Improving Accuracy for Image Fusion in Abdominal Ultrasonography
Abstract
:1. Introduction

2. Experimental Section
2.1. Imaging
2.2. Co-Registration
2.3. Accuracy
2.4. Statistics
3. Results and Discussion
3.1. Results


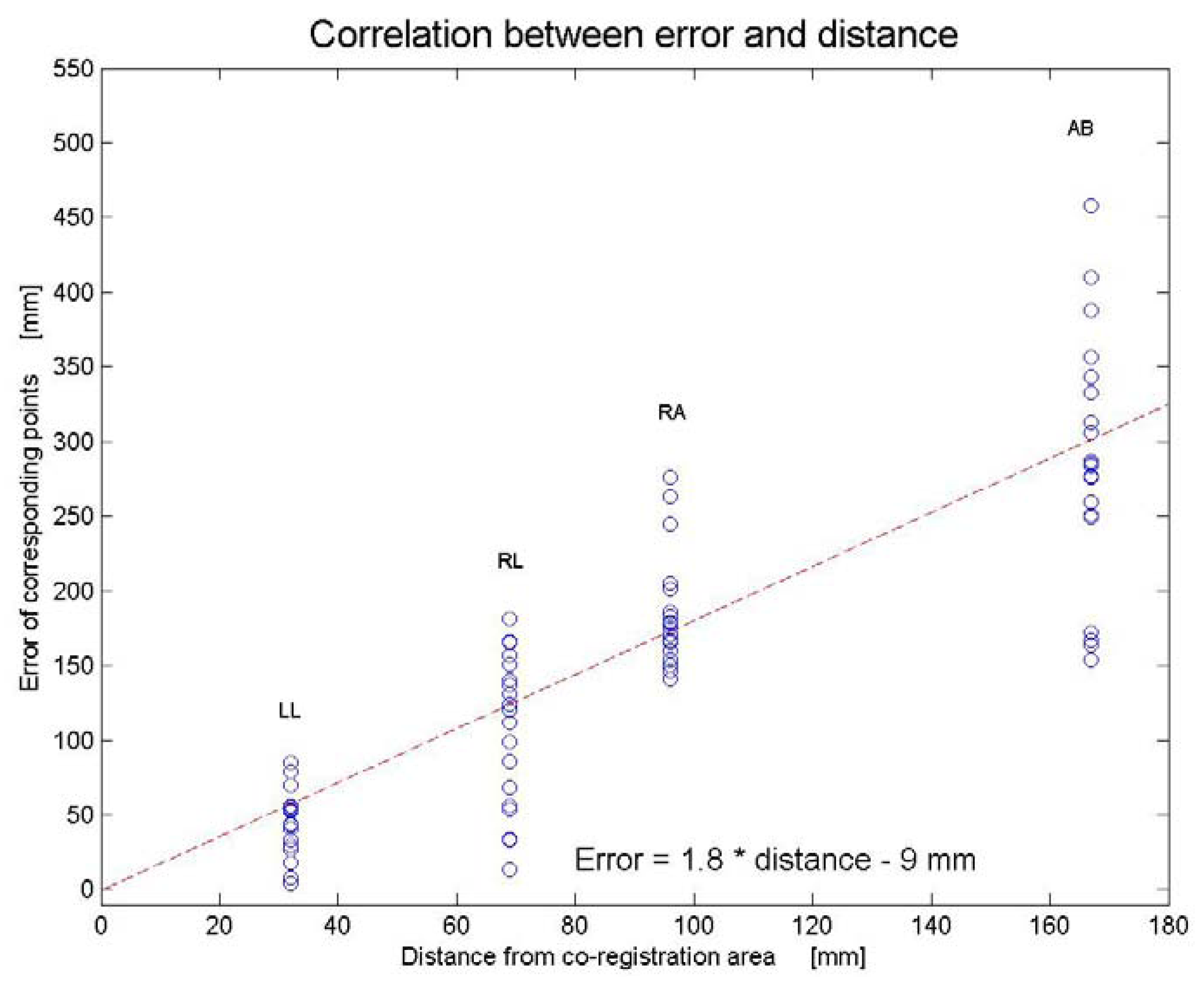
3.2. Discussion
4. Conclusions
References
- Ewertsen, C.; Grossjohann, H.S.; Nielsen, M.B. Image fusion involving ultrasound. Ultraschall Med. 2006, 27, 128–129. [Google Scholar] [CrossRef]
- Crocetti, L.; Lencioni, R.; Debeni, S.; See, T.C.; Pina, C.D.; Bartolozzi, C. Targeting liver lesions for radiofrequency ablation: An experimental feasibility study using a CT-US fusion imaging system. Invest. Radiol. 2008, 43, 33–39. [Google Scholar] [CrossRef]
- Ewertsen, C.; Dencker, D.; Karstrup, S. Core needle biopsy from a small retroperitoneal lymphoma guided by image-fusion and electromagnetic needle tracking. Ultraschall Med. 2012, 33, 1–3. [Google Scholar] [CrossRef]
- Dencker, D.; Ewertsen, C.; Karstrup, S. Drainage of air-containing cavities guided by image fusion involving ultrasound and electromagnetic needle tracking. Ultraschall Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Ewertsen, C.; Ellegaard, K.; Boesen, M.; Torp-Pedersen, S.; Bachmann Nielsen, M. Comparison of two co-registration methods for real-time ultrasonography fused with MRI: A phantom study. Ultraschall Med. 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Hakime, A.; Deschamps, F.; de Carvalho, E.G.; Teriitehau, C.; Auperin, A.; de Baere, T. Clinical evaluation of spatial accuracy of a fusion imaging technique combining previously acquired computed tomography and real-time ultrasound for imaging of liver metastases. Cardiovasc. Interv. Radiol. 2011, 34, 338–344. [Google Scholar] [CrossRef]
- Carrillo, A.; Duerk, J.L.; Lewin, J.S.; Wilson, D.L. Semiautomatic 3-D image registration as applied to interventional MRI liver cancer treatment. IEEE Trans. Med. Imaging 2000, 19, 175–185. [Google Scholar] [CrossRef]
- Lavely, W.C.; Scarfone, C.; Cevikalp, H.; Li, R.; Byrne, D.W.; Cmelak, A.J.; Dawant, B.; Price, R.R.; Hallahan, D.E.; Fitzpatrick, J.M. Phantom validation of coregistration of PET and CT for image-guided radiotherapy. Med. Phys. 2004, 31, 1083–1092. [Google Scholar] [CrossRef]
- Penney, G.P.; Blackall, J.M.; Hamady, M.S.; Sabharwal, T.; Adam, A.; Hawkes, D.J. Registration of freehand 3D ultrasound and magnetic resonance liver images. Med. Image Anal. 2004, 8, 81–91. [Google Scholar] [CrossRef]
- Wein, W.; Brunke, S.; Khamene, A.; Callstrom, M.R.; Navab, N. Automatic CT-ultrasound registration for diagnostic imaging and image-guided intervention. Med. Image Anal. 2008, 12, 577–585. [Google Scholar] [CrossRef]
- Wein, W.; Roper, B.; Navab, N. Automatic registration and fusion of ultrasound with CT for radiotherapy. Med. Image Comput. Comput. Assist. Interv. 2005, 8, 303–311. [Google Scholar] [PubMed]
- Krucker, J.; Xu, S.; Glossop, N.; Viswanathan, A.; Borgert, J.; Schulz, H.; Wood, B.J. Electromagnetic tracking for thermal ablation and biopsy guidance: Clinical evaluation of spatial accuracy. J. Vasc. Interv. Radiol. 2007, 18, 1141–1150. [Google Scholar] [CrossRef]
- Xu, S.; Kruecker, J.; Guion, P.; Glossop, N.; Neeman, Z.; Choyke, P.; Singh, A.K.; Wood, B.J. Closed-loop control in fused MR-TRUS image-guided prostate biopsy. Med. Image Comput. Comput. Assist. Interv. 2007, 10, 128–135. [Google Scholar] [PubMed]
- Kunishi, Y.; Numata, K.; Morimoto, M.; Okada, M.; Kaneko, T.; Maeda, S.; Tanaka, K. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. Am. J. Roentgenol. 2012, 198, 106–114. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ewertsen, C.; Hansen, K.L.; Henriksen, B.M.; Nielsen, M.B. Improving Accuracy for Image Fusion in Abdominal Ultrasonography. Diagnostics 2012, 2, 34-41. https://doi.org/10.3390/diagnostics2030034
Ewertsen C, Hansen KL, Henriksen BM, Nielsen MB. Improving Accuracy for Image Fusion in Abdominal Ultrasonography. Diagnostics. 2012; 2(3):34-41. https://doi.org/10.3390/diagnostics2030034
Chicago/Turabian StyleEwertsen, Caroline, Kristoffer L. Hansen, Birthe M. Henriksen, and Michael B. Nielsen. 2012. "Improving Accuracy for Image Fusion in Abdominal Ultrasonography" Diagnostics 2, no. 3: 34-41. https://doi.org/10.3390/diagnostics2030034




