Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management
Abstract
:1. Introduction
2. Impact of POC Diagnostics on Health Outcomes in LMICs
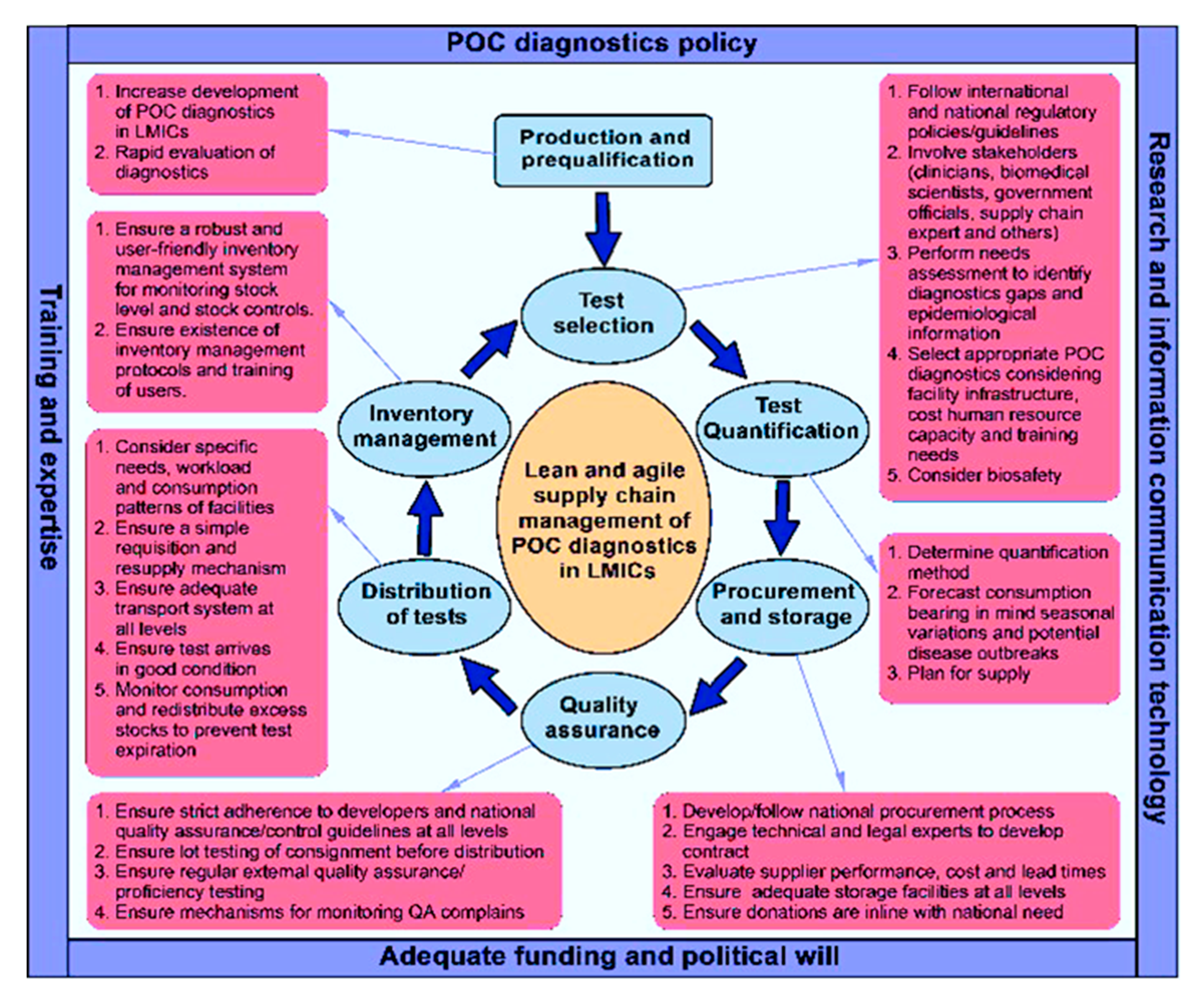
3. Efficient Supply Chain Management for POC Diagnostics in LMICs
4. Quality Systems Management for POC Diagnostics in LMICs
5. Geographical Considerations Regarding Healthcare Access for POC Diagnostics in LMICs
6. Health Infrastructure for POC Diagnostics in LMICs
7. Enabling Policy Framework for POC Diagnostics in LMICs
8. Barriers and Challenges Related to Accessibility of POC Diagnostics in LMICs
8.1. Policy/Regulatory Guidelines and Funding Challenges
8.2. Health System and Infrastructure Barriers
8.3. Challenges with POC Diagnostics Development
8.4. Geographical Barriers to Access
8.5. Supply Chain Barriers
9. Discussion
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Manual for Procurement of Diagnostics and Related Laboratory Items and Equipment; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Global Health Delivery Online. Expert Panel: Advancing Care Delivery: Driving Demand and Supply of Diagnostics. 2015. Available online: https://www.ghdonline.org/global-diagnostics/discussion/driving-demand-and-supply-of-diagnostics/ (accessed on 12 September 2017).
- Peeling, R.W.; Mabey, D. Point-of-care tests for diagnosing infections in the developing world. Clin. Microbiol. Infect. 2010, 16, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- HIV/AIDS JUNPo. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2014. [Google Scholar]
- Drain, P.K.; Hyle, E.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.; Rodriguez, W.; Bassett, I.V. Evaluating Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2015, 14, 239–249. [Google Scholar] [CrossRef]
- Peeling, R.W.; McNerney, R. Emerging technologies in point-of-care molecular diagnostics for resource-limited settings. Expert Rev. Mol. Diagn. 2014, 14, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Müller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Peeling, R.W. Diagnostics in a digital age: An opportunity to strengthen health systems and improve health outcomes. Int. Health 2015, 7, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Sartorius, B. Evaluating the accessibility and utility of HIV-related point-of-care diagnostics for maternal health in rural South Africa: A study protocol. BMJ Open 2016, 6, e011155. [Google Scholar]
- Mashamba-Thompson, T.P.; Sartorius, B.; Drain, P.K. Point-of-Care Diagnostics for Improving Maternal Health in South Africa. Diagnostics 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Rooney, K.D.; Schilling, U.M. Point-of-care testing in the overcrowded emergency department-can it make a difference? Crit. Care 2014, 18, 692. [Google Scholar] [CrossRef] [PubMed]
- Kafkova, J. Rapid diagnostic point of care tests in resource limited settings. Int. J. Infect. Dis. 2016, 45, 56–57. [Google Scholar] [CrossRef]
- Mashamba-Thompson, T.P.; Morgan, R.L.; Sartorius, B.; Dennis, B.; Drain, P.K.; Thabane, L. Effect of Point-of-Care Diagnostics on Maternal Outcomes in Human Immunodeficiency Virus–Infected Women: Systematic Review and Meta-analysis. Point Care 2017, 16, 67–77. [Google Scholar] [CrossRef]
- Luppa, P.B.; Bietenbeck, A.; Beaudoin, C.; Giannetti, A. Clinically relevant analytical techniques, organizational concepts for application and future perspectives of point-of-care testing. Biotechnol. Adv. 2016, 34, 139–160. [Google Scholar]
- De Schacht, C.; Lucas, C.; Sitoe, N.; Machekano, R.; Chongo, P.; Temmerman, M.; Tobaiwa, O.; Guay, L.; Kassaye, S.; Jani, I.V. Implementation of Point-of-Care Diagnostics Leads to Variable Uptake of Syphilis, Anemia and CD4+ T-Cell Count Testing in Rural Maternal and Child Health Clinics. PLoS ONE 2015, 10, e0135744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabey, D.C.; Sollis, K.A.; Kelly, H.A.; Benzaken, A.S.; Bitarakwate, E.; Changalucha, J. Point-of-care tests to strengthen health systems and save newborn lives: The case of syphilis. PLoS Med. 2012, 9, e1001233. [Google Scholar] [CrossRef] [PubMed]
- Ansbro, É.M.; Gill, M.M.; Reynolds, J.; Shelley, K.D.; Strasser, S.; Sripipatana, T. Introduction of syphilis point-of-care tests, from pilot study to national programme implementation in Zambia: A qualitative study of healthcare workers’ perspectives on testing, training and quality assurance. PLoS ONE 2015, 10, e0127728. [Google Scholar] [CrossRef] [PubMed]
- Bonawitz, R.E.; Duncan, J.; Hammond, E.; Hamomba, L.; Nambule, J.; Sambambi, K. Assessment of the impact of rapid syphilis tests on syphilis screening and treatment of pregnant women in Zambia. Int. J. Gynaecol. Obstet. 2015, 130 (Suppl. 1), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Mabey, D.C. The introduction of syphilis point of care tests in resource limited settings. Expert Rev. Mol. Diagn. 2017, 17, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Swartzendruber, A.; Steiner, R.J.; Adler, M.R.; Kamb, M.L.; Newman, L.M. Introduction of rapid syphilis testing in antenatal care: A systematic review of the impact on HIV and syphilis testing uptake and coverage. Int. J. Gynecol. Obstet. 2015, 130, S15–S21. [Google Scholar] [CrossRef] [PubMed]
- Omoding, D.; Katawera, V.; Siedner, M.; Boum, Y., II. Evaluation of the SD Bioline HIV/Syphilis Duo assay at a rural health center in Southwestern Uganda. BMC Res. Notes 2014, 7, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, P.J.; Carcamo, C.P.; Chiappe, M.; Valderrama, M.; La Rosa, S.; Holmes, K.K. Rapid syphilis tests as catalysts for health systems strengthening: A case study from Peru. PLoS ONE 2013, 8, e66905. [Google Scholar] [CrossRef] [PubMed]
- Faal, M.; Naidoo, N.; Glencross, D.K.; Venter, W.D.; Osih, R. Providing immediate CD4 count results at HIV testing improves ART initiation. J. Acquir. Immune Defic. Syndr. 2011, 58, e54–e59. [Google Scholar] [CrossRef] [PubMed]
- Jani, I.V. How point-of-care testing could drive innovation in global health. N. Engl. J. Med. 2013, 368, 2319. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Bien, C.H.; Peeling, R.W. Point-of-care testing for sexually transmitted infections: Recent advances and implications for disease control. Curr. Opin. Infect. Dis. 2013, 26, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Branson, B.M. Point-of-Care Rapid Tests for HIV Antibodies. Lab. Med. 2003, 27, 288–295. [Google Scholar]
- Management Science for Health. CHAPTER 47: Laboratory Services and Medical Supplies; Management Science for Health: Medford, MA, USA, 2012. [Google Scholar]
- Pinna, R.; Carrus, P.P.; Marras, F. Emerging Trends in Healthcare Supply Chain Management—An Italian Experience. In Applications of Contemporary Management Approaches in Supply Chains; Tozan, H., Erturk, A., Eds.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Embrey, M.A.; Management Science for Health. MDS-3: Managing Access to Medicines and Health Technologies; Kumarian Press: Sterling, VA, USA, 2012. [Google Scholar]
- Fonjungo, P.N.; Boeras, D.I.; Zeh, C.; Alexander, H.; Parekh, B.S.; Nkengasong, J.N. Access and quality of HIV-Related point-of-care diagnostic testing in global health programs. Clin. Infect. Dis. 2016, 62, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Dacosta-Claro, I.; Lapierre, S.D. Benchmarking as a tool for the improvement of health services’ supply departments. Health Serv. Manag. Res. 2003, 16, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, S.; Jahagirdar, D.; Miani, C.; Guerin, B.; Nolte, E. Health Services and Delivery Research. Learning for the NHS on Procurement and Supply Chain Management: A Rapid Evidence Assessment; NIHR Journals Library: Southampton, UK, 2014. [Google Scholar]
- Palamountain, K.M.; Baker, J.; Cowan, E.P.; Essajee, S.; Mazzola, L.T.; Metzler, M. Perspectives on introduction and implementation of new point-of-care diagnostic tests. J. Infect. Dis. 2012, 205 (Suppl. 2), S181–S190. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.H.; Bloom, G. Developing world: Bring order to unregulated health markets. Nature 2012, 487, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82 (Suppl. 5), v1–v6. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D.; Herring, A.; Hook, E.W. Why do we need quality-assured diagnostic tests for sexually transmitted infections? Nat. Rev. Microbiol. 2006, 4, S7. [Google Scholar] [CrossRef] [PubMed]
- Parekh, B.S.; Kalou, M.B.; Alemnji, G.; Ou, C.Y.; Gershy-Damet, G.M.; Nkengasong, J.N. Scaling up HIV rapid testing in developing countries: Comprehensive approach for implementing quality assurance. Am. J. Clin. Pathol. 2010, 134, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Alemnji, G.; Nkengasong, J.N.; Parekh, B.S. HIV testing in developing countries: What is required? Indian J. Med. Res. 2011, 134, 779. [Google Scholar] [PubMed]
- Stevens, W.; Gous, N.; Ford, N.; Scott, L.E. Feasibility of HIV point-of-care tests for resource-limited settings: Challenges and solutions. BMC Med. 2014, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- McNerney, R. Diagnostics for developing countries. Diagnostics 2015, 5, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Ronald, A. Diagnostic challenges of sexually transmitted infections in resource-limited settings. Future Microbiol. 2009, 4, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Brondeel, R.; Weill, A.; Thomas, F.; Chaix, B. Use of healthcare services in the residence and workplace neighbourhood: The effect of spatial accessibility to healthcare services. Health Place 2014, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- MacKinney, A.; Coburn, A.; Lundblad, J.; McBride, T.; Mueller, K.; Watson, S. Access to Rural Health Care–A Literature Review and New Synthesis; Policy Report; (Rupri) Rural Policy Research Institute: Iowa City, IA, USA, 2014. [Google Scholar]
- Wang, F. Measurement, optimization, and impact of health care accessibility: A methodological review. Ann. Assoc. Am. Geogr. 2012, 102, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. An integrated approach to measuring potential spatial access to health care services. Socioecon. Plan. Sci. 1992, 26, 275–287. [Google Scholar] [CrossRef]
- Aday, L.A.; Andersen, R.M. Equity of access to medical care: A conceptual and empirical overview. Med. Care 1981, 19, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.E.; Phillips, D.R. Accessibility and Utilization: Geographical Perspectives on Health Care Delivery; Sage: Thousand Oaks, CA, USA, 1984. [Google Scholar]
- Meade, S.M.; Earickson, R.J. Medical Geography, 2nd ed.; Guilford: New York, NY, USA, 2000. [Google Scholar]
- Haines, A.; Horton, R.; Bhutta, Z. Primary health care comes of age. Looking forward to the 30th anniversary of Alma-Ata: Call for papers. Lancet 2007, 370, 911–913. [Google Scholar] [CrossRef]
- World Health Organization. Primary Health Care: Report of International Conference on Primary Health Care, Alma-Ata, USSR, 6–12 September 1978; World Health Organization: Geneva, Switzerland, 1978. [Google Scholar]
- Fleming, K.A.; Naidoo, M.; Wilson, M.; Flanigan, J.; Horton, S.; Kuti, M.; Looi, L.M.; Price, C.; Ru, K.; Ghafur, A.; et al. An Essential Pathology Package for Low- and Middle-Income Countries. Am. J. Clin. Pathol. 2017, 147, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Boeras, D.I.; Nkengasong, J.N.; Peeling, R.W. Implementation science: The laboratory as a command centre. Curr. Opin. HIV Aids 2017, 12, 171–174. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Proposal for a WHO Model List of Essential in Vitro Diagnostics (or the EDL); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-Care Diagnostics for Global Health. Annu. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Wachter, K.; Pai, M.; Gallarda, J.; Boehme, C.; Celentano, I. Addressing the challenges of diagnostics demand and supply: Insights from an online global health discussion platform. BMJ Glob. Health 2016, 1, e000132. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. In Vitro Diagnostics and Laboratory Technology; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mugambi, M.L.; Palamountain, K.M.; Gallarda, J.; Drain, P.K. Exploring the Case for a Global Alliance for Medical Diagnostics Initiative. Diagnostics 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Engel, N.; Ganesh, G.; Patil, M.; Yellappa, V.; Pai, P.N.; Vadnais, C. Barriers to Point-of-Care Testing in India: Results from Qualitative Research across Different Settings, Users and Major Diseases. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Thairu, L.; Katzenstein, D.; Israelski, D. Operational challenges in delivering CD4 diagnostics in sub-Saharan Africa. AIDS Care 2011, 23, 814–821. [Google Scholar] [CrossRef] [PubMed]
- McNerney, R.; Daley, P. Towards a point-of-care test for active tuberculosis: Obstacles and opportunities. Nat. Rev. Microbiol. 2011, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Hecht, R.; Stover, J.; Bollinger, L.; Muhib, F.; Case, K.; de Ferranti, D. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009–31. Lancet 2010, 376, 1254–1260. [Google Scholar] [CrossRef]
- Sharma, S.; Crawley, A. Strategies for overcoming challenges for decentralized diagnostics in resource-limited and catastrophe settings. Expert Rev. Mol. Diagn. 2017, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.K.; de Savigny, D.; Lanata, C.F.; Victora, C.G. How can we achieve and maintain high-quality performance of health workers in low-resource settings? Lancet 2005, 366, 1026–1035. [Google Scholar] [CrossRef]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Johnson, F.A.; Frempong-Ainguah, F.; Nyarko, P.; Baschieri, A.; Aboagye, P. Geographical access to care at birth in Ghana: A barrier to safe motherhood. BMC Public Health 2012, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Kujinga, T. Access to HIV diagnostics in Sub-Sahara Africa. WHO Meeting with Diagnostics Manufacturers and Stakeholders Global Forecast of Diagnostics Demand for 2014–2018. 2015. Available online: http://www.who.int/hiv/amds/206_Access-to-HIV-diagnostics-in-sSA.pdf?ua=1 (accessed on 12 September 2017).
- Linard, C.; Gilbert, M.; Snow, R.W.; Noor, A.M.; Tatem, A.J. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS ONE 2012, 7, e31743. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.H.; Brooks, E.T.; Semrau, K.; Pilingana, P.; MacLeod, W.B.; Siazeele, K. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathog. Glob. Health 2012, 106, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hasselback, L.; Crawford, J.; Chaluco, T.; Rajagopal, S.; Prosser, W.; Watson, N. Rapid diagnostic test supply chain and consumption study in Cabo Delgado, Mozambique: Estimating stock shortages and identifying drivers of stock-outs. Malar. J. 2014, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Kyabayinze, D.J.; Achan, J.; Nakanjako, D.; Mpeka, B.; Mawejje, H.; Mugizi, R. Parasite-based malaria diagnosis: Are Health Systems in Uganda equipped enough to implement the policy? BMC Public Health 2012, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R. Bringing diagnostics to developing countries: An interview with Rosanna Peeling. Expert Rev. Mol. Diagn. 2015, 15, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Sabidó, M.; Camey, E.; Batres, A.; Casabona, J. Lessons learned from integrating simultaneous triple point-of-care screening for syphilis, hepatitis B, and HIV in prenatal services through rural outreach teams in Guatemala. Int. J. Gynecol. Obstet. 2015, 130, S70–S72. [Google Scholar] [CrossRef] [PubMed]
- Biza, A.; Jille-Traas, I.; Colomar, M.; Belizan, M.; Requejo Harris, J.; Crahay, B. Challenges and opportunities for implementing evidence-based antenatal care in Mozambique: A qualitative study. BMC Pregnancy Childbirth 2015, 15, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badman, S.G.; Vallely, L.M.; Toliman, P.; Kariwiga, G.; Lote, B.; Pomat, W. A novel point-of-care testing strategy for sexually transmitted infections among pregnant women in high-burden settings: Results of a feasibility study in Papua New Guinea. BMC Infect. Dis. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kania, D.; Bekale, A.; Nagot, N.; Mondain, A.M.; Ottomani, L.; Meda, N. Combining rapid diagnostic tests and dried blood spot assays for point-of-care testing of human immunodeficiency virus, hepatitis B and hepatitis C infections in Burkina Faso, West Africa. Clin. Microbiol. Infect. 2013, 19, E533. [Google Scholar] [CrossRef] [PubMed]
- Sokhna, C.; Mediannikov, O.; Fenollar, F.; Bassene, H.; Diatta, G.; Tall, A. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl. Trop. Dis. 2013, 7, e1999. [Google Scholar] [CrossRef] [PubMed]
- Chandra, C.; Grabis, J. Supply Chain Configuration: Concepts, Solutions, and Applications; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- World Health Organization. Good Practices for Selecting and Procuring Rapid Diagnostic Tests for Malaria; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Tiven, G.; Frist, L.; Chang, M.; Engelhard, Y. Management for the World Module: Assessing Point-of-Care Diagnostics for Resource-Limited Settings; Global Health MIT: Cambridge, MA, USA, 2011. [Google Scholar]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyei, S.; Nettey, O.E.; Zandoh, C.; Sulemana, A.; Adda, R.; Amenga-Etego, S. Demographic patterns and trends in Central Ghana: Baseline indicators from the Kintampo Health and Demographic Surveillance System. Glob. Health Action 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oduro, A.R.; Wak, G.; Azongo, D.; Debpuur, C.; Wontuo, P.; Kondayire, F. Profile of the Navrongo Health and Demographic Surveillance System. Int. J. Epidemiol. 2012, 41, 968–976. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. https://doi.org/10.3390/diagnostics7040058
Kuupiel D, Bawontuo V, Mashamba-Thompson TP. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics. 2017; 7(4):58. https://doi.org/10.3390/diagnostics7040058
Chicago/Turabian StyleKuupiel, Desmond, Vitalis Bawontuo, and Tivani P. Mashamba-Thompson. 2017. "Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management" Diagnostics 7, no. 4: 58. https://doi.org/10.3390/diagnostics7040058




