Noninvasive Models to Assess Liver Inflammation and Fibrosis in Chronic HBV Infected Patients with Normal or Mildly Elevated Alanine Transaminase Levels: Which One Is Most Suitable?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Liver Histological Examination
2.3. Noninvasive Models
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Validation of Noninvasive Models in All Patients
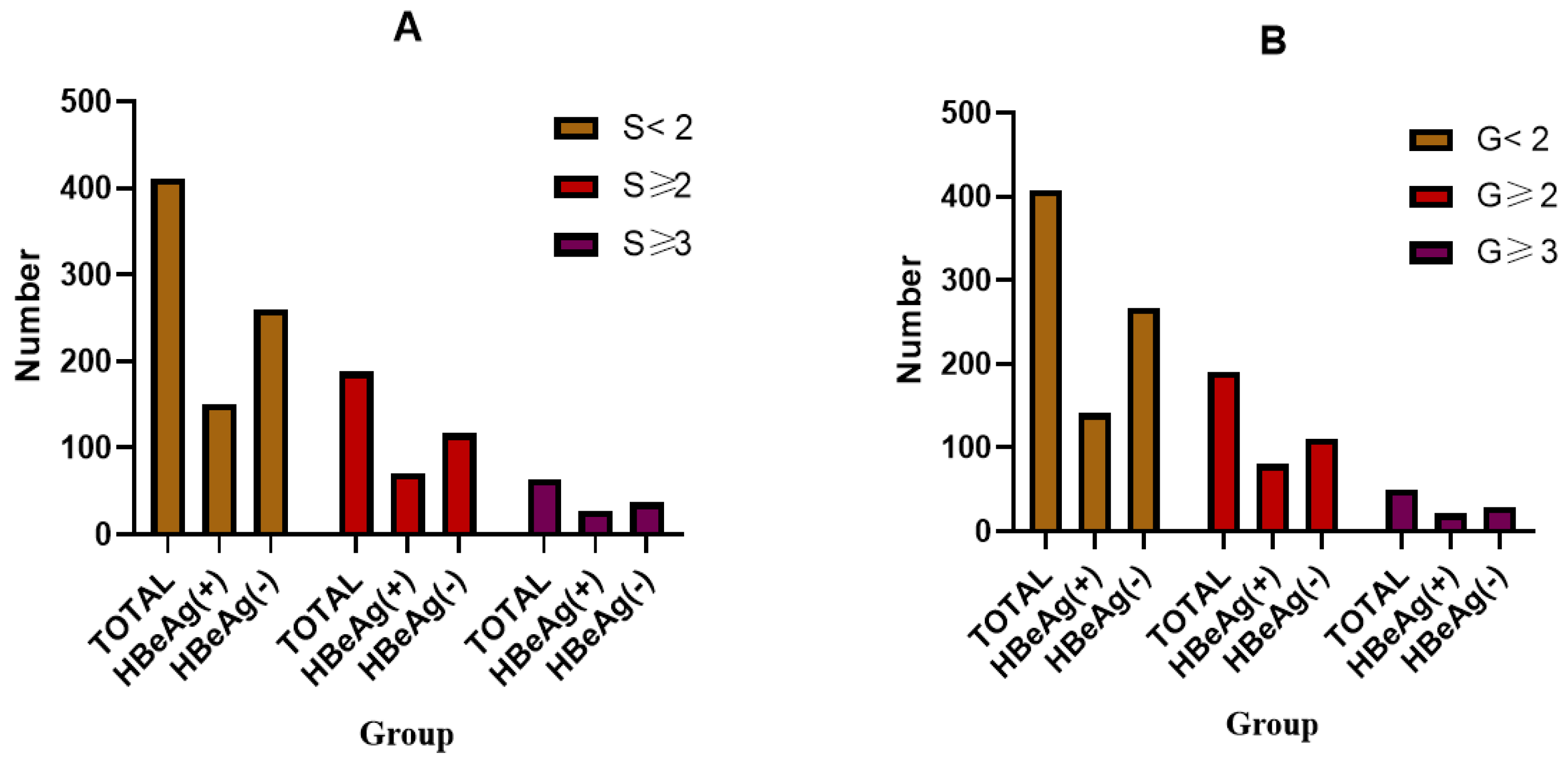
3.3. Evaluation and Comparison of Noninvasive Models in the HBeAg-Negative and HBeAg-Positive Groups
3.4. Reassessment and Comparison of Noninvasive Models in Patients with Varying Levels of ALT below Two Times the ULN (ULN = 40 U/L)
3.5. Evaluation and Comparison of Noninvasive Models Developed in Cohorts with CHB versus Those in Other Chronic Liver Diseases (CLDs)
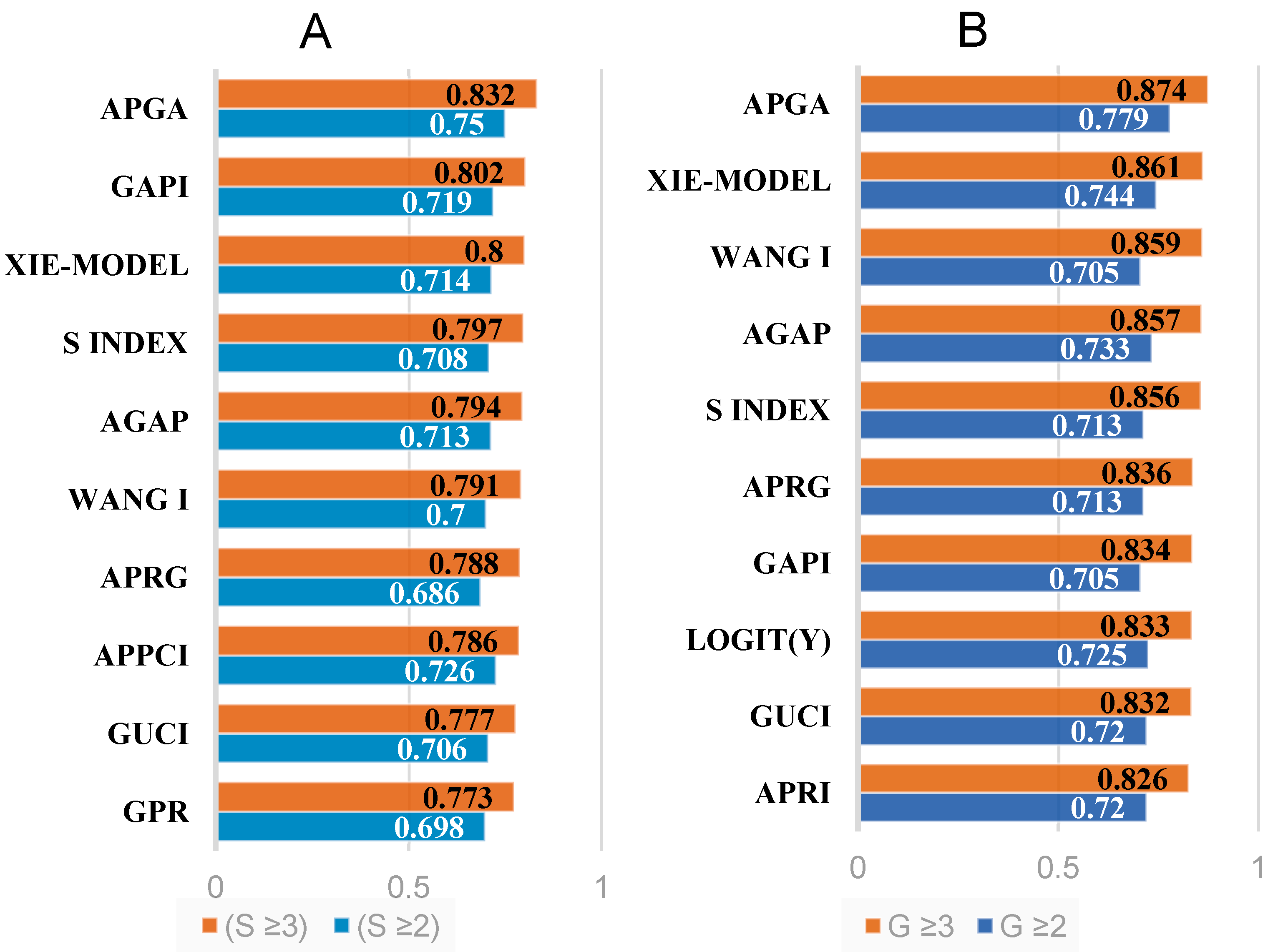
3.6. Comprehensive Evaluation of Noninvasive Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hsu, Y.C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin. Liver Dis. 2018, 12, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Park, S.Y.; Moon, J.E.; Lee, Y.R.; Lee, H.A.; Lee, J.; Kim, Y.S.; Seo, Y.S.; Kim, S.G. Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune-tolerant phase. Clin. Mol. Hepatol. 2023, 29, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lim, Y.S.; Han, S.; Choi, J.; Shim, J.H.; Kim, K.M.; Lee, H.C.; Lee, Y.S. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut 2018, 67, 945–952. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, A.L.; Xie, Q.; Zhang, X.Q.; Cheng, J.; Zhang, D.Z.; Wang, G.Q.; Zhao, H. Significant histological changes and satisfying antiviral efficacy in chronic hepatitis B virus infection patients with normal alanine aminotransferase. Antiviral therapy decision in chronic HBV patients with normal ALT. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101463. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.W.; Wu, Z.; Zhao, H.; Wang, G.Q. Significant Histologic Changes Are Not Rare in Treatment-naive Hepatitis B Patients with Normal Alanine Aminotransferase Level: A Meta-analysis. J. Clin. Transl. Hepatol. 2021, 9, 615–625. [Google Scholar] [CrossRef]
- You, H.; Sun, Y.M.; Zhang, M.Y.; Nan, Y.M.; Xu, X.Y.; Li, T.S.; Wang, G.Q.; Hou, J.L.; Duan, Z.P.; Wei, L.; et al. Interpretation of the essential updates in guidelines for the prevention and treatment of chronic hepatitis B (Version 2022). Chin. J. Hepatol. 2023, 31, 385–388. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Li, L.; Chen, E. Expanding antiviral therapy indications for HBeAg-negative chronic hepatitis B patients with normal ALT and positive HBV DNA. Precis. Clin. Med. 2022, 5, pbac030. [Google Scholar] [CrossRef]
- Kawanaka, M.; Nishino, K.; Kawamoto, H.; Haruma, K. Hepatitis B: Who should be treated?-managing patients with chronic hepatitis B during the immune-tolerant and immunoactive phases. World J. Gastroenterol. 2021, 27, 7497–7508. [Google Scholar] [CrossRef]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.N.; Han, K.H.; Kim, S.U. Clinical application of transient elastography in patients with chronic viral hepatitis receiving antiviral treatment. Liver Int. Off. J. Int. Assoc. Study Liver 2015, 35, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Liao, M.; Zheng, J.; Huang, Z.P.; Xie, S.D. Two-dimensional shear wave elastography utilized in patients with ascites: A more reliable method than transient elastography for noninvasively detecting the liver stiffness-an original study with 170 patients. Ann. Transl. Med. 2023, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Adams, L.A. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Serra-Burriel, M.; Graupera, I.; Toran, P.; Thiele, M.; Roulot, D.; Wai-Sun Wong, V.; Neil Guha, I.; Fabrellas, N.; Arslanow, A.; Exposito, C.; et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J. Hepatol. 2019, 71, 1141–1151. [Google Scholar] [CrossRef]
- Williams, A.L.; Hoofnagle, J.H. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 1988, 95, 734–739. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Bonacini, M.; Hadi, G.; Govindarajan, S.; Lindsay, K.L. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 1997, 92, 1302–1304. [Google Scholar]
- Lemoine, M.; Shimakawa, Y.; Nayagam, S.; Khalil, M.; Suso, P.; Lloyd, J.; Goldin, R.; Njai, H.F.; Ndow, G.; Taal, M.; et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2016, 65, 1369–1376. [Google Scholar] [CrossRef]
- Zeng, D.W.; Huang, Z.X.; Lin, M.X.; Kang, N.L.; Lin, X.; Li, Y.N.; Zhu, Y.Y.; Liu, Y.R. A novel HBsAg-based model for predicting significant liver fibrosis among Chinese patients with immune-tolerant phase chronic hepatitis B: A multicenter retrospective study. Ther. Adv. Gastroenterol. 2021, 14, 17562848211010675. [Google Scholar] [CrossRef]
- Seto, W.K.; Lee, C.F.; Lai, C.L.; Ip, P.P.; Fong, D.Y.; Fung, J.; Wong, D.K.; Yuen, M.F. A new model using routinely available clinical parameters to predict significant liver fibrosis in chronic hepatitis B. PLoS ONE 2011, 6, e23077. [Google Scholar] [CrossRef]
- Wang, J.; Yan, X.; Yang, Y.; Chang, H.; Jia, B.; Zhao, X.A.; Chen, G.; Xia, J.; Liu, Y.; Chen, Y.; et al. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget 2017, 8, 59257–59267. [Google Scholar] [CrossRef]
- Fung, J.; Lai, C.L.; Fong, D.Y.; Yuen, J.C.; Wong, D.K.; Yuen, M.F. Correlation of liver biochemistry with liver stiffness in chronic hepatitis B and development of a predictive model for liver fibrosis. Liver Int. Off. J. Int. Assoc. Study Liver 2008, 28, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Li, N.; Fan, X.; Li, C.; Zhang, P.; Han, Q.; Liu, Z. A Noninvasive Score to Predict Liver Fibrosis in HBeAg-Positive Hepatitis B Patients with Normal or Minimally Elevated Alanine Aminotransferase Levels. Dis. Markers 2018, 2018, 3924732. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Grading and staging the histopathological lesions of chronic hepatitis: The Knodell histology activity index and beyond. Hepatology 2000, 31, 241–246. [Google Scholar] [CrossRef]
- Ahmed Hassan, E.; Sharaf El-Din Abd El-Rehim, A.; Ahmed Sayed, Z.E.; Farah Mohamed Kholef, E.; Sabry, A.; Abd El-Rehim Abo Elhagag, N. VAP score as a novel non-invasive liver fibrosis model in patients with chronic hepatitis C. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 1408–1416. [Google Scholar] [CrossRef]
- Zeng, M.D.; Lu, L.G.; Mao, Y.M.; Qiu, D.K.; Li, J.Q.; Wan, M.B.; Chen, C.W.; Wang, J.Y.; Cai, X.; Gao, C.F.; et al. Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology 2005, 42, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Mahtab, M.A.; Rahman, S.; Karim, M.F.; Khan, M.; Akther, T.; Manik, A.H.; Debnath, C.R. Significance of Age-Spleen-Platelet Ratio Index (ASPRI) to Exclude Cirrhosis in Patients with Chronic Hepatitis B. Mymensingh Med. J. MMJ 2017, 26, 868–873. [Google Scholar]
- Li, J.; Mao, R.C.; Li, X.L.; Zheng, J.W.; Qi, X.; Yuan, Q.; Zhang, J.; Zhang, J.M.; Xia, N.S. A novel noninvasive index for the prediction of moderate to severe fibrosis in chronic hepatitis B patients. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 482–489. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Du, H.; Zao, X.; Gan, D.; Yang, X.; Li, X.; Xing, Y.; Ye, Y. Identification of pseudo-immune tolerance for chronic hepatitis B patients: Development and validation of a non-invasive prediction model. Front. Public Health 2023, 11, 1137738. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wu, J.; Yu, X.; Li, J.; Yang, S.; Qi, X.; Mao, R.; Zhang, Y.; Yu, J.; Zhu, H.; et al. Validation and comparison of seventeen noninvasive models for evaluating liver fibrosis in Chinese hepatitis B patients. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, Y.; Zhou, D.; Xiao, H.; Zhou, Z.; Han, Z.; Sun, X.; Li, S.; Zhang, L.; Li, Z.; et al. A Non-invasive Model for Predicting Liver Inflammation in Chronic Hepatitis B Patients With Normal Serum Alanine Aminotransferase Levels. Front. Med. 2021, 8, 688091. [Google Scholar] [CrossRef] [PubMed]
- Toy, M.; Hutton, D.; Jia, J.; So, S. Costs and health impact of delayed implementation of a national hepatitis B treatment program in China. J. Glob. Health 2022, 12, 04043. [Google Scholar] [CrossRef] [PubMed]
- Kranidioti, H.; Manolakopoulos, S.; Khakoo, S.I. Outcome after discontinuation of nucleot(s)ide analogues in chronic hepatitis B: Relapse rate and associated factors. Ann. Gastroenterol. 2015, 28, 173–181. [Google Scholar] [PubMed]
- Erdogan, S.; Dogan, H.O.; Sezer, S.; Uysal, S.; Ozhamam, E.; Kayacetin, S.; Koca, Y. The diagnostic value of non-invasive tests for the evaluation of liver fibrosis in chronic hepatitis B patients. Scand. J. Clin. Lab. Investig. 2013, 73, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yi, W.; Zhang, L.; Lu, Y.; Hao, H.X.; Gao, Y.J.; Ran, C.P.; Lu, H.H.; Chen, Q.Q.; Shen, G.; et al. Evaluation of a logistic regression model for predicting liver necroinflammation in hepatitis B e antigen-negative chronic hepatitis B patients with normal and minimally increased alanine aminotransferase levels. J. Viral Hepat. 2019, 26 (Suppl. 1), 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kayadibi, H.; Yilmaz, B.; Ozgur Yeniova, A.; Koseoglu, H.; Simsek, Z. Development and evaluation of a novel noninvasive index for predicting significant fibrosis, advanced fibrosis, and cirrhosis in patients with chronic hepatitis B infection. Eur. J. Gastroenterol. Hepatol. 2021, 33, e121–e130. [Google Scholar] [CrossRef]
- Feng, L.; Sun, K.; Zhang, J.; Feng, G.; Zhao, Y. A novel non-invasive index using AFP and APTT is associated with liver fibrosis in patients with chronic hepatitis B infection: A retrospective cohort study. BMJ Open 2015, 5, e008032. [Google Scholar] [CrossRef]
- Wang, H.W.; Peng, C.Y.; Lai, H.C.; Su, W.P.; Lin, C.H.; Chuang, P.H.; Chen, S.H.; Chen, C.H.; Hsu, W.F.; Huang, G.T. New noninvasive index for predicting liver fibrosis in Asian patients with chronic viral hepatitis. Sci. Rep. 2017, 7, 3259. [Google Scholar] [CrossRef]
- Omran, M.M.; Farid, K.; Emran, T.M.; Attallah, A.A. Fibro-alpha score as a simple and useful non-invasive test for predicting significant liver fibrosis in chronic hepatitis C patients. Arab. J. Gastroenterol. Off. Publ. Pan Arab. Assoc. Gastroenterol. 2011, 12, 74–79. [Google Scholar] [CrossRef]
- Shiha, G.; Soliman, R.; Mikhail, N.N.H.; Alswat, K.; Abdo, A.; Sanai, F.; Derbala, M.F.; Ormeci, N.; Dalekos, G.N.; Al-Busafi, S.; et al. Development and multicenter validation of FIB-6: A novel, machine learning, simple bedside score to rule out liver cirrhosis and compensated advanced chronic liver disease in patients with chronic hepatitis C. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2022, 52, 165–175. [Google Scholar] [CrossRef]
- Fontana, R.J.; Kleiner, D.E.; Bilonick, R.; Terrault, N.; Afdhal, N.; Belle, S.H.; Jeffers, L.J.; Ramcharran, D.; Ghany, M.G.; Hoofnagle, J.H.; et al. Modeling hepatic fibrosis in African American and Caucasian American patients with chronic hepatitis C virus infection. Hepatology 2006, 44, 925–935. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Javed, F.T.; Gull, S.; Kausar, H.; Sarwar, M.T.; Asad, S.; Shahid, I.; Sumrin, A.; Khaliq, S.; et al. A comparison of four fibrosis indexes in chronic HCV: Development of new fibrosis-cirrhosis index (FCI). BMC Gastroenterol. 2011, 11, 44. [Google Scholar] [CrossRef]
- Islam, S.; Antonsson, L.; Westin, J.; Lagging, M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand. J. Gastroenterol. 2005, 40, 867–872. [Google Scholar] [CrossRef]
- Forns, X.; Ampurdanes, S.; Llovet, J.M.; Aponte, J.; Quinto, L.; Martinez-Bauer, E.; Bruguera, M.; Sanchez-Tapias, J.M.; Rodes, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002, 36, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Bellon, J.M.; Miralles, P.; Alvarez, E.; Sanchez-Conde, M.; Cosin, J.; Lopez, J.C.; Alvarez, F.; Catalan, P.; Resino, S. Identification of liver fibrosis in HIV/HCV-coinfected patients using a simple predictive model based on routine laboratory data. J. Viral Hepat. 2007, 14, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Attallah, A.M.; Shiha, G.E.; Omran, M.M.; Zalata, K.R. A discriminant score based on four routine laboratory blood tests for accurate diagnosis of severe fibrosis and/or liver cirrhosis in Egyptian patients with chronic hepatitis C. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2006, 34, 163–169. [Google Scholar] [CrossRef]
- Meng, J.; Xu, H.; Liu, X.; Wu, R.; Niu, J. Increased red cell width distribution to lymphocyte ratio is a predictor of histologic severity in primary biliary cholangitis. Medicine 2018, 97, e13431. [Google Scholar] [CrossRef] [PubMed]
- Andres-Otero, M.J.; De-Blas-Giral, I.; Puente-Lanzarote, J.J.; Serrano-Aullo, T.; Morandeira, M.J.; Lorente, S.; Lou-Bonafonte, J.M. Multiple approaches to assess fourteen non-invasive serum indexes for the diagnosis of liver fibrosis in chronic hepatitis C patients. Clin. Biochem. 2016, 49, 560–565. [Google Scholar] [CrossRef]
- Resino, S.; Bellon, J.M.; Asensio, C.; Micheloud, D.; Miralles, P.; Vargas, A.; Catalan, P.; Lopez, J.C.; Alvarez, E.; Cosin, J.; et al. Can serum hyaluronic acid replace simple non-invasive indexes to predict liver fibrosis in HIV/Hepatitis C coinfected patients? BMC Infect. Dis. 2010, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Yang, K.; Zhang, H.; Wang, Q.; Lv, X.; Guan, S. Comparison and evaluation of non-invasive models in predicting liver inflammation and fibrosis of chronic hepatitis B virus-infected patients with high hepatitis B virus DNA and normal or mildly elevated alanine transaminase levels. Medicine 2020, 99, e20548. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Q.; Wu, Z.; Zhao, H.; Wang, G.Q.; China HepB-Related Fibrosis Assessment Research Group. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in Chinese hepatitis B patients. J. Viral Hepat. 2019, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, B.; Li, S.; Li, J.; Shen, Z.; Zheng, Y.; Zhao, W.; Tan, D.; Wu, J.; Zhang, X.; et al. Development and Validation of a Novel Model to Predict Liver Histopathology in Patients with Chronic Hepatitis B. BioMed. Res. Int. 2019, 2019, 1621627. [Google Scholar] [CrossRef]


| Histology Stages | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| S ≥ 2 or G ≥ 2 | <0.70 | 0.70–0.75 | 0.75–0.80 | ≥0.80 |
| S ≥ 3 or G ≥ 3 | <0.70 | 0.70–0.75 | 0.75–0.80 | ≥0.80 |
| Variables | Total (n = 599) | S0–1 (n = 411) | S2–4 (n = 188) | p-Value (S0–1 vs. S2–4) |
|---|---|---|---|---|
| Age, years # | 37(31, 43) | 36(31, 43) | 37(32, 43) | 0.357 |
| Male, n (%) | 411(68.6) | 269(65.5) | 142(75.5) | 0.014 * |
| Log10[HBsAg], IU/mL # | 3.36(2.88, 3.86) | 3.32(2.86, 3.91) | 3.43(3.0, 3.81) | 0.656 |
| HBeAg positive, n (%) | 221(35.2%) | 150(36.5%) | 71(37.8%) | 0.781 |
| Log10[HBVDNA], IU/mL # | 4.67(3.38, 6.81) | 4.425(3.2975, 7.8025) | 5.16(3.675, 6.11) | 0.773 |
| CP (mg/dL) # | 0.201 ± 0.039 | 0.204 ± 0.037 | 0.197 ± 0.043 | 0.143 |
| AFP (ng/mL) # | 2.58(1.8, 4.0) | 2.29(1.74, 3.50) | 3.28(1.98, 5.98) | 0.000 * |
| PT (s) # | 13.3(12.9, 13.9) | 13.2(12.8, 13.675) | 13.55(13.1, 14.1) | 0.000 * |
| PTA (%) & | 97.8 ± 12.1 | 99.5 ± 12.4 | 94.0 ± 10.9 | 0.000 * |
| INR # | 1.01(0.97, 1.06) | 1.01(0.96, 1.05) | 1.04(1.01, 1.09) | 0.000 * |
| AST (U/L) # | 26(21, 31) | 24(21, 29) | 28(24, 35) | 0.000 * |
| ALT (U/L) # | 31(22, 41) | 28(21, 40) | 36(25, 43.75) | 0.001 * |
| GGT (U/L) # | 22(16, 32) | 20(15, 29) | 28(19,38.75) | 0.000 * |
| ALP (U/L) # | 63(53, 76) | 62(52, 73) | 66.5(55, 83.25) | 0.001 * |
| TBA (µmol/L) # | 4.3(2.4, 8.6) | 3.6(2.1, 7.7) | 5.8(3.55, 12.55) | 0.000 * |
| CHE (U/L) # | 8616 ± 2047 | 8833 ± 2079 | 8135 ± 1893 | 0.000 * |
| Glu (mmol/L) # | 5.04(4.64, 5.525) | 5.04(4.65, 5.49) | 5.02(4.6125, 5.49) | 0.848 |
| TCHO (mmol/L) # | 4.81(4.3, 5.48) | 4.85(4.32, 5.45) | 4.77(4.22, 5.58) | 0.743 |
| ApoA1 (g/L) # | 1.3(1.2, 1.5) | 1.3(1.2, 1.5) | 1.4(1.2, 1.5) | 0.020 * |
| TBIL (µmol/L) # | 10.3(7.5, 14.35) | 10.1(7.2325, 14.675) | 11(8.25, 13.8) | 0.172 |
| DBIL (µmol/L) # | 2.8(1.9, 4.2) | 2.6(1.8, 4.1) | 3.2(2.3, 4.3) | 0.001 * |
| ALB (g/L) & | 46.1 ± 3.5 | 46.5 ± 3.2 | 45.3 ± 4.0 | 0.000 * |
| GLO (g/L) & | 27.5 ± 3.8 | 27.2 ± 3.6 | 28.0 ± 4.0 | 0.01 * |
| PLT (×109/L) # | 216(184, 250) | 222(192, 258) | 198(163, 231) | 0.000 * |
| N (×109/L) # | 3.25(2.59, 4.18) | 3.36(2.64, 4.29) | 3.115(2.4725, 3.9225) | 0.035 * |
| L (×109/L) # | 1.74(1.45, 2.12) | 1.74(1.44, 2.09) | 1.75(1.4725, 2.175) | 0.364 |
| RDW-SD (fl) # | 40.2(38.4, 42.4) | 40(38.3, 42.0) | 40.75(38.525, 43.175) | 0.015 * |
| Noninvasive Models | Significant Fibrosis (S ≥ 2) | Advanced Fibrosis (S ≥ 3) | Significant Inflammation G ≥ 2 | Advanced Inflammation G ≥ 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 188) | HBeAg (+) (n = 71) | HBeAg (−) (n = 117) | Total (n = 64) | HBeAg (+) (n = 27) | HBeAg (−) (n = 37) | Total (n = 191) | HBeAg (+) (n = 80) | HBeAg (−) (n = 110) | Total (n = 50) | HBeAg (+) (n = 21) | HBeAg (−) (n = 29) | |
| AA index | 0.600 | 0.559 | 0.621 | 0.684 | 0.681 | 0.684 | 0.630 | 0.573 | 0.662 | 0.673 | 0.662 | 0.677 |
| AAR | 0.533 | 0.581 | 0.505 | 0.594 | 0.689 | 0.529 | 0.488 | 0.517 | 0.479 | 0.597 | 0.697 | 0.529 |
| AARPRI | 0.616 | 0.644 | 0.599 | 0.687 | 0.752 | 0.643 | 0.577 | 0.592 | 0.581 | 0.710 | 0.753 | 0.682 |
| ABA | 0.570 | 0.607 | 0.549 | 0.651 | 0.696 | 0.636 | 0.596 | 0.634 | 0.594 | 0.689 | 0.692 | 0.709 |
| AGAP | 0.713 | 0.750 | 0.695 | 0.794 | 0.823 | 0.784 | 0.733 | 0.754 | 0.736 | 0.857 | 0.841 | 0.881 |
| AGPR | 0.698 | 0.710 | 0.691 | 0.768 | 0.812 | 0.743 | 0.701 | 0.724 | 0.696 | 0.812 | 0.801 | 0.827 |
| ALRI | 0.590 | 0.553 | 0.607 | 0.652 | 0.634 | 0.658 | 0.606 | 0.597 | 0.606 | 0.691 | 0.629 | 0.730 |
| APGA | 0.750 | 0.761 | 0.745 | 0.832 | 0.846 | 0.824 | 0.779 | 0.781 | 0.785 | 0.874 | 0.878 | 0.873 |
| AP index | 0.627 | 0.687 | 0.594 | 0.666 | 0.724 | 0.645 | 0.636 | 0.694 | 0.626 | 0.695 | 0.703 | 0.714 |
| APRI | 0.692 | 0.690 | 0.689 | 0.760 | 0.731 | 0.772 | 0.720 | 0.711 | 0.722 | 0.826 | 0.772 | 0.857 |
| APPCI | 0.726 | 0.682 | 0.757 | 0.786 | 0.806 | 0.777 | 0.720 | 0.717 | 0.725 | 0.737 | 0.737 | 0.736 |
| APPR | 0.670 | 0.668 | 0.674 | 0.721 | 0.773 | 0.692 | 0.673 | 0.690 | 0.670 | 0.761 | 0.741 | 0.784 |
| APRG | 0.686 | 0.701 | 0.678 | 0.788 | 0.812 | 0.776 | 0.713 | 0.729 | 0.714 | 0.836 | 0.811 | 0.863 |
| ATPI model | 0.649 | 0.628 | 0.658 | 0.737 | 0.740 | 0.732 | 0.650 | 0.655 | 0.646 | 0.771 | 0.742 | 0.788 |
| CDS | 0.634 | 0.649 | 0.625 | 0.707 | 0.749 | 0.678 | 0.613 | 0.615 | 0.617 | 0.716 | 0.759 | 0.687 |
| Doha score | 0.656 | 0.684 | 0.642 | 0.730 | 0.749 | 0.722 | 0.672 | 0.679 | 0.677 | 0.760 | 0.748 | 0.773 |
| eLIFT | 0.676 | 0.714 | 0.655 | 0.749 | 0.811 | 0.713 | 0.688 | 0.716 | 0.691 | 0.783 | 0.800 | 0.780 |
| FCI | 0.670 | 0.643 | 0.684 | 0.736 | 0.743 | 0.730 | 0.652 | 0.658 | 0.652 | 0.747 | 0.699 | 0.781 |
| FI | 0.662 | 0.677 | 0.650 | 0.761 | 0.758 | 0.763 | 0.694 | 0.670 | 0.716 | 0.821 | 0.795 | 0.839 |
| FIB-4 | 0.659 | 0.717 | 0.630 | 0.726 | 0.790 | 0.698 | 0.672 | 0.719 | 0.671 | 0.769 | 0.797 | 0.769 |
| mFIB-4 | 0.610 | 0.677 | 0.572 | 0.675 | 0.771 | 0.616 | 0.595 | 0.650 | 0.585 | 0.691 | 0.765 | 0.650 |
| FIB-5 | 0.368 | 0.390 | 0.355 | 0.332 | 0.409 | 0.276 | 0.326 | 0.361 | 0.300 | 0.279 | 0.394 | 0.196 |
| FIB-6 | 0.647 | 0.689 | 0.627 | 0.737 | 0.779 | 0.727 | 0.700 | 0.725 | 0.709 | 0.823 | 0.803 | 0.866 |
| FibroQ | 0.623 | 0.687 | 0.588 | 0.692 | 0.789 | 0.634 | 0.604 | 0.657 | 0.597 | 0.701 | 0.777 | 0.662 |
| Forns | 0.643 | 0.713 | 0.615 | 0.729 | 0.805 | 0.715 | 0.639 | 0.707 | 0.638 | 0.743 | 0.755 | 0.799 |
| Fibro-α | 0.674 | 0.696 | 0.665 | 0.746 | 0.818 | 0.705 | 0.649 | 0.670 | 0.654 | 0.773 | 0.854 | 0.720 |
| GAPI | 0.719 | 0.756 | 0.701 | 0.802 | 0.859 | 0.772 | 0.705 | 0.725 | 0.708 | 0.834 | 0.850 | 0.836 |
| Gao-2 | 0.637 | 0.703 | 0.643 | 0.689 | 0.752 | 0.729 | 0.652 | 0.700 | 0.672 | 0.741 | 0.771 | 0.787 |
| Gao-1 | 0.677 | 0.770 | 0.619 | 0.750 | 0.851 | 0.675 | 0.689 | 0.794 | 0.662 | 0.793 | 0.873 | 0.750 |
| GqHBsR | 0.650 | 0.695 | 0.633 | 0.684 | 0.774 | 0.643 | 0.632 | 0.653 | 0.634 | 0.728 | 0.784 | 0.707 |
| GP | 0.651 | 0.673 | 0.637 | 0.738 | 0.745 | 0.737 | 0.671 | 0.682 | 0.671 | 0.787 | 0.787 | 0.788 |
| GPR | 0.698 | 0.731 | 0.684 | 0.773 | 0.817 | 0.753 | 0.695 | 0.697 | 0.705 | 0.818 | 0.835 | 0.817 |
| GUCI | 0.706 | 0.700 | 0.706 | 0.777 | 0.750 | 0.787 | 0.720 | 0.712 | 0.719 | 0.832 | 0.782 | 0.857 |
| HBeAg(+)model | 0.637 | 0.703 | 0.619 | 0.689 | 0.752 | 0.675 | 0.652 | 0.700 | 0.662 | 0.741 | 0.771 | 0.750 |
| HB-F | 0.659 | 0.686 | 0.641 | 0.743 | 0.815 | 0.693 | 0.648 | 0.662 | 0.650 | 0.738 | 0.790 | 0.704 |
| HGM-1 | 0.643 | 0.619 | 0.660 | 0.732 | 0.735 | 0.738 | 0.659 | 0.638 | 0.680 | 0.784 | 0.746 | 0.823 |
| HGM-2 | 0.443 | 0.416 | 0.457 | 0.431 | 0.418 | 0.425 | 0.429 | 0.399 | 0.432 | 0.391 | 0.416 | 0.356 |
| IT model | 0.570 | 0.638 | 0.549 | 0.577 | 0.676 | 0.537 | 0.542 | 0.628 | 0.522 | 0.569 | 0.657 | 0.539 |
| INPR | 0.667 | 0.689 | 0.656 | 0.734 | 0.758 | 0.721 | 0.659 | 0.682 | 0.653 | 0.738 | 0.741 | 0.740 |
| King’s score | 0.690 | 0.718 | 0.677 | 0.754 | 0.767 | 0.754 | 0.719 | 0.747 | 0.714 | 0.807 | 0.773 | 0.839 |
| Lok index | 0.664 | 0.673 | 0.656 | 0.745 | 0.796 | 0.707 | 0.640 | 0.634 | 0.649 | 0.739 | 0.771 | 0.715 |
| Logit(Y) | 0.691 | 0.661 | 0.703 | 0.751 | 0.708 | 0.773 | 0.725 | 0.687 | 0.744 | 0.833 | 0.758 | 0.879 |
| Mehdi’s model | 0.592 | 0.370 | 0.718 | 0.619 | 0.350 | 0.751 | 0.646 | 0.417 | 0.783 | 0.680 | 0.376 | 0.858 |
| NLR | 0.453 | 0.427 | 0.469 | 0.496 | 0.499 | 0.499 | 0.426 | 0.455 | 0.408 | 0.449 | 0.399 | 0.488 |
| NIKEI | 0.566 | 0.636 | 0.526 | 0.629 | 0.740 | 0.567 | 0.557 | 0.634 | 0.539 | 0.648 | 0.751 | 0.596 |
| PAPAS | 0.639 | 0.697 | 0.616 | 0.670 | 0.731 | 0.653 | 0.675 | 0.746 | 0.674 | 0.700 | 0.731 | 0.704 |
| PGA | 0.542 | 0.553 | 0.536 | 0.560 | 0.623 | 0.513 | 0.532 | 0.539 | 0.528 | 0.606 | 0.600 | 0.613 |
| PNALT | 0.586 | 0.531 | 0.618 | 0.587 | 0.449 | 0.670 | 0.625 | 0.575 | 0.652 | 0.649 | 0.495 | 0.745 |
| RPR | 0.654 | 0.681 | 0.640 | 0.729 | 0.725 | 0.735 | 0.654 | 0.668 | 0.652 | 0.750 | 0.730 | 0.764 |
| RLR | 0.490 | 0.487 | 0.490 | 0.549 | 0.571 | 0.533 | 0.483 | 0.517 | 0.461 | 0.520 | 0.534 | 0.511 |
| S index | 0.708 | 0.737 | 0.695 | 0.797 | 0.827 | 0.782 | 0.713 | 0.702 | 0.732 | 0.856 | 0.864 | 0.858 |
| Virahep-C model | 0.652 | 0.687 | 0.636 | 0.707 | 0.768 | 0.686 | 0.694 | 0.737 | 0.693 | 0.771 | 0.737 | 0.826 |
| Wang I | 0.700 | 0.766 | 0.680 | 0.791 | 0.838 | 0.786 | 0.705 | 0.774 | 0.689 | 0.859 | 0.894 | 0.854 |
| Wang II | 0.695 | 0.725 | 0.678 | 0.758 | 0.809 | 0.726 | 0.663 | 0.678 | 0.658 | 0.769 | 0.769 | 0.772 |
| XIE-model | 0.714 | 0.670 | 0.739 | 0.800 | 0.769 | 0.816 | 0.744 | 0.696 | 0.770 | 0.861 | 0.796 | 0.897 |
| Group | Grade A (9–12 Points) | Grade B (5–8 Points) | Grade C (0–4 Points) |
|---|---|---|---|
| Total | APGA | AGPR, APRI, APPCI, APRG, FI, FIB-6, GAPI, GPR, GUCI, King’s score, Logit(Y), S index, Wang I, XIE-model | AA index, AAR, AARPRI, ABA, ALRI, AP index, APPR, ATPI model, CDS, Doha score, eLIFT, FCI, FIB-4, mFIB-4, FIB-5, FIB-6, FibroQ, Forns, Fibro-α, Gao-2, Gao-1, GqHBsR, GP, HB-F, HBeAg(+)model, HGM-1, HGM-2, IT model, INPR, Lok index, Mehdi’s model, NIKEI, NLR, PAPAS, PGA, PNALT, RPR, RLR, Virahep-C model, Wang II |
| HBeAg(+)group | APGA, AGAP, GAPI, Gao-1Wang I | AGPR, APPCI, APRG, eLIFT, FIB-4 Forns, Gao-2, GPR, GUCI, S index King’s score, HBeAg(+) model, Wang II | AA index, AAR, AARPRI, ABA, ALRI, AP index, APRI, APPR, ATPI model, CDS, Doha score, FCI, FI, mFIB-4, FIB-5, FIB-6, FibroQ, Fibro-α, GqHBsR, GP, HB-F, HGM-1, HGM-2, IT model INPR, Lok index, Logit(Y), Mehdi’s model, NLR, NIKEI, PAPAS PGA, PNALT, RPR, RLR, Virahep-C model, XIE-model |
| HBeAg(−)group | APGA, XIE-model | AGAP, APRI, APPCI, APRG, FI FIB-6, GAPI, GPR, GUCI, Logit(Y), King’s score, Mehdi’s model, S index Wang I | AA index, AAR, AARPRI, ABA, AGPR, ALRI, AP index, APPR ATPI model, CDS, Doha score, eLIFT, FCI, FIB-4, mFIB-4, FIB-5 FibroQ, Forns, Fibro-α, Gao-2, Gao-1, GqHBsR, GP HBeAg(+)model, HB-F, HGM-1, HGM-2, IT model, INPR, Lok index, NLR, NIKEI, PAPAS, PGA, PNALT, RPR, RLR Virahep-C model, Wang II |
| Noninvasive Models or Indices | Inflammation Activity | Fibrosis Stage | Noninvasive Models or Indices | Inflammation Activity | Fibrosis Stage | ||||
|---|---|---|---|---|---|---|---|---|---|
| Spearman’s r | p Value | Spearman’s r | p Value | Spearman’s r | p Value | Spearman’s r | p Value | ||
| AFP | 0.291 | <0.001 | 0.223 | <0.001 | GLB | 0.0.95 | 0.021 | 0.116 | 0.005 |
| PT | 0.188 | <0.001 | 0.224 | <0.001 | PLT | −0.245 | <0.001 | −0.233 | <0.001 |
| AST | 0.304 | <0.001 | 0.265 | <0.001 | N | −0.129 | 0.002 | −0.086 | 0.035 |
| ALT | 0.207 | <0.001 | 0.139 | <0.001 | RDW-SD | 0.131 | 0.001 | 0.099 | 0.015 |
| GGT | 0.251 | <0.001 | 0.255 | <0.001 | APGA | 0.452 | <0.001 | 0.405 | <0.001 |
| ALP | 0.149 | <0.001 | 0.153 | <0.001 | GAPI | 0.331 | <0.001 | 0.352 | <0.001 |
| DBIL | 0.081 | 0.051 | 0.140 | 0.001 | XIE-model | 0.392 | <0.001 | 0.344 | <0.001 |
| ALB | −0.224 | <0.001 | −0.125 | <0.001 | |||||
| Models | AUROC (95% CI) | Cutoff | Se (%) | Sp (%) | PLR | NLR | PPV (%) | NPV (%) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| S ≥ 2 | |||||||||
| APGA | 0.750(0.702–0.798) | 6.72 | 69.1 | 69.8 | 2.2 | 0.4 | 52.2 | 82.5 | <0.001 |
| GAPI | 0.719(0.674–0.763) | 1.81 | 52.7 | 82.3 | 2.8 | 0.6 | 57.5 | 78.1 | <0.001 |
| XIE model | 0.714(0.668–0.761) | −0.94 | 61.5 | 74.2 | 2.2 | 0.6 | 51.6 | 78.5 | <0.001 |
| S ≥ 3 | |||||||||
| APGA | 0.832(0.778–0.885) | 7.27 | 76.8 | 73.4 | 3.1 | 0.3 | 28.4 | 95.8 | <0.001 |
| GAPI | 0.802(0.746–0.858) | 1.84 | 74.6 | 77.4 | 3.3 | 0.4 | 28.5 | 95.7 | <0.001 |
| XIE model | 0.800(0.736–0.850) | −0.72 | 74.2 | 75.4 | 3.0 | 0.4 | 27.2 | 95.6 | <0.001 |
| G ≥ 2 | |||||||||
| APGA | 0.779(0.734–0.823) | 6.72 | 70.8 | 70.8 | 2.3 | 0.4 | 52.2 | 83.6 | <0.001 |
| GAPI | 0.705(0.658–0.751) | 1.86 | 50.5 | 83.5 | 3.0 | 0.6 | 57.6 | 78.0 | <0.001 |
| XIE model | 0.744(0.697–0.790) | −0.69 | 56.8 | 83.0 | 3.2 | 0.5 | 59.1 | 80.1 | <0.001 |
| G ≥ 3 | |||||||||
| APGA | 0.874(0.823–0.926) | 8.53 | 65.9 | 91.8 | 7.1 | 0.4 | 42.1 | 96.3 | <0.001 |
| GAPI | 0.834(0.776–0.892) | 1.91 | 77.6 | 79.5 | 3.5 | 0.3 | 24.4 | 97.4 | <0.001 |
| XIE model | 0.861(0.806–0.917) | −0.43 | 79.2 | 81.0 | 4.3 | 0.3 | 28.7 | 97.5 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Zhou, L.; Lin, S.; Li, M.; Luo, J.; Chen, L. Noninvasive Models to Assess Liver Inflammation and Fibrosis in Chronic HBV Infected Patients with Normal or Mildly Elevated Alanine Transaminase Levels: Which One Is Most Suitable? Diagnostics 2024, 14, 456. https://doi.org/10.3390/diagnostics14050456
Ma S, Zhou L, Lin S, Li M, Luo J, Chen L. Noninvasive Models to Assess Liver Inflammation and Fibrosis in Chronic HBV Infected Patients with Normal or Mildly Elevated Alanine Transaminase Levels: Which One Is Most Suitable? Diagnostics. 2024; 14(5):456. https://doi.org/10.3390/diagnostics14050456
Chicago/Turabian StyleMa, Shasha, Lian Zhou, Shutao Lin, Mingna Li, Jing Luo, and Lubiao Chen. 2024. "Noninvasive Models to Assess Liver Inflammation and Fibrosis in Chronic HBV Infected Patients with Normal or Mildly Elevated Alanine Transaminase Levels: Which One Is Most Suitable?" Diagnostics 14, no. 5: 456. https://doi.org/10.3390/diagnostics14050456






