Implementation of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics in South Africa: Perspectives of Key Stakeholders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
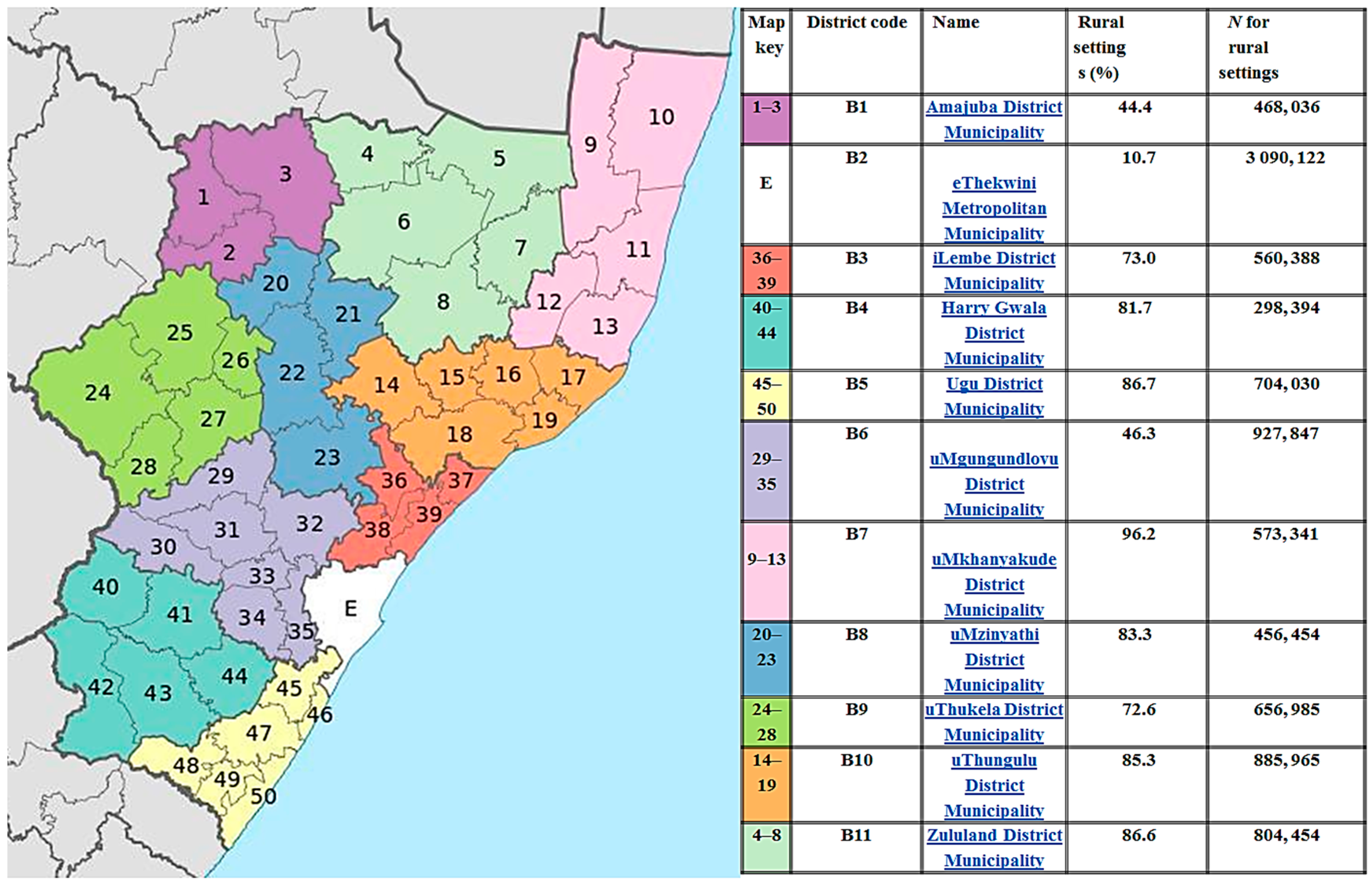
2.2. Study Sample and Setting
2.3. Data Collection
2.4. Data Entry and Analysis
3. Results
3.1. Theme 1: Accessibility of POC Diagnostics in Rural PHC Clinics
3.2. Theme 2: Effectiveness of POC Diagnostics in Rural PHC Clinics
3.3. Theme 3: Reliability of POC Diagnostics in Rural PHC Clinics
4. Discussion
4.1. Main Summary of Findings
4.2. Strengths and Limitations
4.3. Comparison with Existing Literature
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gaede, B.; Versteeg, M. The state of the right to health in rural South Africa. S. Afr. Health Rev. 2011, 9, 99–106. [Google Scholar]
- Boniface, R.; Moshabela, M.; Zulliger, R.; MacPherson, P.; Nyasulu, P. Correlates of delayed diagnosis amongst human immunodeficiency virus-infected pulmonary HIV clinic, South Africa. Tuberc. Res. Treat. 2012. [Google Scholar] [CrossRef] [PubMed]
- Omoding, D.; Katawera, V.; Siedner, M.; Boum, Y. Evaluation of the SD BIOLINE HIV/syphilis Duo assay at a rural health center in Southwestern Uganda. BMC Res. Notes 2014, 7, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melvin, A.J.; Alarcon, J.; Velasquez, C.; Rodriguez, C.; Piscoya, J.; Giraldo, A.; Dinh, P.; Frenkel, L.M. Rapid HIV type 1 testing of women presenting in late pregnancy with unknown HIV status in Lima, Peru. AIDS Res. Hum. Retrovir. 2004, 20, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.J.; Carcamo, C.P.; Chiappe, M.; Valderrama, M.; La Rosa, S.; Holmes, K.K.; Mabey, D.C.; Peeling, R.W. Rapid syphilis tests as catalysts for health systems strengthening: A case study from Peru. PLoS ONE 2013, 8, e66905. [Google Scholar] [CrossRef] [PubMed]
- Faal, M.; Naidoo, N.; Glencross, D.K.; Venter, W.D.; Osih, R. Providing immediate cd4 count results at HIV testing improves art initiation. J. Acquir. Immune Defic. Syndr. 2011, 58, e54–e59. [Google Scholar] [CrossRef] [PubMed]
- Pai, N.P.; Pai, M. Point-of-care diagnostics for HIV and tuberculosis: Landscape, pipeline, and unmet needs. Discov. Med. 2012, 13, 35–45. [Google Scholar] [PubMed]
- Lathrop, E.; Jamieson, D.J.; Danel, I. HIV and maternal mortality. Int. J. Gynaecol. Obstet. 2014, 127, 213–215. [Google Scholar] [CrossRef]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report. Available online: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf (accessed on 16 June 2016).
- Wang, S.; Chinnasamy, T.; Lifson, M.A.; Inci, F.; Demirci, U. Flexible substrate-based devices for point-of-care diagnostics. Trends Biotechnol. 2016, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Mabude, Z.; Smit, J.; Kotze, P.; Arbuckle, D.; Wu, J.; van Niekerk, N.; van de Wijgert, J. HIV incidence remains high in Kwazulu-Natal, South Africa: Evidence from three districts. PLoS ONE 2012, 7, e35278. [Google Scholar] [CrossRef] [PubMed]
- Mashamba-Thompson, T.P.; Sartorius, B.; Drain, P.K. Point-of-care diagnostics for improving maternal health in South Africa. Diagnostics 2016, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Policy: Sustainable development goals for people and planet. Nature 2013, 495, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Keeler, E.; Perkins, M.D.; Small, P.; Hanson, C.; Reed, S.; Cunningham, J.; Aledort, J.E.; Hillborne, L.; Rafael, M.E.; Girosi, F. Reducing the global burden of tuberculosis: The contribution of improved diagnostics. Nature 2006, 444, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mackillop, L.H.; Bartlett, K.; Birks, J.; Farmer, A.J.; Gibson, O.J.; Kevat, D.A.; Kenworthy, Y.; Levy, J.C.; Loerup, L.; Tarassenko, L. Trial protocol to compare the efficacy of a smartphone-based blood glucose management system with standard clinic care in the gestational diabetic population. BMJ Open 2016, 6, e009702. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D.; Herring, A.; Hook, E.W. Why do we need quality-assured diagnostic tests for sexually transmitted infections? Nat. Rev. Microbiol. 2006, 4, S7–S19. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Centers for Disease Control. Rapid HIV Tests: Guidelines for Use in HIV Testing and Counselling Services in Resource-Constrained Settings; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Mashamba-Thompson, T.P.; Sartorius, B.; Stevens, F.C.; Drain, P.K. Experiential bloom’s taxonomy learning framework for point-of-care diagnostics training of primary healthcare workers. Afr. J. Lab. Med. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Stekler, J.D.; Ure, G.; O’Neal, J.D.; Lane, A.; Swanson, F.; Maenza, J.; Stevens, C.; Coombs, R.W.; Dragavon, J.; Swenson, P.D. Performance of determine combo and other point-of-care HIV tests among seattle MSM. J. Clin. Virol. 2016, 76, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Šprongl, L. Quality Assurance for the Poct Systems; Intech: Sumperk, Czech Republic, 2012. [Google Scholar]
- Engel, N.; Ganesh, G.; Patil, M.; Yellappa, V.; Pai, N.P.; Vadnais, C.; Pai, M. Barriers to point-of-care testing in india: Results from qualitative research across different settings, users and major diseases. PLoS ONE 2015, 10, e0135112. [Google Scholar] [CrossRef] [PubMed]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low-and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Dominique, J.K.; Ortiz-Osorno, A.A.; Fitzgibbon, J.; Gnanashanmugam, D.; Gilpin, C.; Tucker, T.; Peel, S.; Peter, T.; Kim, P.; Smith, S. Implementation of HIV and tuberculosis diagnostics: The importance of context. Clin. Infect. Dis. 2015, 61, S119–S125. [Google Scholar] [CrossRef] [PubMed]
- Jaya, Z.; Mashamba-Thompson, T.P. Lean and agile point-of-care diagnostic services quality systems management for low-and middle-income countries. Point Care 2016, 15, 152–157. [Google Scholar] [CrossRef]
- Nabyonga, J.; Orem, J. From knowledge to policy: Lessons from Africa. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Mashamba-Thompson, T.P.; Drain, P.K.; Sartorius, B. Evaluating the accessibility and utility of HIV-related point-of-care diagnostics for maternal health in rural South Africa: a study protocol. BMJ Open 2016, 6, e011155. [Google Scholar] [CrossRef] [PubMed]
- KwaZulu-Natal. Available online: https://en.Wikipedia.Org/wiki/KwaZulu-Natal (accessed on 30 November 2016).
- Provintial Profile 2004 Kwazulu-Natal. Available online: http://www.statssa.gov.za/publications/Report-00-91-05/Report-00-91-052004.pdf (accessed on 30 November 2016).
- Ministry of Rural Development and Land Reform. The Comprehensive Rural Development Programme Framework; Ministry of Rural Development and Land Reform: Pretoria, South Africa, 2009.
- The Presidency Republic of South Africa. Together Doing More and Better Medium Term Strategic Framework: A Framework to Guide Government’s Programme in the Electoral Mandate Period (2009–2014); South African Government: Pretoria, South Africa, 2009.
- Engel, N.; Davids, M.; Blankvoort, N.; Pai, N.P.; Dheda, K.; Pai, M. Compounding diagnostic delays: A qualitative study of point-of-care testing in South Africa. Trop. Med. Int. Health 2015, 20, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Novick, G. Is there a bias against telephone interviews in qualitative research? Res. Nurs. Health 2008, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- South African National AIDS Council. National Strategic Plan on HIV, STIs and TB 2012–2016; South African National AIDS Council: Pretoria, South Africa, 2012. [Google Scholar]
- McNerney, R. Diagnostics for developing countries. Diagnostics 2015, 5, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90: An Ambitious Treatment Target to Help End the Aids Epidemic; UNAIDS: Geneva, Switzerland, 2014. [Google Scholar]
- Garcia, P.J.; You, P.; Fridley, G.; Mabey, D.; Peeling, R. Point-of-care diagnostic tests for low-resource settings. Lancet Glob. Health 2015, 3, e257–e258. [Google Scholar] [CrossRef]
- Jones, C.H.; Howick, J.; Roberts, N.W.; Price, C.P.; Heneghan, C.; Plüddemann, A.; Thompson, M. Primary care clinicians’ attitudes towards point-of-care blood testing: A systematic review of qualitative studies. BMC Fam. Pract. 2013, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Nakyanzi, J.K.; Kitutu, F.E.; Oria, H.; Kamba, P.F. Expiry of medicines in supply outlets in uganda. Bull. World Health Organ. 2010, 88, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Schito, M.L.; Peter, T.F.; Cavanaugh, S.; Piatek, A.S.; Young, G.J.; Alexander, H.; Coggin, W.; Domingo, G.J.; Ellenberger, D.; Ermantraut, E. Opportunities and challenges for cost-efficient implementation of new point-of-care diagnostics for HIV and tuberculosis. J. Infecti. Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- Butsashvili, M.; Preble, E.; Kamkamidze, G.; Robinson, J.; Chubinishvili, O.; Sukhiashvili, R. Uptake of an HIV voluntary counseling and testing program for pregnant women in georgia. AIDS Care 2008, 20, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.L.; Negron, T.J.; Lindsay, M.; Nesheim, S.R.; Lee, F.K.; Jamieson, D.J. Rapid human immunodeficiency virus testing in labor and delivery: A comparison of implementation models between 2 hospitals. J. Perinat. Neonatal Nurs. 2007, 21, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Granade, T.C.; Parekh, B.S.; Tih, P.M.; Welty, T.; Welty, E.; Bulterys, M.; Ndikintum, G.; Nkuoh, G.; Tancho, S. Evaluation of rapid prenatal human immunodeficiency virus testing in rural cameroon. Clin. Diagn. Lab. Immunol. 2005, 12, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Heller, T.; Kunthea, S.; Bunthoeun, E.; Sok, K.; Seuth, C.; Killam, W.P.; Sovanna, T.; Sathiarany, V.; Kanal, K. Point-of-care HIV testing at antenatal care and maternity sites: Experience in battambang province, cambodia. Int. J. STD AIDS 2011, 22, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, G.V.; Febo, I.; Díaz, C. The rapid diagnosis of HIV-1 infection in mothers in puerto rico: A crucial testing strategy for maximal reduction of perinatal transmission. Puerto Rico Health Sci. J. 2002, 21, 133–135. [Google Scholar]
- Kissin, D.M.; Akatova, N.; Rakhmanova, A.G.; Vinogradova, E.N.; Voronin, E.E.; Jamieson, D.J.; Glynn, M.K.; Yakovlev, A.; Robinson, J.; Miller, W.C.; et al. Rapid HIV testing and prevention of perinatal HIV transmission in high-risk maternity hospitals in St. Petersburg, Russia. Am. J. Obstet. Gynecol. 2008, 198, 183.e1–183.e7. [Google Scholar] [CrossRef] [PubMed]
- Kizito, D.; Woodburn, P.W.; Kesande, B.; Ameke, C.; Nabulime, J.; Muwanga, M.; Grosskurth, H.; Elliott, A.M. Uptake of HIV and syphilis testing of pregnant women and their male partners in a programme for prevention of mother-to-child HIV transmission in uganda. Trop. Med. Int. Health 2008, 13, 680–682. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; World Health Organization. Towards Universal Access: Scaling Up Priority HI; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization; Centers for Disease Control and Prevention. Guidelines for Assuring the Accuracy and Reliability of HIV Rapid Testing: Applying a Quality System Approach; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
| Criteria | Characteristics |
|---|---|
| Affordable | Purchasable price for settings comprised of a population at risk of infection |
| Sensitive | Results contain minimal false negatives (99%) |
| Specific | Results contain minimal false positives (99%) |
| User-friendly | Required minimal steps to carry test |
| Rapid and robust | Short turnaround time and no need for refrigerated storage |
| Equipment free | No need for complex equipment |
| Delivered | Made accessible to end users |
| Stakeholder | Role in POC Diagnostics Implementation |
|---|---|
| 3 public health officials from three KwaZulu-Natal (KZN) districts | District PHC managers responsible for deployment and overseeing POC diagnostics services in rural PHC clinics. Although the district managers are based in the district offices rather than in rural PHC clinics, they maintain regular contact with the clinics to monitor to day-to-day running of clinic services, including POC diagnostic services. |
| POC developers-researcher for the most accessible, available and used POC test in rural South Africa | Research and development of HIV rapid test. This stakeholder was part of the team of scientists that were involved in the development and deployment of HIV rapid tests in South Africa. Although the stakeholder is based in the USA, the stakeholder still plays a significant role in the implementation of HIV-related POC diagnostics in South Africa. |
| POC developer-manager for all POC diagnostics for resource-limited settings | Managing research, development and deployment of POC diagnostics to resource-limited settings. Although this stakeholder is based in the USA, the stakeholder is responsible for coordinating the development and deployment of POC diagnostics in resource-limited settings. |
| 2 POC diagnostics users based on rural PHC clinics | Patient testing in rural PHC clinics. In additions these stakeholders are responsible to maintaining the supply chain management for POC diagnostics services from the clinic side. |
| 2 Clinic managers for rural PHC clinics | Managing the smooth running of POC diagnostic services in POC clinics. Clinic managers are responsible for managing POC diagnostics users in the clinic and for reporting to the district managers. |
| 2 NHLS managers from KwaZulu-Natal | Not directly involved in the PHC clinic POC diagnostics implementation, but involved in laboratory-based pathology service provision. All discordant POC test results require a follow up with laboratory testing. Therefore, we perceive laboratories as one of the key stakeholders of these services. |
| Relevance | Recommendations | Alignment with the WHO Quality ASSURED Criteria |
|---|---|---|
| Research | POC diagnostics developer: “What you should really ask to be done or maybe to it yourself, is go do a proper outcome study with the economics involved, identify all the costs, work out all the labour and workflow issues, follow the patient to see if anything gets better and only then start to recommend that point-of-care could be done…” | Affordability |
| POC diagnostics development | POC diagnostics developer: “It is just a trade-off there, if cost is really that critical, we could design a system that is really cheap, you could do two at the time, five at a time or 10 at a time and you could share some of the common things such as a screen, a heater you only need one instead of two for two machines but the workflow is going to be impacted.” | Affordability |
| Stakeholder collaboration | POC diagnostics developer: “So, if we can have better communication between those who are on these data and the developers, I think that we can really make systems more cost effective.” Laboratory manager: “There must be collaboration between the departments, I mean among the whole stakeholders whenever the point-of-care testing is introduced. So that it can be validated before it will be implemented because most of the time they just implement it and then when there is a problem… imagine now you bought a number of machines, only to find that those machines, they are not producing what they are supposed to produce.” | Sensitivity and Specificity |
| Quality Management | Laboratory manager: “Like I said, provided it is done, and we are getting true results, if facility chooses, the only reason I can agree with point-of-care testing to be done in the facility, is when you need urgent results. If you look at lab machines there are internal quality controls that are run to ensure accuracy of the results to make sure that all instrumentation is performing well. Regular maintenance is done in labs by competent staff.” | Sensitivity and Specificity |
| Monitoring and evaluation | PHC manager: “I think if it can be closely monitored and evaluated and challenges can be closely addressed, I don’t think it will be a serious problem.” | Sensitivity and Specificity |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashamba-Thompson, T.P.; Jama, N.A.; Sartorius, B.; Drain, P.K.; Thompson, R.M. Implementation of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics in South Africa: Perspectives of Key Stakeholders. Diagnostics 2017, 7, 3. https://doi.org/10.3390/diagnostics7010003
Mashamba-Thompson TP, Jama NA, Sartorius B, Drain PK, Thompson RM. Implementation of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics in South Africa: Perspectives of Key Stakeholders. Diagnostics. 2017; 7(1):3. https://doi.org/10.3390/diagnostics7010003
Chicago/Turabian StyleMashamba-Thompson, Tivani P., Ngcwalisa A. Jama, Benn Sartorius, Paul K. Drain, and Rowan M. Thompson. 2017. "Implementation of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics in South Africa: Perspectives of Key Stakeholders" Diagnostics 7, no. 1: 3. https://doi.org/10.3390/diagnostics7010003






