A Rapid, Multiplexed, High-Throughput Flow-Through Membrane Immunoassay: A Convenient Alternative to ELISA
Abstract
:1. Introduction

2. Materials and Methods
2.1. Test Samples
2.2. Conventional ELISA
2.2.1. Preparation of ELISA Capture Plates for the Indirect IgM Assay
2.2.2. Sample Preparation and IgG “Mop-Up”
2.2.3. ELISA Procedure for the Indirect IgM Assay
2.3. Flow-Through Membrane Immunoassay (FMIA)
2.3.1. Preparation of FMIA Capture Membrane for the Indirect IgM Assay
2.3.2. Sample Preparation and IgG Removal
2.3.3. FMIA Procedure for the Indirect IgM Assay
2.3.4. Image Capture and Quantification
3. Results
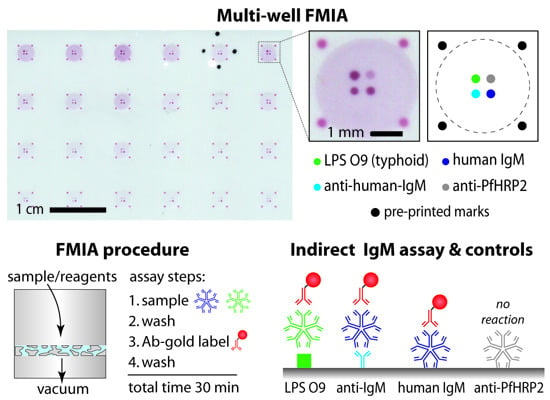
3.1. The Flow-Through Membrane Immunoassay (FMIA) Format
3.2. Representative Images from the FMIA

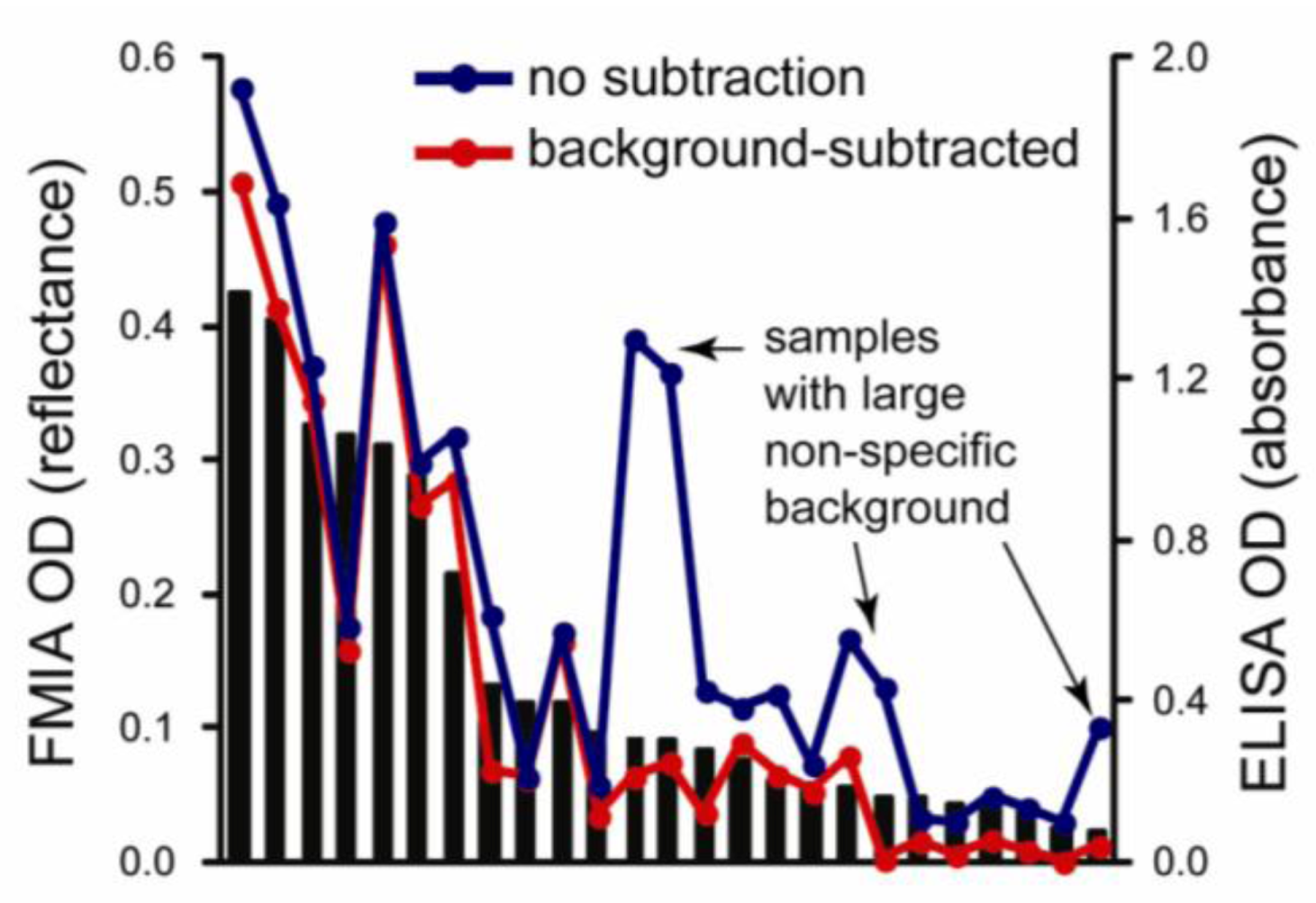
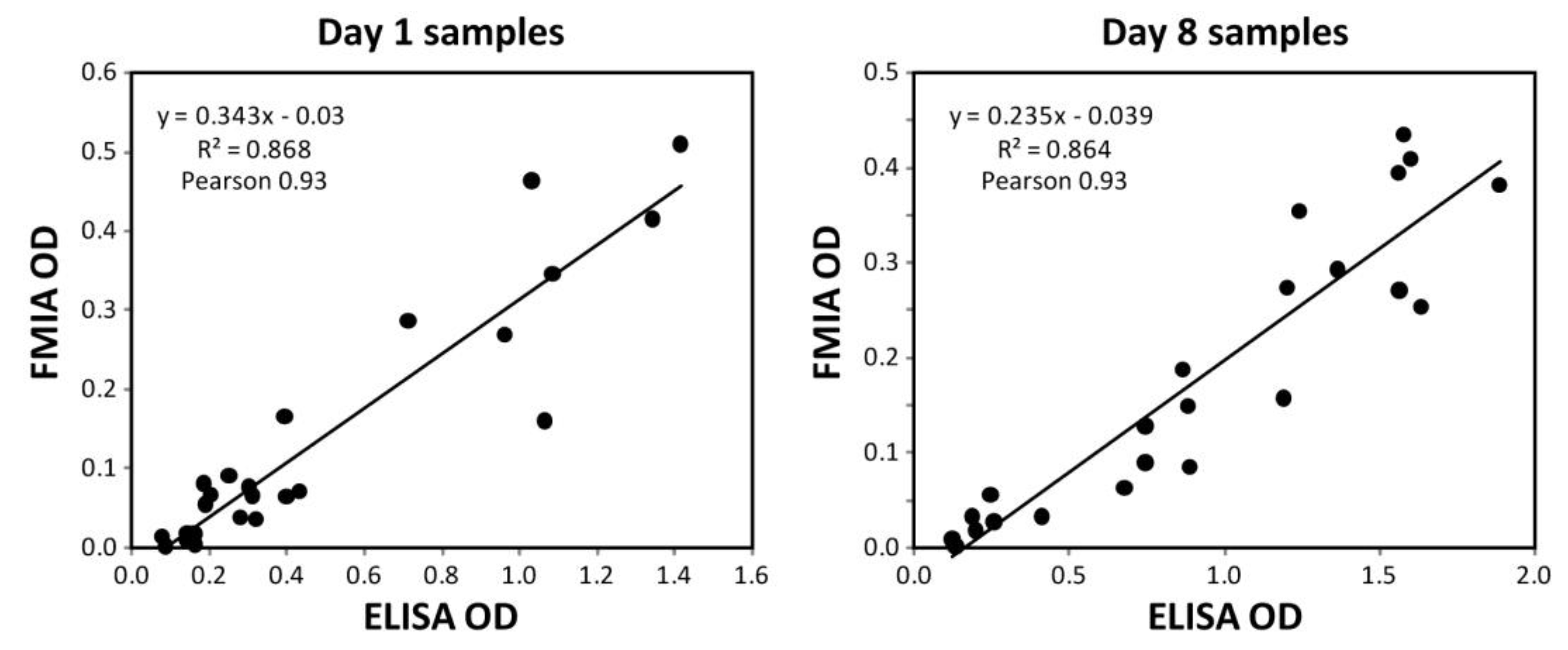
3.3. Comparison between FMIA and ELISA
4. Discussion and Conclusion
 represent optical density (OD) from ELISA (absorbance measurements), and the line plots
represent optical density (OD) from ELISA (absorbance measurements), and the line plots  represent the background subtracted OD from the FMIA (reflectance measurements). (B) Log-log correlation plot of scaled FMIA OD versus ELISA OD for all samples reported in Panel A. The lower plots in Panel B show analysis of error distribution across the measured range (Bland-Altman analysis on scaled FMIA data). The error is well distributed and falls within the limits of agreement (dashed lines; 1.96 times standard deviation of errors).
represent the background subtracted OD from the FMIA (reflectance measurements). (B) Log-log correlation plot of scaled FMIA OD versus ELISA OD for all samples reported in Panel A. The lower plots in Panel B show analysis of error distribution across the measured range (Bland-Altman analysis on scaled FMIA data). The error is well distributed and falls within the limits of agreement (dashed lines; 1.96 times standard deviation of errors).
 represent optical density (OD) from ELISA (absorbance measurements), and the line plots
represent optical density (OD) from ELISA (absorbance measurements), and the line plots  represent the background subtracted OD from the FMIA (reflectance measurements). (B) Log-log correlation plot of scaled FMIA OD versus ELISA OD for all samples reported in Panel A. The lower plots in Panel B show analysis of error distribution across the measured range (Bland-Altman analysis on scaled FMIA data). The error is well distributed and falls within the limits of agreement (dashed lines; 1.96 times standard deviation of errors).
represent the background subtracted OD from the FMIA (reflectance measurements). (B) Log-log correlation plot of scaled FMIA OD versus ELISA OD for all samples reported in Panel A. The lower plots in Panel B show analysis of error distribution across the measured range (Bland-Altman analysis on scaled FMIA data). The error is well distributed and falls within the limits of agreement (dashed lines; 1.96 times standard deviation of errors).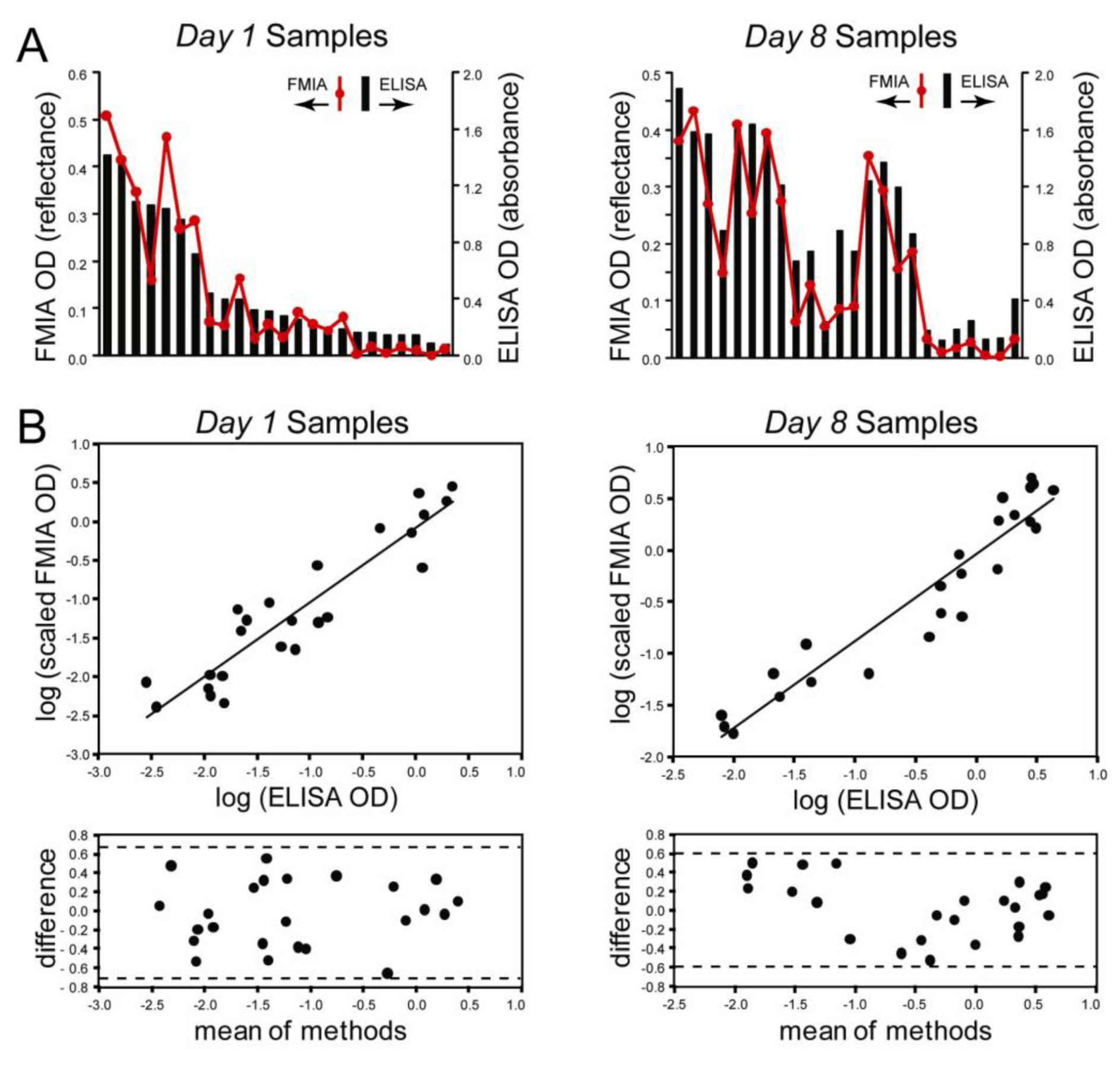
| ELISA | FMIA | |
|---|---|---|
| Assay time | 2.5 h a | 30 min |
| Number of user steps | 12 a | 5 |
| Substrate | Flat microplate | High surface area membrane |
| Detection reagent | Two-step enzyme detection | One-step gold detection |
| Readout method | Microplate reader | camera, or scanner |
| Targets per well | Single | Multiple |
| Assay controls | Separate wells | Integrated within each sample well |
Appendix


Acknowledgments
References
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef]
- Spicar-Mihalic, P.; Stevens, D.Y.; Yager, P. Progress toward a Flow-Through Membrane ELISA in a Microfluidic Format. In Proceedings of the 11th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2007), Paris, France, 7–11 October 2007; pp. 667–669.
- Stevens, D.Y.; Petri, C.R.; Osborn, J.L.; Spicar-Mihalic, P.; McKenzie, K.G.; Yager, P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip 2008, 8, 2038–2045. [Google Scholar] [CrossRef]
- Stevens, D.Y.; Petri, C.R.; Osborn, J.L.; Spicar-Mihalic, P.; McKenzie, K.G.; Yager, P. Rapid and Quantitative Detection of Malarial Antigen for Microfluidic Point-of-Care Diagnosis in the Developing World. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2008), San Diego, CA, USA, 12–16 October 2008; pp. 1768–1770.
- Sumi, S.; Mathai, A.; Radhakrishnan, V.V. Dot-immunobinding assay. Methods Mol. Biol. 2009, 536, 89–93. [Google Scholar] [CrossRef]
- Cardosa, M.J.; Baharudin, F.; Hamid, S.; Hooi, T.P.; Nimmanitya, S. A nitrocellulose membrane based IgM capture enzyme immunoassay for etiological diagnosis of dengue virus infections. Clin. Diagn. Virol. 1995, 3, 343–350. [Google Scholar] [CrossRef]
- Cardona-Castro, N.; Agudelo-Florez, P. Immunoenzymatic dot-blot test for the diagnosis of enteric fever caused by Salmonella typhi in an endemic area. Clin. Microbiol. Infect. 1998, 4, 64–69. [Google Scholar] [CrossRef]
- Van Vooren, J.P.; Turneer, M.; Yernault, J.C.; de Bruyn, J.; Burton, E.; Legros, F.; Farber, C.M. A multidot immunobinding assay for the serodiagnosis of tuberculosis. Comparison with an enzyme-linked immunosorbent assay. J. Immunol. Methods 1988, 113, 45–49. [Google Scholar] [CrossRef]
- Zalis, M.; Jaffe, C.L. Routine dot-blot assay of multiple serum samples using a simple apparatus. J. Immunol. Meth. 1987, 101, 261–264. [Google Scholar] [CrossRef]
- House, D.; Wain, J.; Ho, V.A.; Diep, T.S.; Chinh, N.T.; Bay, P.V.; Vinh, H.; Duc, M.; Parry, C.M.; Dougan, G.; et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J. Clin. Microbiol. 2001, 39, 1002–1007. [Google Scholar] [CrossRef]
- House, D.; Chinh, N.T.; Diep, T.S.; Parry, C.M.; Wain, J.; Dougan, G.; White, N.J.; Hien, T.T.; Farrar, J.J. Use of paired serum samples for serodiagnosis of typhoid fever. J. Clin. Microbiol. 2005, 43, 4889–4890. [Google Scholar] [CrossRef]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [PubMed]
- Parry, C.M.; Hien, T.T.; Dougan, G.; White, N.J.; Farrar, J.J. Typhoid fever. New Engl. J. Med. 2002, 347, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Favorov, M.; Dougan, G. Searching for the elusive typhoid diagnostic. BMC Infect. Dis. 2010, 10. [Google Scholar] [CrossRef]
- Martins, T.B.; Jaskowski, T.D.; Mouritsen, C.L.; Hill, H.R. An evaluation of the effectiveness of three immunoglobulin G (IgG) removal procedures for routine IgM serological testing. Clin. Diagn. Lab. Immunol. 1995, 2, 98–103. [Google Scholar] [PubMed]
- Steward, M.W.; Steensgaard, J. Antibody Affinity: Thermodynamic Aspects and Biological Significance; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- McKenzie, K.G.; Lafleur, L.K.; Lutz, B.R.; Yager, P. Rapid protein depletion from complex samples using a bead-based microfluidic device for the point of care. Lab Chip 2009, 9, 3543–3548. [Google Scholar] [CrossRef]
- Lafleur, L.; Stevens, D.; McKenzie, K.; Ramachandran, S.; Spicar-Mihalic, P.; Singhal, M.; Arjyal, A.; Osborn, J.; Kauffman, P.; Yager, P.; et al. Progress toward multiplexed sample-to-result detection in low resource settings using microfluidic immunoassay cards. Lab Chip 2012, 12, 1119–1127. [Google Scholar] [CrossRef]
- ImageJ. U.S. National Institutes of Health: Bethesda, MD, USA. Available online: http://rsb.info.nih.gov/ij/ (accessed on 1 February 2013).
- Stevens, D.Y.; Petri, C.R.; Yager, P. On-Card Dry Reagent Storage for Disposable Microfluidic Immunoassays. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2008), San Diego, CA, USA, 12–16 October 2008; pp. 188–190.
- Lafleur, L.K.; Lutz, B.R.; Stevens, D.Y.; Spicar-Mihalic, P.; Osborn, J.L.; McKenzie, K.G.; Yager, P. Rapid Air-Driven Point-of-Care Malaria Detection. In Proceedings of the 13th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2009), Jeju, Korea, 1–5 November 2009; pp. 1698–1700.
- Strother, K.O.; Zsak, L. Development of an enzyme-linked immunosorbent assay to detect chicken parvovirus-specific antibodies. Avian Dis. 2009, 53, 585–591. [Google Scholar] [CrossRef]
- Wong, R.; Favaloro, E.; Pollock, W.; Wilson, R.; Hendle, M.; Adelstein, S.; Baumgart, K.; Homes, P.; Smith, S.; Steele, R.; et al. A multi-centre evaluation of the intra-assay and inter-assay variation of commercial and in-house anti-cardiolipin antibody assays. Pathology 2004, 36, 182–192. [Google Scholar] [CrossRef]
- Stone, R.; Coppock, J.S.; Dawes, P.T.; Bacon, P.A.; Scott, D.L. Clinical value of ELISA assays for IgM and IgG rheumatoid factors. J. Clin. Pathol. 1987, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.A.; Mawhinney, K.A.; Elvander, M.; Adair, B.M.; Merza, M. Evaluation of an IgM-specific indirect enzyme-linked immunosorbent assay for serodiagnosis of bovine respiratory syncytial virus infection: Influence of IgM rheumatoid factor on test results with field sera. J. Vet. Diagn. Invest. 1998, 10, 331–337. [Google Scholar] [CrossRef]
- Barbulovic-Nad, I.; Lucente, M.; Sun, Y.; Zhang, M.J.; Wheeler, A.R.; Bussmann, M. Bio-microarray fabrication techniques—A review. Crit. Rev. Biotechnol. 2006, 26, 237–259. [Google Scholar] [CrossRef]
- Nishioka, G.M.; Markey, A.A.; Holloway, C.K. Protein damage in drop-on-demand printers. J. Am. Chem. Soc. 2004, 126, 16320–16321. [Google Scholar] [CrossRef]
- Zheng, Q.A.; Lu, J.G.; Chen, H.; Huang, L.; Cai, J.N.; Xu, Z.N. Application of inkjet printing technique for biological material delivery and antimicrobial assays. Anal. Biochem. 2011, 410, 171–176. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Shimoda, T. Inkjet printing of functional materials. MRS Bull. 2003, 28, 802–803. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramachandran, S.; Singhal, M.; McKenzie, K.G.; Osborn, J.L.; Arjyal, A.; Dongol, S.; Baker, S.G.; Basnyat, B.; Farrar, J.; Dolecek, C.; et al. A Rapid, Multiplexed, High-Throughput Flow-Through Membrane Immunoassay: A Convenient Alternative to ELISA. Diagnostics 2013, 3, 244-260. https://doi.org/10.3390/diagnostics3020244
Ramachandran S, Singhal M, McKenzie KG, Osborn JL, Arjyal A, Dongol S, Baker SG, Basnyat B, Farrar J, Dolecek C, et al. A Rapid, Multiplexed, High-Throughput Flow-Through Membrane Immunoassay: A Convenient Alternative to ELISA. Diagnostics. 2013; 3(2):244-260. https://doi.org/10.3390/diagnostics3020244
Chicago/Turabian StyleRamachandran, Sujatha, Mitra Singhal, Katherine G. McKenzie, Jennifer L. Osborn, Amit Arjyal, Sabina Dongol, Stephen G. Baker, Buddha Basnyat, Jeremy Farrar, Christiane Dolecek, and et al. 2013. "A Rapid, Multiplexed, High-Throughput Flow-Through Membrane Immunoassay: A Convenient Alternative to ELISA" Diagnostics 3, no. 2: 244-260. https://doi.org/10.3390/diagnostics3020244