Current Staging Procedures in Urinary Bladder Cancer
Abstract
:1. Introduction
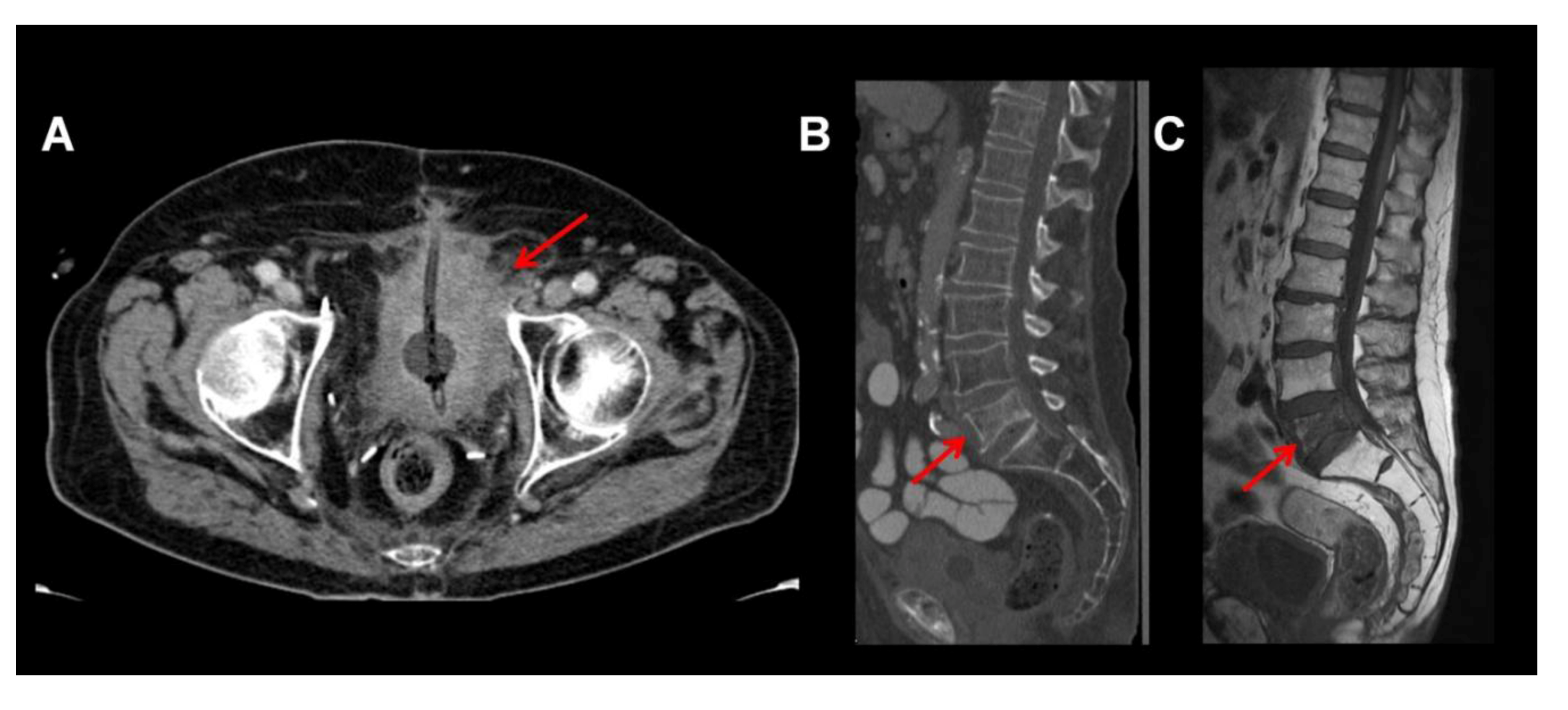
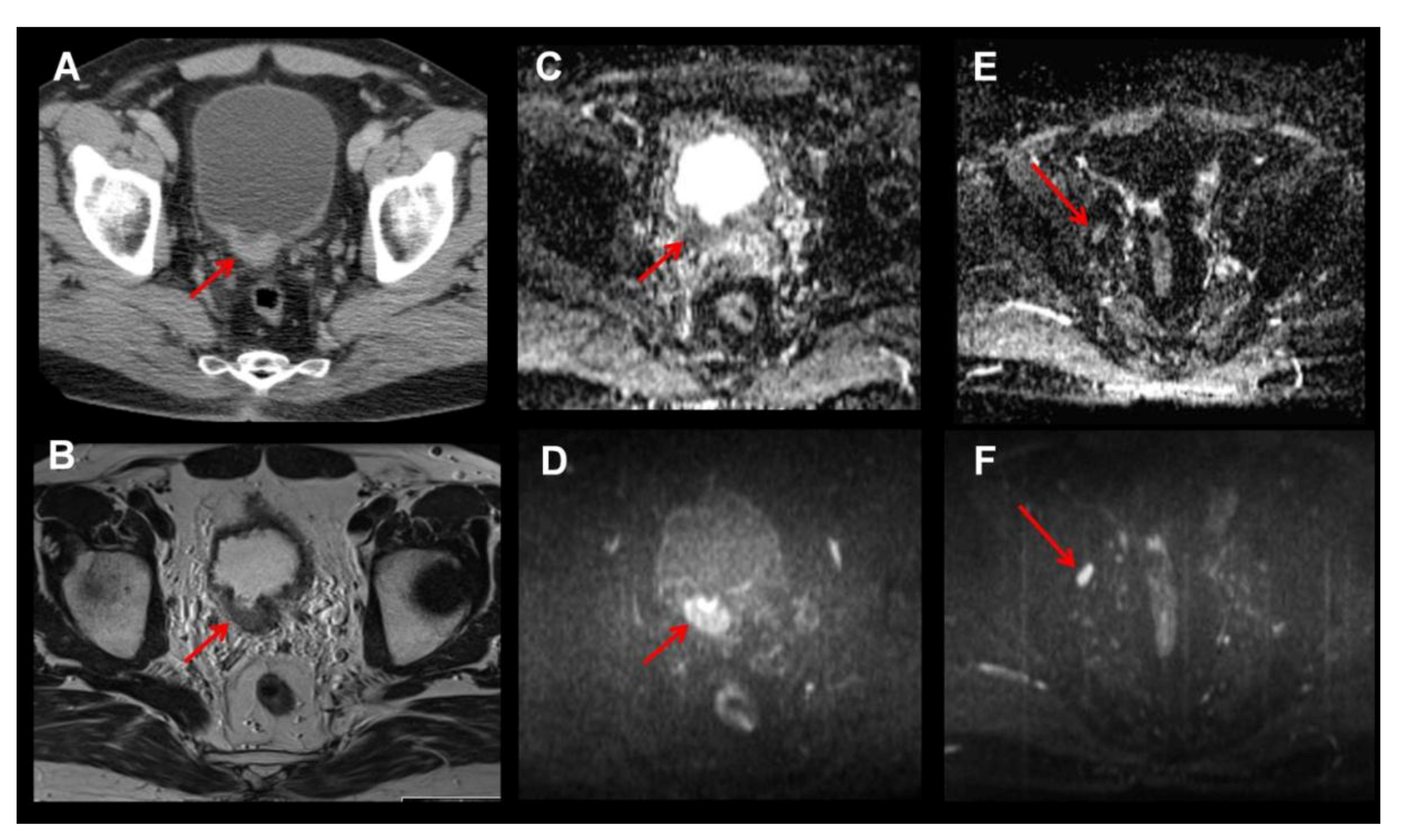
2. MRI
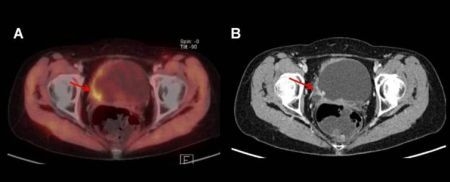
3. PET and PET/CT
3.1. 18F-FDG
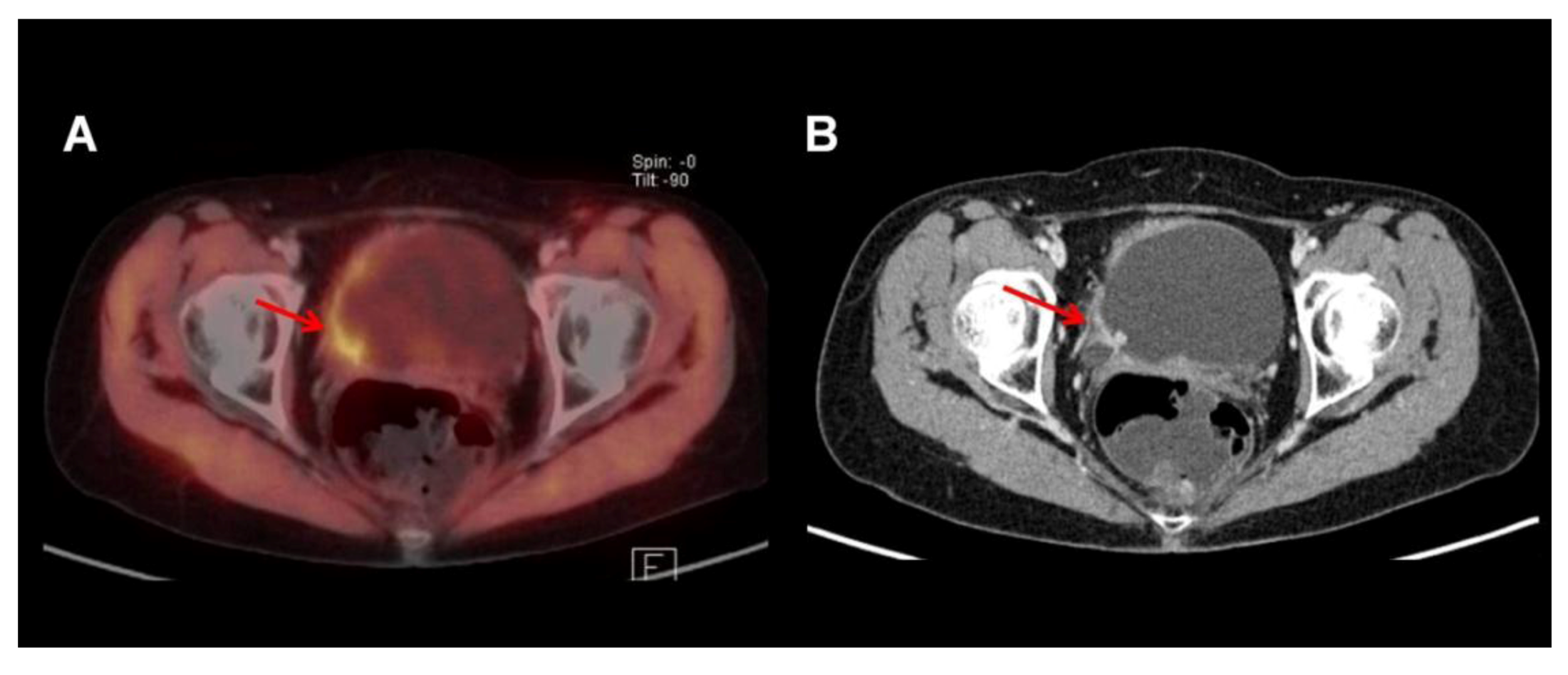
3.2. 11C-Choline
3.3. 11C-Acetate
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Stenzl, A.; Witjes, J.A.; Cowan, N.C.; De Santis, M.; Kuczyk, M.; Lebret, T.; Merseburger, A.S.; Ribal, M.J.; Sherif, A. Guidelines on Bladder Cancer Muscle-Invasive and Metastatic. Available online: http://www.uroweb.org/gls/pdf/07_%20Bladder%20Cancer.pdf (accessed on 17 May 2013).
- Shariat, S.F.; Ehdaie, B.; Rink, M.; Cha, E.K.; Svatek, R.S.; Chromecki, T.F.; Fajkovic, H.; Novara, G.; David, S.G.; Daneshmand, S.; et al. Clinical nodal staging scores for bladder cancer: A proposal for preoperative risk assessment. Eur. Urol. 2012, 61, 237–242. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Vale, C.; Advanced-Bladder-Cancer-Meta-Analysis-Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis. Lancet 2003, 361, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Saksena, M.A.; Dahl, D.M.; Harisinghani, M.G. New imaging modalities in bladder cancer. World J. Urol. 2006, 24, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tekes, A.; Kamel, I.; Imam, K.; Szarf, G.; Schoenberg, M.; Nasir, K.; Thompson, R.; Bluemke, D. Dynamic MRI of bladder cancer: Evaluation of staging accuracy. Am. J. Roentgenol. 2005, 184, 121–127. [Google Scholar] [CrossRef]
- Liedberg, F.; Bendahl, P.O.; Davidsson, T.; Gudjonsson, S.; Holmer, M.; Mansson, W.; Wallengren, N.O. Preoperative staging of locally advanced bladder cancer before radical cystectomy using 3 tesla magnetic resonance imaging with a standardized protocol. Scand. J. Urol. 2013, 47, 108–112. [Google Scholar] [CrossRef]
- Kobayashi, S.; Koga, F.; Yoshida, S.; Masuda, H.; Ishii, C.; Tanaka, H.; Komai, Y.; Yokoyama, M.; Saito, K.; Fujii, Y.; et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: Potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur. Radiol. 2011, 21, 2178–2186. [Google Scholar] [CrossRef]
- Daggulli, M.; Onur, M.R.; Firdolas, F.; Onur, R.; Kocakoc, E.; Orhan, I. Role of diffusion MRI and apparent diffusion coefficient measurement in the diagnosis, staging and pathological classification of bladder tumors. Urol. Int. 2011, 87, 346–352. [Google Scholar] [CrossRef]
- Daneshmand, S.; Ahmadi, H.; Huynh, L.N.; Dobos, N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: Results from a prospective study. Urology 2012, 80, 1313–1318. [Google Scholar] [CrossRef]
- Saokar, A.; Islam, T.; Jantsch, M.; Saksena, M.A.; Hahn, P.F.; Harisinghani, M.G. Detection of lymph nodes in pelvic malignancies with computed tomography and magnetic resonance imaging. Clin. Imaging 2010, 34, 361–366. [Google Scholar] [CrossRef]
- Beer, A.J.; Eiber, M.; Souvatzoglou, M.; Holzapfel, K.; Ganter, C.; Weirich, G.; Maurer, T.; Kubler, H.; Wester, H.J.; Gaa, J.; et al. Restricted water diffusibility as measured by diffusion-weighted MR imaging and choline uptake in 11C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol. Imaging Biol. 2011, 13, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koga, F.; Kobayashi, S.; Ishii, C.; Tanaka, H.; Komai, Y.; Saito, K.; Masuda, H.; Fujii, Y.; Kawakami, S.; et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e21–e27. [Google Scholar] [CrossRef]
- Tuncbilek, N.; Kaplan, M.; Altaner, S.; Atakan, I.H.; Sut, N.; Inci, O.; Demir, M.K. Value of dynamic contrast-enhanced mri and correlation with tumor angiogenesis in bladder cancer. Am. J. Roentgenol. 2009, 192, 949–955. [Google Scholar] [CrossRef]
- Deserno, W.M.; Harisinghani, M.G.; Taupitz, M.; Jager, G.J.; Witjes, J.A.; Mulders, P.F.; Hulsbergen van de Kaa, C.A.; Kaufmann, D.; Barentsz, J.O. Urinary bladder cancer: Preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology 2004, 233, 449–456. [Google Scholar] [CrossRef]
- Froehlich, J.M.; Triantafyllou, M.; Fleischmann, A.; Vermathen, P.; Thalmann, G.N.; Thoeny, H.C. Does quantification of USPIO uptake-related signal loss allow differentiation of benign and malignant normal-sized pelvic lymph nodes? Contrast Media Mol. Imaging 2012, 7, 346–355. [Google Scholar] [CrossRef]
- Triantafyllou, M.; Studer, U.E.; Birkhauser, F.D.; Fleischmann, A.; Bains, L.J.; Petralia, G.; Christe, A.; Froehlich, J.M.; Thoeny, H.C. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur. J. Cancer 2013, 49, 616–624. [Google Scholar] [CrossRef]
- Green, D.A.; Durand, M.; Gumpeni, N.; Rink, M.; Cha, E.K.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Role of magnetic resonance imaging in bladder cancer: Current status and emerging techniques. BJU Int. 2012, 110, 1463–1470. [Google Scholar] [CrossRef]
- Mertens, L.S.; Bruin, N.M.; Vegt, E.; de Blok, W.M.; Fioole-Bruining, A.; van Rhijn, B.W.; Horenblas, S.; Vogel, W.V. Catheter-assisted 18F-FDG-PET/CT imaging of primary bladder cancer: A prospective study. Nucl. Med. Commun. 2012, 33, 1195–1201. [Google Scholar] [CrossRef]
- Vicente, A.M.; Castrejon, A.S.; Munoz, A.P.; Woll, P.P.; Garcia, A.N. Impact of 18F-FDG PET/CT with retrograde filling of the urinary bladder in patients with suspected pelvic malignancies. J. Nucl. Med. Technol. 2010, 38, 128–137. [Google Scholar] [CrossRef]
- Kibel, A.S.; Dehdashti, F.; Katz, M.D.; Klim, A.P.; Grubb, R.L.; Humphrey, P.A.; Siegel, C.; Cao, D.; Gao, F.; Siegel, B.A. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J. Clin. Oncol. 2009, 27, 4314–4320. [Google Scholar] [CrossRef]
- Lodde, M.; Lacombe, L.; Friede, J.; Morin, F.; Saourine, A.; Fradet, Y. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int. 2010, 106, 658–663. [Google Scholar] [CrossRef]
- Jensen, T.K.; Holt, P.; Gerke, O.; Riehmann, M.; Svolgaard, B.; Marcussen, N.; Bouchelouche, K. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: Correlation with histopathology. Scand. J. Urol. 2011, 45, 122–128. [Google Scholar] [CrossRef]
- Swinnen, G.; Maes, A.; Pottel, H.; Vanneste, A.; Billiet, I.; Lesage, K.; Werbrouck, P. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur. Urol. 2010, 57, 641–647. [Google Scholar] [CrossRef]
- Apolo, A.B.; Riches, J.; Schoder, H.; Akin, O.; Trout, A.; Milowsky, M.I.; Bajorin, D.F. Clinical value of fluorine-18 2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography in bladder cancer. J. Clin. Oncol. 2010, 28, 3973–3978. [Google Scholar] [CrossRef]
- Jadvar, H.; Quan, V.; Henderson, R.W.; Conti, P.S. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int. J. Clin. Oncol. 2008, 13, 42–47. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Chen, J.H.; Liang, J.A.; Wang, H.Y.; Lin, C.C.; Lin, W.Y.; Kao, C.H. Clinical value of FDG PET or PET/CT in urinary bladder cancer: A systemic review and meta-analysis. Eur. J. Radiol. 2012, 81, 2411–2416. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Mishani, E.; Orevi, M.; Klein, M.; Freedman, N.; Pode, D.; Shapiro, A.; Katz, R.; Libson, E.; Chisin, R. Contribution of 11C-choline positron emission tomography/computerized tomography to preoperative staging of advanced transitional cell carcinoma. J. Urol. 2006, 176, 940–944. [Google Scholar] [CrossRef]
- Maurer, T.; Souvatzoglou, M.; Kubler, H.; Opercan, K.; Schmidt, S.; Herrmann, K.; Stollfuss, J.; Weirich, G.; Haller, B.; Gschwend, J.E.; et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur. Urol. 2012, 61, 1031–1038. [Google Scholar] [CrossRef]
- Golan, S.; Sopov, V.; Baniel, J.; Groshar, D. Comparison of 11C-choline with 18F-FDG in positron emission tomography/computerized tomography for staging urothelial carcinoma: A prospective study. J. Urol. 2011, 186, 436–441. [Google Scholar] [CrossRef]
- Orevi, M.; Klein, M.; Mishani, E.; Chisin, R.; Freedman, N.; Gofrit, O.N. 11C-acetate PET/CT in bladder urothelial carcinoma: Intraindividual comparison with 11C-choline. Clin. Nucl. Med. 2012, 37, e67–e72. [Google Scholar] [CrossRef]
- Schoder, H.; Ong, S.C.; Reuter, V.E.; Cai, S.; Burnazi, E.; Dalbagni, G.; Larson, S.M.; Bochner, B.H. Initial results with 11C-acetate positron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol. Imaging Biol. 2012, 14, 245–251. [Google Scholar] [CrossRef]
- Vargas, H.A.; Akin, O.; Schoder, H.; Olgac, S.; Dalbagni, G.; Hricak, H.; Bochner, B.H. Prospective evaluation of MRI, 11C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur. J. Radiol. 2012, 81, 4131–4137. [Google Scholar] [CrossRef]
- Hafeez, S.; Huddart, R. Advances in bladder cancer imaging. BMC Med. 2013, 11. [Google Scholar] [CrossRef]
- Drzezga, A.; Souvatzoglou, M.; Eiber, M.; Beer, A.J.; Furst, S.; Martinez-Moller, A.; Nekolla, S.G.; Ziegler, S.; Ganter, C.; Rummeny, E.J.; et al. First clinical experience with integrated whole-body PET/MR: Comparison to PET/CT in patients with oncologic diagnoses. J. Nucl. Med. 2012, 53, 845–855. [Google Scholar] [CrossRef]
- Judenhofer, M.S.; Wehrl, H.F.; Newport, D.F.; Catana, C.; Siegel, S.B.; Becker, M.; Thielscher, A.; Kneilling, M.; Lichy, M.P.; Eichner, M.; et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat. Med. 2008, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maurer, T.; Horn, T.; Heck, M.; Gschwend, J.E.; Eiber, M.; Beer, A.J. Current Staging Procedures in Urinary Bladder Cancer. Diagnostics 2013, 3, 315-324. https://doi.org/10.3390/diagnostics3030315
Maurer T, Horn T, Heck M, Gschwend JE, Eiber M, Beer AJ. Current Staging Procedures in Urinary Bladder Cancer. Diagnostics. 2013; 3(3):315-324. https://doi.org/10.3390/diagnostics3030315
Chicago/Turabian StyleMaurer, Tobias, Thomas Horn, Matthias Heck, Jürgen E. Gschwend, Matthias Eiber, and Ambros J. Beer. 2013. "Current Staging Procedures in Urinary Bladder Cancer" Diagnostics 3, no. 3: 315-324. https://doi.org/10.3390/diagnostics3030315
APA StyleMaurer, T., Horn, T., Heck, M., Gschwend, J. E., Eiber, M., & Beer, A. J. (2013). Current Staging Procedures in Urinary Bladder Cancer. Diagnostics, 3(3), 315-324. https://doi.org/10.3390/diagnostics3030315