Robotic-Assisted Bronchoscopy: A Comprehensive Review of System Functions and Analysis of Outcome Data
Abstract
:1. Introduction
2. Robotic-Assisted Bronchoscopy Technologies
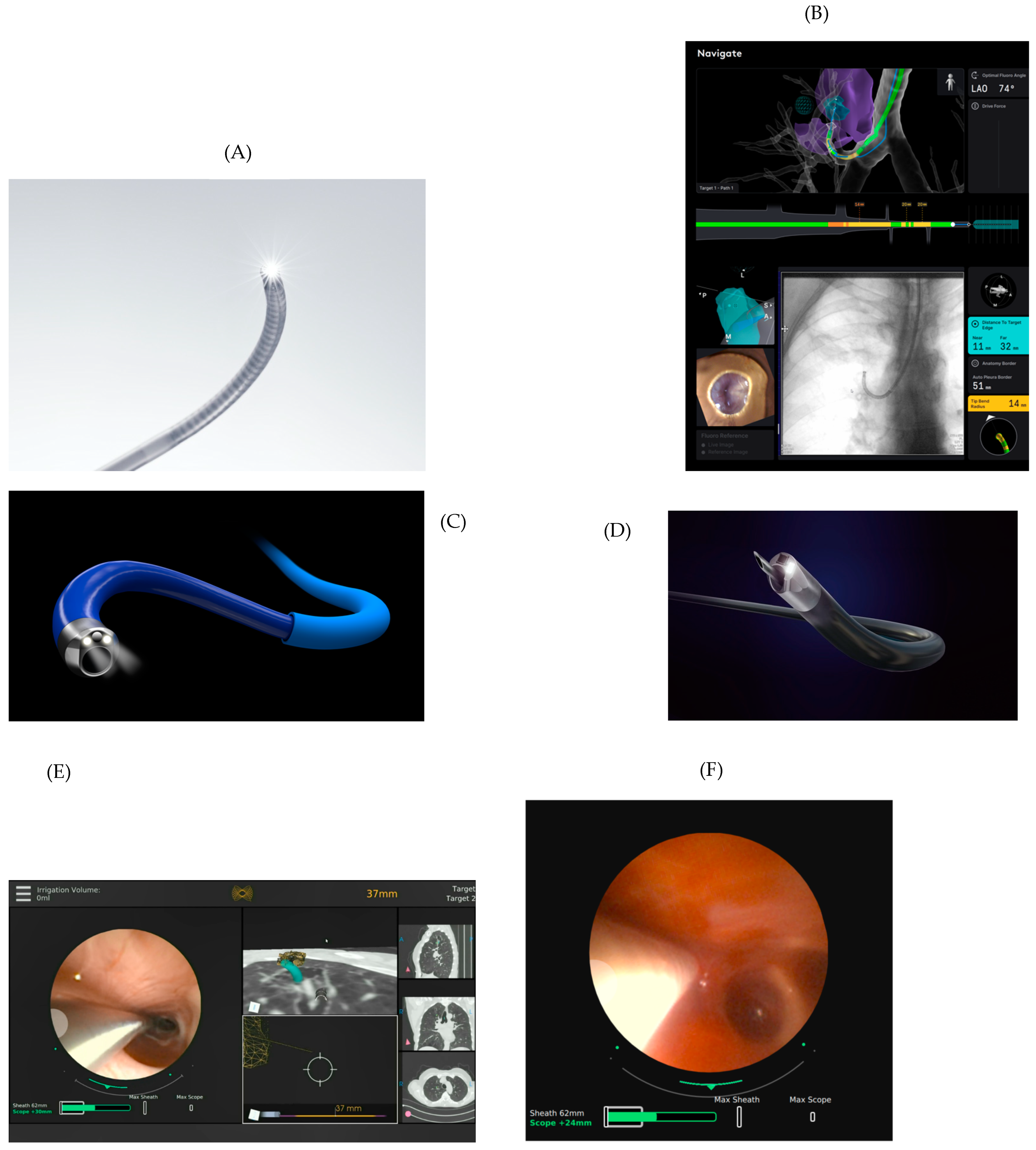
2.1. Navigation
2.2. Catheter/Bronchoscope
2.3. Controls
2.4. Display Screens
3. Procedure Setting and Sedation/Anesthesia
3.1. Location Setting
3.2. Sedation/Anesthesia
4. Literature
4.1. Ion™
4.2. Monarch™
4.3. Galaxy™
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, M.P.; Mehta, A.C. Initial Diagnosis of Lung Cancer. Chest 2007, 132, 131S–148S. [Google Scholar] [CrossRef] [PubMed]
- Baaklini, W.A.; Reinoso, M.A.; Gorin, A.B.; Sharafkaneh, A.; Manian, P. Diagnostic Yield of Fiberoptic Bronchoscopy in Evaluating Solitary Pulmonary Nodules. Chest 2000, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chee, C.G.; Cho, J.; Kim, Y.; Yoon, M.A. Diagnostic accuracy and complication rate of image-guided percutaneous transthoracic needle lung biopsy for subsolid pulmonary nodules: A systematic review and meta-analysis. Br. J. Radiol. 2021, 94, 20210065. [Google Scholar] [CrossRef] [PubMed]
- Heerink, W.J.; De Bock, G.H.; De Jonge, G.J.; Groen, H.J.M.; Vliegenthart, R.; Oudkerk, M. Complication rates of CT-guided transthoracic lung biopsy: Meta-analysis. Eur. Radiol. 2017, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wallace, M.J.; Cardella, J.F.; Kundu, S.; Miller, D.L.; Rose, S.C. Quality Improvement Guidelines for Percutaneous Needle Biopsy. J. Vasc. Interv. Radiol. 2010, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.A.; Casal, R.F.; Jimenez, C.A.; Eapenten, G.A.; Uzbeck, M.; Sarkiss, M.; Rice, D.; Morice, R.C.; Ost, D.E. Quality Gaps and Comparative Effectiveness in Lung Cancer Staging. Chest 2013, 144, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Hurter, T.; Hanrath, P. Endobronchial sonography: Feasibility and preliminary results. Thorax 1992, 47, 565–567. [Google Scholar] [CrossRef]
- Panchabhai, T.S.; Mehta, A.C. Historical Perspectives of Bronchoscopy. Connecting the Dots. Ann. Am. Thorac. Soc. 2015, 12, 631–641. [Google Scholar] [CrossRef]
- Wang Memoli, J.S.; Nietert, P.J.; Silvestri, G.A. Meta-analysis of Guided Bronchoscopy for the Evaluation of the Pulmonary Nodule. Chest 2012, 142, 385–393. [Google Scholar] [CrossRef]
- Shaller, B.D.; Almeida, F.A. I Now Walk into the Wild. Chest 2020, 158, 2268–2269. [Google Scholar] [CrossRef]
- Nadig, T.R.; Thomas, N.; Nietert, P.J.; Lozier, J.; Tanner, N.T.; Memoli, J.S.W.; Pastis, N.J.; Silvestri, G.A. Guided Bronchoscopy for the Evaluation of Pulmonary Lesions. Chest 2023, 163, 1589–1598. [Google Scholar] [CrossRef]
- Reisenauer, J.; Duke, J.D.; Kern, R.; Fernandez-Bussy, S.; Edell, E. Combining Shape-Sensing Robotic Bronchoscopy with Mobile Three-Dimensional Imaging to Verify Tool-in-Lesion and Overcome Divergence: A Pilot Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 177–185. [Google Scholar] [CrossRef]
- Donna, E.; Wu, S.; Arias, S. Proof of concept: Shape-sensing robotic-assisted bronchoscopy performed under moderate sedation. Respir. Med. Case Rep. 2023, 41, 101787. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Bhadra, K.; Calcutt, M.; Folch, E. Virtual or reality: Divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J. Thorac. Dis. 2020, 12, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, M.A. Prospective Analysis of a Novel Endobronchial Augmented Fluoroscopic Navigation System for Diagnosis of Peripheral Pulmonary Lesions. J. Bronchol. Interv. Pulmonol. 2021, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.-E.S.; Sabath, B.F.; Eapen, G.A.; Song, J.; Marcoux, M.; Sarkiss, M.; Arain, M.H.; Grosu, H.B.; Ost, D.E.; Jimenez, C.A.; et al. Incidence and Location of Atelectasis Developed During Bronchoscopy Under General Anesthesia. Chest 2020, 158, 2658–2666. [Google Scholar] [CrossRef]
- Pritchett, M.A.; Lau, K.; Skibo, S.; Phillips, K.A.; Bhadra, K. Anesthesia considerations to reduce motion and atelectasis during advanced guided bronchoscopy. BMC Pulm. Med. 2021, 21, 240. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.; Sarkiss, M.; Sagar, A.-E.S.; Vlahos, I.; Chang, C.H.; Shah, A.; Sabath, B.F.; Lin, J.; Song, J.; Moon, T.; et al. Ventilatory Strategy to Prevent Atelectasis During Bronchoscopy Under General Anesthesia. Chest 2022, 162, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Seijo, L.M.; de Torres, J.P.; Lozano, M.D.; Bastarrika, G.; Alcaide, A.B.; Lacunza, M.M.; Zulueta, J.J. Diagnostic Yield of Electromagnetic Navigation Bronchoscopy Is Highly Dependent on the Presence of a Bronchus Sign on CT Imaging. Chest 2010, 138, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Fielding, D.I.; Bashirzadeh, F.; Son, J.H.; Todman, M.; Chin, A.; Tan, L.; Steinke, K.; Windsor, M.N.; Sung, A.W. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019, 98, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Simoff, M.J.; Pritchett, M.A.; Reisenauer, J.S.; Ost, D.E.; Majid, A.; Keyes, C.; Casal, R.F.; Parikh, M.S.; Diaz-Mendoza, J.; Fernandez-Bussy, S.; et al. Shape-sensing robotic-assisted bronchoscopy for pulmonary nodules: Initial multicenter experience using the IonTM Endoluminal System. BMC Pulm. Med. 2021, 21, 322. [Google Scholar] [CrossRef]
- Benn, B.S.; Romero, A.O.; Lum, M.; Krishna, G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021, 199, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.-H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. [Google Scholar] [CrossRef]
- Reisenauer, J.; Simoff, M.J.; Pritchett, M.A.; Ost, D.E.; Majid, A.; Keyes, C.; Casal, R.F.; Parikh, M.S.; Diaz-Mendoza, J.; Fernandez-Bussy, S.; et al. Ion: Technology and Techniques for Shape-sensing Robotic-assisted Bronchoscopy. Ann. Thorac. Surg. 2022, 113, 308–315. [Google Scholar] [CrossRef]
- Styrvoky, K.; Schwalk, A.; Pham, D.; Chiu, H.T.; Rudkovskaia, A.; Madsen, K.; Carrio, S.; Kurian, E.M.; Casas, L.D.L.; Abu-Hijleh, M. Shape-Sensing Robotic-Assisted Bronchoscopy with Concurrent use of Radial Endobronchial Ultrasound and Cone Beam Computed Tomography in the Evaluation of Pulmonary Lesions. Lung 2022, 200, 755–761. [Google Scholar] [CrossRef]
- Chambers, J.; Knox, D.; Leclair, T. O-arm CT for Confirmation of Successful Navigation During Robotic Assisted Bronchoscopy. J. Bronchol. Interv. Pulmonol. 2023, 30, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Low, S.-W.; Lentz, R.J.; Chen, H.; Katsis, J.; Aboudara, M.C.; Whatley, S.; Paez, R.; Rickman, O.B.; Maldonado, F. Shape-Sensing Robotic-Assisted Bronchoscopy vs Digital Tomosynthesis-Corrected Electromagnetic Navigation Bronchoscopy. Chest 2023, 163, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Lee-Mateus, A.Y.; Reisenauer, J.; Garcia-Saucedo, J.C.; Abia-Trujillo, D.; Buckarma, E.H.; Edell, E.S.; Grage, R.A.; Bowman, A.W.; Labarca, G.; Johnson, M.M.; et al. Robotic-assisted bronchoscopy versus CT-guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology 2023, 28, 66–73. [Google Scholar] [CrossRef]
- Rojas-Solano, J.R.; Ugalde-Gamboa, L.; Machuzak, M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J. Bronchol. Interv. Pulmonol. 2018, 25, 168–175. [Google Scholar] [CrossRef]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P.; Murgu, S. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef]
- Chen, A.C.; Pastis, N.J.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic Bronchoscopy for Peripheral Pulmonary Lesions. Chest 2021, 159, 845–852. [Google Scholar] [CrossRef]
- Agrawal, A.; Ho, E.; Chaddha, U.; Demirkol, B.; Bhavani, S.V.; Hogarth, D.K.; Murgu, S. Factors Associated with Diagnostic Accuracy of Robotic Bronchoscopy With 12-Month Follow-up. Ann. Thorac. Surg. 2023, 115, 1361–1368. [Google Scholar] [CrossRef]
- Cumbo-Nacheli, G.; Velagapudi, R.K.; Enter, M.; Egan, J.P.; Conci, D. Robotic-assisted Bronchoscopy and Cone-beam CT: A Retrospective Series. J. Bronchol. Interv. Pulmonol. 2022, 29, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Seaman, J.; Hunter, T.D.; Ribeiro, D.; Laxmanan, B.; Kalsekar, I.; Cumbo-Nacheli, G. Diagnostic outcomes of robotic-assisted bronchoscopy for pulmonary lesions in a real-world multicenter community setting. BMC Pulm. Med. 2023, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Pyarali, F.F.; Hakami-Majd, N.; Sabbahi, W.; Chaux, G. Robotic-assisted Navigation Bronchoscopy: A Meta-Analysis of Diagnostic Yield and Complications. J. Bronchol. Interv. Pulmonol. 2024, 31, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dibardino, D.M.; Ma, K.C. Bronchoscopic treatment of thoracic malignancy. AME Med. J. 2023, 8, 36. [Google Scholar] [CrossRef]
- Mondoni, M.; Sotgiu, G. Bronchoscopic management of peripheral pulmonary lesions: Robotic approach paves the way to the future. BMC Pulm. Med. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Puchalski, J.; Sterman, D. Recent Advances in Bronchoscopic Treatment of Peripheral Lung Cancers. Chest 2017, 151, 674–685. [Google Scholar] [CrossRef]




| Robotic-Assisted Bronchoscopy Features | Ion™ Intuitive | Monarch™ Auris | Galaxy™ Noah Medical |
|---|---|---|---|
| Navigation technology | Shape sensing | Electromagnetic navigation | Electromagnetic navigation augmented fluoroscopy |
| Bronchoscopy | Catheter with removable vision probe (disposable after 5 uses) * | Sheath and bronchoscope with built-in camera (disposable after 2 uses) * | Bronchoscope with built-in camera (single use) |
| Catheter articulation | 180° | 180° (sheath: 130°) | 180° |
| Catheter outer diameter | 3.5 mm (catheter) | Outer sheath: 6 mm/inner scope: 4.2 mm | 4.0 mm |
| Working channel diameter | 2.0 mm | 2.1 mm | 2.1 mm |
| Irrigation and aspiration | No | Yes | Yes |
| CT-to-body divergence correction system | Yes (only with Cios Spin) | No | Yes |
| Augmented fluoroscopy | No | No | Yes |
| Tool-in-lesion confirmation | Yes (only when utilizing Cios Spin) | No | Yes |
| Tactile feedback | No | No | No |
| Article: Author (Year) | Prospective versus Retrospective Single Center or Multicentric | Number of Lesions (Number of Patients) | Diagnostic Performance | Strict versus Lenient Diagnostic Performance Definition | X-ray Imaging | Mean Diameter of Lesions (mm) | Number of Lesions with Positive Bronchus Sign (%) | Number of Lesions in the Lower Lobes (%) | Number of Radial EBUS Confirmations (%) | Number of Pneumothorax (%) Number Requiring Drinage (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fielding et al. (2019) [20] | Prospective Single center | 29 (29) | 79.3% | Not strict | Two-dimensional fluoroscopy | 14.8 | 17 (58.6) | 7 (24.1) | 27 (93.1) | 0 |
| Simoff et al. (2021) [21] | Prospective Multicentric | 67 (60) | Not described | N/A | Two-dimensional fluoroscopy | 20 | 25 (37.3) | 29 (43.2) | 59 (89.4) | 0 |
| Benn et al. (2021) [22] * | Prospective Single center | 59 (52) | 86% | Less strict | CBCT | 21.9 | 27 (46) | 14 (23.7) | Not used | 2 (3.8) 1 (1.9) |
| Kalchiem-Dekel et al. (2022) [23] | Prospective Single center | 159 (131) | 81.7% | Strict | Two-dimensional fluoroscopy in 79.9%: two- and three-dimensional fluoroscopy in 20.1% | 18 (median) | 100 (62.9) | 54 (34) | 124 (91.2) | 2 (1.5) 2 (1.5) |
| Reisenauer et al. (2022) [24] | Prospective Multicentric | 270 (241) | Not described | N/A | Not reported | 18.8 | ND | 83 (30.9) | 232 (86.6) | 8 (3.3) 1 (0.4) |
| Styrvoky et al. (2022) [25] | Retrospective Single center | 209 (198) | Not described 89% (calculated based on provided data; 83.2% if all inflammation findings had an alternative diagnosis) | Not strict | CBCT (98.6%) | 22.6 | 126 (60.3) | 67 (32.1) | 183 (87.5) | 2 (1) 1 (0.5) |
| Reisenauer et al. (2022) [12] † | Prospective Single center | 30 (30) | 93.3% | Strict | Three-dimensional fluoroscopy | 17.5 (median) | 12 (40) | 9 (30) | 23 (76.7) | 0 |
| Chambers et al. (2022) [26] | Retrospective Single center | 79 (75) | 77% | Strict | Three-dimensional fluoroscopy | 20 (median) | 44 (56) | 23 (29) | Not used | 2 (2.5) 1 (1.3) |
| Low et al. (2022) [27] | Retrospective Single center | 143 (133) | 77% | Strict | Two-dimensional fluoroscopy | 17 | 57 (39.9) | 51 (35.6) | 127 (88.8) | 2 (1.5) 2 (1.5) |
| Yu Lee-Mateus et al. (2023) [28] | Retrospective Multicentric | 113 (113) | 87.6% (no less than 81.4% if all inflammation cases with incorrect diagnosis) | Not strict | Two-dimensional fluoroscopy | ND | ND | 35 (30.9) | ND | 4 (3.5) ND |
| Article: Author (Year) | Prospective versus Retrospective Single Center or Multicentric | Number of Lesions (Number of Patients) | Diagnostic Performance | Strict versus Lenient Diagnostic Performance Definition | X-ray Imaging | Mean Diameter of Lesions (mm) | Number of Lesions with Positive Bronchus Sign (%) | Number of Lesions in the Lower Lobes (%) | Number of Radial EBUS Confirmations (%) | Number of Pneumothorax (%) Number Requiring Drainage (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rojas-Solano et al. (2018) [29] | Prospective Single center | 15 (15) | Not described | N/A | Two-dimensional fluoroscopy | 26 | 12 (80) * | 7 (46.7) | Not used | 0 |
| Chaddha et al. (2019) [30] | Retrospective Multicentric | 167 (165) | 69.1 a 77% † | Strict | Two-dimensional fluoroscopy (presumed) | 25 | 106 (63.5) | 59 (35.3) | 109 (89.4) | 6 (3.6) 4 (2.4) |
| Chen et al. (2021) [31] | Prospective Multicentric | 54 (54) | 74.1% ‡ | Not strict | Two-dimensional fluoroscopy (presumed) | 23.2 | 32 (59.3) | 17 (31.5) | 51 (96.2) | 2 (3.7) 1 (1.9) |
| Cumbo-Nacheli et al. (2022) [33] | Retrospective Single center | 20 (20) | 70% (calculated based on provided data) | N/A | CBCT | 22 | 10 (50%) | 6 (30) | 15 (75) | 0 |
| Agrawal et al. (2023) [32] | Retrospective Single center | 124 (124) | 77% § | Strict | Two-dimensional fluoroscopy | 24 | 93 (75%) | 38 (30.6) | 102 (82.2) | 2 (1.6) 0 (0) |
| Khan et al. x (2023) [34] | Retrospective Multicentric | 264 (264) | 79.4% (at 12 months) | Not strict | Two-dimensional fluoroscopy (99.6%) CBCT (3.4%) | 19.3 (median) | 78/259 y (30.1%) | 86 (32.6) | 248 (93.9) | 15 (5.7) 10 (3.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado, R.M.G.; Cicenia, J.; Almeida, F.A. Robotic-Assisted Bronchoscopy: A Comprehensive Review of System Functions and Analysis of Outcome Data. Diagnostics 2024, 14, 399. https://doi.org/10.3390/diagnostics14040399
Prado RMG, Cicenia J, Almeida FA. Robotic-Assisted Bronchoscopy: A Comprehensive Review of System Functions and Analysis of Outcome Data. Diagnostics. 2024; 14(4):399. https://doi.org/10.3390/diagnostics14040399
Chicago/Turabian StylePrado, Renan Martins Gomes, Joseph Cicenia, and Francisco Aécio Almeida. 2024. "Robotic-Assisted Bronchoscopy: A Comprehensive Review of System Functions and Analysis of Outcome Data" Diagnostics 14, no. 4: 399. https://doi.org/10.3390/diagnostics14040399





