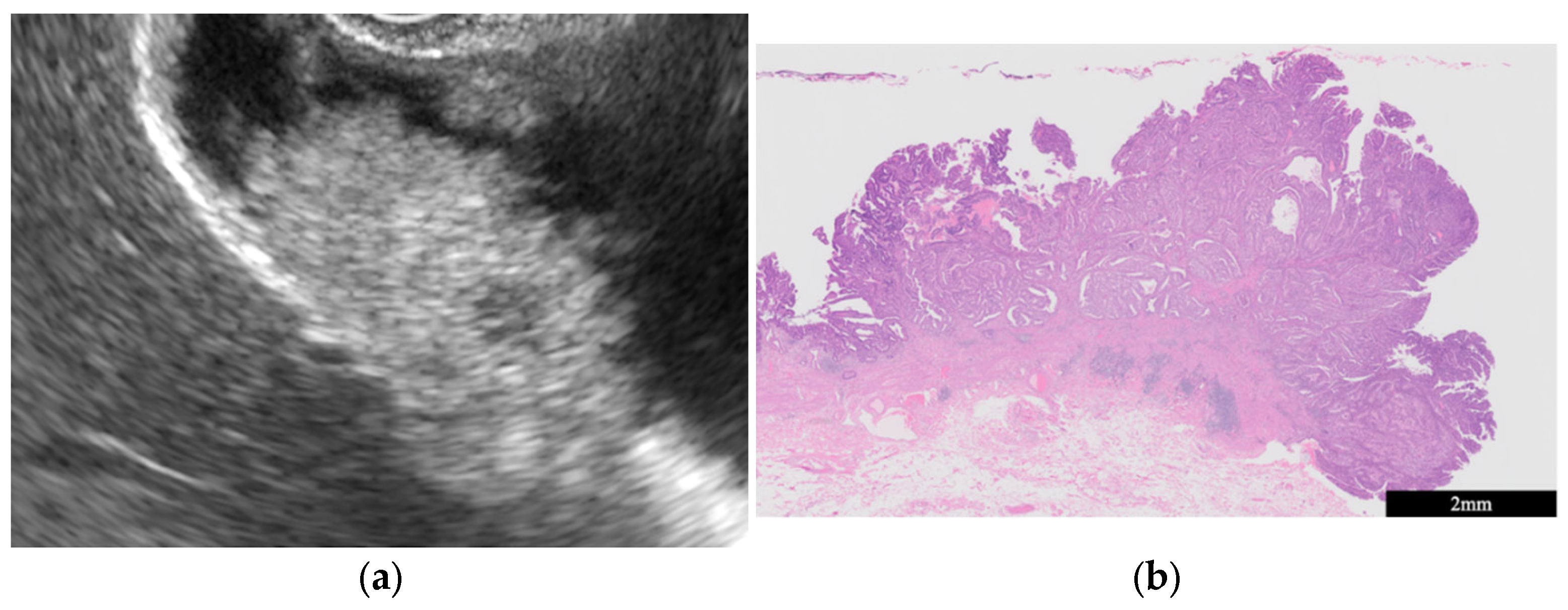
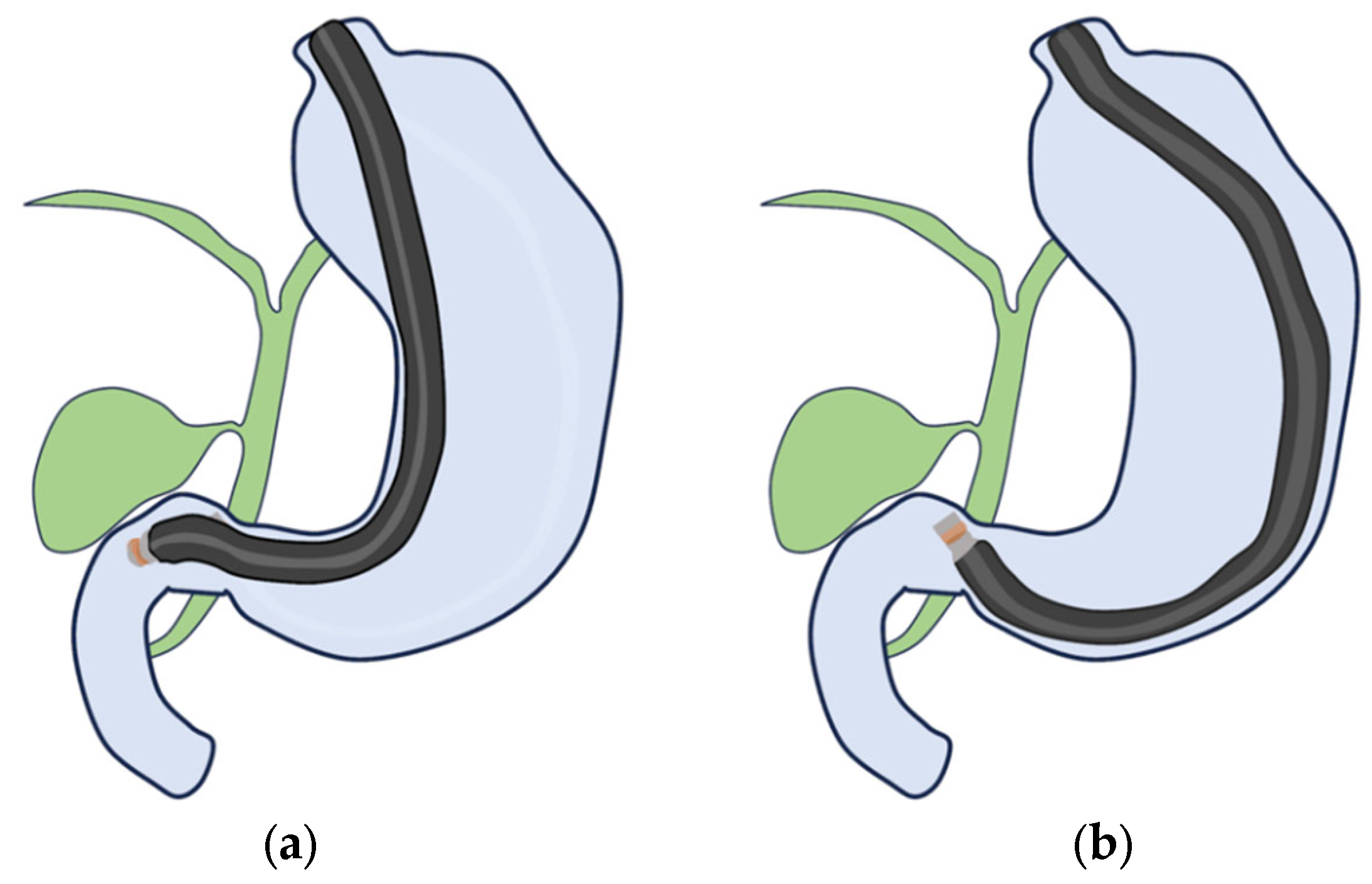


| Surface | Internal Echo | Layer Structure | |
|---|---|---|---|
| Adenomyomatosis | Smooth | Multiple anechoic areas comet tail artifact | Preserved |
| Acute cholecystitis | Smooth | No distinctive findings | Preserved |
| Chronic cholecystitis | Smooth | No distinctive findings | Preserved |
| Xanthogranulomatous cholecystitis | Smooth | Mixed hyperechoic and hypoechoic echotexture | Irregular or disrupted |
| Immunoglobulin G4-related sclerosing cholecystitis | Smooth | No distinctive findings | Preserved |
| GB mucosal hyperplasia associated with pancreaticobiliary maljunction | Smooth | Uniform hypoechogenicity | Preserved |
| GB carcinoma | Irregular or papillated | Uneven hypoechogenicity | Irregular or disrupted |
| Form | Pedunculation | Surface | Internal Echo | |
|---|---|---|---|---|
| Cholesterol polyp | Morular or oval | Pedunculated | Granular | Rough or granular Hyperechoic spots |
| Hyperplastic polyp | Papillated or lobulated | Pedunculated | Smooth | Uniform low echogenicity |
| Adenoma | Oval | Pedunculated or subpedunculated | Smooth or nodular | Homogenous isoechogenicity Multiple microcystic spaces |
| Carcinoma | Oval or irregular | Sessile > Pedunculated | Smooth or nodular | Heterogenous dense echogenicity Hypoechoic areas at the core |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Ozawa, E.; Shimakura, A.; Mori, T.; Miyaaki, H.; Nakao, K. Recent Advances in Endoscopic Ultrasound for Gallbladder Disease Diagnosis. Diagnostics 2024, 14, 374. https://doi.org/10.3390/diagnostics14040374
Takahashi K, Ozawa E, Shimakura A, Mori T, Miyaaki H, Nakao K. Recent Advances in Endoscopic Ultrasound for Gallbladder Disease Diagnosis. Diagnostics. 2024; 14(4):374. https://doi.org/10.3390/diagnostics14040374
Chicago/Turabian StyleTakahashi, Kosuke, Eisuke Ozawa, Akane Shimakura, Tomotaka Mori, Hisamitsu Miyaaki, and Kazuhiko Nakao. 2024. "Recent Advances in Endoscopic Ultrasound for Gallbladder Disease Diagnosis" Diagnostics 14, no. 4: 374. https://doi.org/10.3390/diagnostics14040374






