Computed Tomography and Ultrasounds for the Follow-up of Hepatocellular Carcinoma Ablation: What You Need to Know
Abstract
:1. Introduction
2. Assessment of Tumor Response

3. Ultrasounds

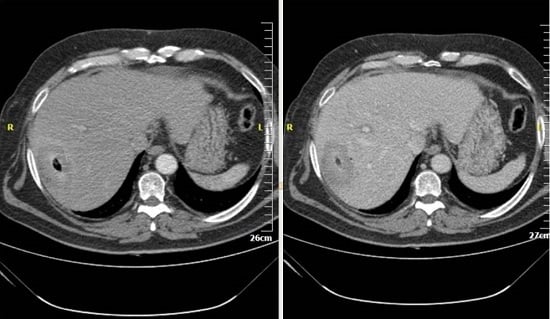
4. Computed Tomography (CT)

5. Conclusions
Acknowledgments
Conflicts of Interest
References
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar]
- Galun, D.; Basaric, D.; Zuvela, M.; Bulajic, P.; Bogdanovic, A.; Bidzic, N.; Milicevic, M. Hepatocellular carcinoma: From clinical practice to evidence-based treatment protocols. World J. Hepatol. 2015, 7, 2274–2291. [Google Scholar] [CrossRef] [PubMed]
- Sofocleous, C.T.; Sideras, P.; Petre, E.N. “How We Do it”—A Practical Approach to Hepatic Metastases Ablation Techniques. Tech. Vasc. Interv. Radiol. 2013, 16, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Dommermuth, A.; Heinle, B.; Nour-Eldin, N.E.; Lehnert, T.; Eichler, K.; Zangos, S.; Bechstein, W.O.; Naguib, N.N. Colorectal Cancer Liver Metastases: Long-Term Survival and Progression-Free Survival After Thermal Ablation Using Magnetic Resonance-Guided Laser-Induced Interstitial Thermotherapy in 594 Patients: Analysis of Prognostic Factors. Investig. Radiol. 2014, 49, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Silk, M.T.; Wimmer, T.; Lee, K.S.; Srimathveeravalli, G.; Brown, K.T.; Kingham, P.T.; Fong, Y.; Durack, J.C.; Sofocleous, C.T.; Solomon, S.B. Percutaneous Ablation of Peribiliary Tumors with Irreversible Electroporation. J. Vasc. Interv. Radiol. 2014, 25, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Cioni, D.; Della Pina, C.; Crocetti, L. Hepatocellular carcinoma: New Options for Image-Guided Ablation. J. Hepatobiliary Pancreat. Sci. 2010, 17, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Cioni, D.; Lencioni, R.; Bartolozzi, C. Percutaneous ablation of liver malignancies: Imaging Evaluation of Treatment Response. Eur. J. Ultrasound 2001, 13, 73–93. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Grassi, C.J.; Cardella, J.F.; Charboneau, J.W.; Dodd, G.D., 3rd; Dupuy, D.E.; Gervais, D.; Gillams, A.R.; Kane, R.A.; Lee, F.T., Jr.; et al. Society of Interventional Radiology Technology Assessment Committee. Image-guided tumor ablation: Standardization of Terminology and Reporting Criteria. J. Vasc. Interv. Radiol. 2005, 16, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; de Baere, T.; Lencioni, R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Intervent. Radiol. 2010, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.J.; Locklin, J.K.; Viswanathan, A.; Kruecker, J.; Haemmerich, D.; Cebral, J.; Sofer, A.; Cheng, R.; McCreedy, E.; Cleary, K.; et al. Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future. J. Vasc. Interv. Radiol. 2007, 18, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R. New data supporting modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Vossen, J.A.; Buijs, M.; Geschwind, J.F.; Liapi, E.; Prieto Ventura, V.; Lee, K.H.; Bluemke, D.A.; Kamel, I.R. DWI and GD-EOB-DTPA contrast enhanced MR imaging for characterization of tumor necrosis in an animal model. JCAT 2009, 33, 626–630. [Google Scholar]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—Update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med. Biol. 2013, 39, 187–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yang, W.; Yan, K.; Zou, M.W.; Solbiati, L.; Liu, J.B.; Dai, Y. Large Liver Tumors: Protocol for Radiofrequency Ablation and its Clinical Application in 110 Patients—Mathematic Model, Overlapping Mode, and Electrode Placement Process. Radiology 2004, 232, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.F.; Andreano, A.; Zimbaro, F.; Lava, M.; Lazzaroni, S.; Sironi, S. Contrast enhanced ultrasound: Roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J. Ultrasound 2012, 15, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Numata, K.; Morimoto, M.; Oshima, T.; Ueda, M.; Okada, M.; Takebayashi, S.; Zhou, X.; Tanaka, K. Role of Sonazoid-Enhanced Three-Dimensional Ultrasonography in the Evaluation of Percutaneous Radiofrequency Ablation of Hepatocellular Carcinoma. Eur. J. Radiol. 2010, 75, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lim, H.K.; Kim, S.H.; Lee, W.J.; Jang, H.J.; Kim, H.; Lee, S.J.; Lim, J.H. Assessment of therapeutic response in hepatocellular carcinoma treated with percutaneous radio frequency ablation: Comparison of multiphase helical computed tomography and power Doppler ultrasonography with a microbubble contrast agent. J. Ultrasound Med. 2002, 21, 391–401. [Google Scholar] [PubMed]
- Schima, W.; Ba-Ssalamah, A.; Kurtaran, A.; Schindl, M.; Gruenberger, T. Post-treatment imaging of liver tumours. Cancer Imaging 2007, 7, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, J.M.; Choi, B.I. Assessment of the treatment response of HCC. Abdom. Imaging 2011, 36, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Sainani, N.I.; Gervais, D.A.; Mueller, P.R.; Arellano, R.S. Imaging after Percutaneous Radiofrequency Ablation of Hepatic Tumors: Part 2, Abnormal Findings. Am. J. Roentgenol. 2013, 200, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; de Baere, T.; Elias, D.; Kuoch, V.; Ducreux, M.; Boige, V.; Petrow, P.; Roche, A.; Sigal, R. Hepatic tumors treated with percutaneous RFA: CT and MR imaging follow-up. Radiology 2002, 223, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Klotz, E.; Schreiber, S.; Bruners, P.; Isfort, P.; Günther, R.W.; Wildberger, J.E. Volumetric arterial enhancement fraction predicts tumor recurrence after hepatic radiofrequency ablation of liver metastases: Initial results. Am. J. Roentgenol. 2011, 196, W573–W579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Schwartz, L.H.; Jiang, L.; Colville, J.; Moskowitz, C.; Wang, L.; Leftowitz, R.; Liu, F.; Kalaigian, J. Shape-constrain region—Growing for delineation of hepatic metastasis on contrast enhanced CT scans. Investig. Radiol. 2006, 41, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, J.M.; Klotz, E.; Park, H.S.; Lee, D.H.; Kim, J.Y.; Kim, S.J.; Kim, S.H.; Lee, J.Y.; Han, J.K.; et al. Quantitative CT color mapping of the arterial enhancement fraction of the liver to detect hepatocellular carcinoma. Radiology 2009, 250, 425–434. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelekis, A.; Filippiadis, D. Computed Tomography and Ultrasounds for the Follow-up of Hepatocellular Carcinoma Ablation: What You Need to Know. Diagnostics 2016, 6, 9. https://doi.org/10.3390/diagnostics6010009
Kelekis A, Filippiadis D. Computed Tomography and Ultrasounds for the Follow-up of Hepatocellular Carcinoma Ablation: What You Need to Know. Diagnostics. 2016; 6(1):9. https://doi.org/10.3390/diagnostics6010009
Chicago/Turabian StyleKelekis, Alexios, and Dimitrios Filippiadis. 2016. "Computed Tomography and Ultrasounds for the Follow-up of Hepatocellular Carcinoma Ablation: What You Need to Know" Diagnostics 6, no. 1: 9. https://doi.org/10.3390/diagnostics6010009
APA StyleKelekis, A., & Filippiadis, D. (2016). Computed Tomography and Ultrasounds for the Follow-up of Hepatocellular Carcinoma Ablation: What You Need to Know. Diagnostics, 6(1), 9. https://doi.org/10.3390/diagnostics6010009