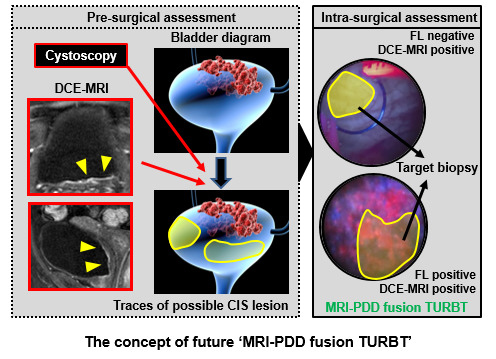
A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection of the Patients
2.2. Procedure of ALA-PDD Assisted TURBT
2.3. Immunohistochemical Staining
2.4. DCE-MRI Scan Protocol
2.5. Image Interpretation for Bladder Lesions
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Tissue Specimens Obtained from TURBT
3.2. Hypervascularity and Hyperproliferation of the Bladder CIS Lesions
3.3. Preoperative Urinary Cytology and Pathological CIS
3.4. Detection of the CIS Lesions by Multiple Imaging Modalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malats, N.; Real, F.X. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Fujimoto, K.; Hirao, Y. Active surveillance for nonmuscle invasive bladder cancer. Investig. Clin. Urol. 2016, 57, S4–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, Y.; Inoue, K.; Tsuzuki, T.; Shimamoto, T.; Shuin, T.; Nagao, K.; Matsuyama, H.; Oyama, M.; Furuse, H.; Ozono, S.; et al. Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. Int. J. Urol. 2018, 25, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Gotoh, D.; Shimada, K.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Konishi, N.; et al. Exploration of risk factors predicting outcomes for primary T1 high-grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: Long-term follow-up experience at a single institute. Int. J. Urol. 2015, 22, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, Y.; Nakaigawa, N. Essential content of evidence-based clinical practice guidelines for bladder cancer: The Japanese Urological Association 2015 update. Int. J. Urol. 2016, 23, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Clark, P.E.; Downs, T.M.; Efstathiou, J.A.; Flaig, T.W.; Friedlander, T.; et al. Bladder cancer, version 5. 2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 1240–1267. [Google Scholar] [CrossRef]
- Lamm, D.L. Carcinoma in situ. Urol. Clin. N. Am. 1992, 19, 499–508. [Google Scholar]
- Inoue, K.; Fukuhara, H.; Shimamoto, T.; Kamada, M.; Iiyama, T.; Miyamura, M.; Kurabayashi, A.; Furihata, M.; Tanimura, M.; Watanabe, H.; et al. Comparison between intravesical and oral administration of 5-aminolevulinic acid in the clinical benefit of photodynamic diagnosis for nonmuscle invasive bladder cancer. Cancer 2012, 118, 1062–1074. [Google Scholar] [CrossRef]
- Rink, M.; Babjuk, M.; Catto, J.W.; Jichlinski, P.; Shariat, S.F.; Stenzl, A.; Stepp, H.; Zaak, D.; Witjes, J.A. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle invasive bladder cancer: A critical review of the current literature. Eur. Urol. 2013, 64, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Geavlete, B.; Multescu, R.; Georgescu, D.; Jecu, M.; Stanescu, F.; Geavlete, P. Treatment changes and long-term recurrence rates after hexaminolevulinate (HAL) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (NMIBC)? BJU Int. 2012, 109, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hungerhuber, E.; Stepp, H.; Kriegmair, M.; Stief, C.; Hofstetter, A.; Hartmann, A.; Knuechel, R.; Karl, A.; Tritschler, S.; Zaak, D. Seven years’ experience with 5-aminolevulinic acid in detection of transitional cell carcinoma of the bladder. Urology 2007, 69, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Filbeck, T.; Roessler, W.; Knuechel, R.; Straub, M.; Kiel, H.J.; Wieland, W.F. 5-aminolevulinic acid- induced fluorescence endoscopy applied at secondary transurethral resection after conventional resection of primary superficial bladder tumors. Urology 1999, 53, 77–81. [Google Scholar] [CrossRef]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; Bosch, J.L. Predictors of false positives in 5-aminolevulinic acid-induced photodynamic diagnosis of bladder carcinoma: Identification of patient groups that may benefit most from highly specific optical diagnostics. Urology 2009, 74, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Jichlinski, P.; Forrer, M.; Mizeret, J.; Glanzmann, T.; Braichotte, D.; Wagnières, G.; Zimmer, G.; Guillou, L.; Schmidlin, F.; Graber, P.; et al. Clinical evaluation of a method for detecting superficial surgical transitional cell carcinoma of the bladder by light-induced fluorescence of protoporphyrin 5-aminolevulinic acid: Preliminary results. Lasers Surg. Med. 1997, 20, 402–408. [Google Scholar] [CrossRef]
- Draga, R.O.; Grimbergen, M.C.; Kok, E.T.; Jonges, T.N.; van Swol, C.F.; Bosch, J.L. Photodynamic diagnosis (5-aminolevulinic acid) of transitional cell carcinoma after bacillus Calmette- Guérin immunotherapy and mitomycin C intravesical therapy. Eur. Urol. 2010, 57, 655–660. [Google Scholar] [CrossRef]
- Ray, E.R.; Chatterton, K.; Khan, M.S.; Chandra, A.; Thomas, K.; Dasgupta, P.; O’Brien, T.S. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guérin. BJU Int. 2010, 105, 789–794. [Google Scholar] [CrossRef]
- Blake, M.A.; Kalra, M.K. Imaging of urinary tract tumors. Cancer Treat. Res. 2008, 143, 299–317. [Google Scholar]
- Green, D.A.; Durand, M.; Gumpeni, N.; Rink, M.; Cha, E.K.; Karakiewicz, P.I.; Scherr, D.S.; Shariat, S.F. Role of magnetic resonance imaging in bladder cancer: current status and emerging techniques. BJU Int. 2012, 110, 1463–1470. [Google Scholar] [CrossRef]
- Gandhi, N.; Krishna, S.; Booth, C.M.; Breau, R.; Flood, T.A.; Morgan, S.C.; Schieda, N.; Salameh, J.P.; McGrath, T.A.; McInnes, M.D.F. Diagnostic accuracy of magnetic resonance imaging for tumour staging of bladder cancer: Systematic review and meta-analysis. BJU Int. 2018, 122, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Naito, S. General Rule for Clinical and Pathological Studies on Renal Pelvic, Ureteral and Bladder Cancer, 1st ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2011; pp. 85–86. (In Japanese) [Google Scholar]
- Miyake, M.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Shimada, K.; et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekes, A.; Kamel, I.; Imam, K.; Szarf, G.; Schoenberg, M.; Nasir, K.; Thompson, R.; Bluemke, D. Dynamic MRI of bladder cancer: Evaluation of staging accuracy. AJR Am. J. Roentgenol. 2005, 184, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sasaki, S.; Ito, M.; Okada, S.; Takahashi, S.; Kawai, T.; Suzuki, K.; Oshima, H.; Hara, M.; Shibamoto, Y. Urinary bladder cancer: Diffusion-weighted MR imaging—Accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009, 251, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Marugami, N.; Hirai, T.; Takahama, J.; Kichikawa, K.; Fujimoto, K. Usefulness of US, CT and MRI in diagnosis of bladder cancer Development and clinical roles of imaging modalities in clinical practice guidelines. Hinyoukigeka 2015, 28, 127–134. (In Japanese) [Google Scholar]
- Wang, H.J.; Pui, M.H.; Guan, J.; Li, S.R.; Lin, J.H.; Pan, B.; Guo, Y. Comparison of Early Submucosal Enhancement and Tumor Stalk in Staging Bladder Urothelial Carcinoma. AJR Am. J. Roentgenol. 2016, 207, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. 2015, 33, e25–e66. [Google Scholar] [CrossRef] [PubMed]
- Têtu, B. Diagnosis of urothelial carcinoma from urine. Mod. Pathol. 2009, 22, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Garufi, A.; Priolo, G.D.; Pennisi, M. Multiparametric MRI/TRUS Fusion Prostate Biopsy: Advantages of a Transperineal Approach. Anticancer Res. 2017, 37, 3291–3294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnier, C.; Ke, W.; Dillenseger, J.L. Bladder segmentation in MRI images using active region growing model. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 2011, 5702–5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variable | n = 45 |
|---|---|
| Gender, n (%) | |
| Male | 41 (91%) |
| Female | 4 (8.9%) |
| Age at TURBT (years-old) | |
| Mean ± SD | 72 ± 8.5 |
| Median (range) | 73 (41–88) |
| Pathological findings, n (%) | |
| No malignant lesion | 6 (13%) |
| Ta, Low grade | 11 (24%) |
| Ta, High grade | 2 (4.4%) |
| T1, High grade | 15 (33%) |
| Concomitant CIS with Ta or T1 tumor | 9 (20%) |
| Pure CIS (no papillary lesion) | 9 (20%) |
| ≥T2 (MIBC) | 2 (4.4%) # |
| Patohological Finding of Biopsy Specimen | Total n = 45 | WL Source | FL Source (ALA-PDD) | DCE-MRI | FL and DCE-MRI Combination 1 † | FL and DCE-MRI Combination 2 ‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||
| 1. Bladder neck | |||||||||||
| CIS-positive | 4 | 2 | 2 | 3 | 1 | 0 | 4 | 3 | 1 | 0 | 4 |
| CIS-negative | 41 | 3 | 38 | 4 | 37 | 9 | 32 | 11 | 30 | 2 | 39 |
| Sensitivity | 50.0% (8.8–91.1) | 75.0% (30.0–98.7) | 0.0% (0.0–49.0) | 75.0% (30.1–98.7) | 0.0% (0.0–49.0) | ||||||
| Specificity | 92.7% (80.6–97.5) | 90.2% (77.5–96.1) | 78.1% (63.3–88.0) | 73.2% (58.1–84.3) | 95.2% (83.9–99.1) | ||||||
| PPV | 40.0% (71.1–76.9) | 42.9% (15.8–75.0) | 0.0% (0.0–29.9) | 21.4% (7.6–47.6) | 0.0% (0.0–82.2) | ||||||
| NPV | 95.0% (83.5–99.1) | 97.4% (86.5–99.9) | 88.9% (74.7–95.6) | 96.8% (83.8–99.8) | 90.7% (78.4–96.3) | ||||||
| 2. Trigon (including ureteral orifice) | |||||||||||
| CIS-positive | 7 | 6 | 1 | 6 | 1 | 7 | 0 | 7 | 0 | 6 | 1 |
| CIS-negative | 38 | 10 | 28 | 13 | 25 | 14 | 24 | 20 | 18 | 7 | 31 |
| Sensitivity | 85.7% (48.7–99.3) | 85.7% (48.7–99.3) | 100% (64.6–100) | 100% (64.6–100) | 85.7% (48.7–99.3) | ||||||
| Specificity | 73.7% (58.0–85.0) | 65.8% (49.9–78.8) | 63.2% (47.3–76.6) | 47.3% (32.5–62.7) | 81.6% (66.6–90.8) | ||||||
| PPV | 37.5% (18.5–61.4) | 31.6% (15.4–54.0) | 33.3% (17.2–54.6) | 25.9% (13.2–44.7) | 46.2% (23.2–70.9) | ||||||
| NPV | 96.6% (82.8–99.8) | 96.2% (81.1–99.8) | 100% (86.2–100) | 100% (82.4–100) | 96.9% (84.3–99.8) | ||||||
| 3. Posterior | |||||||||||
| CIS-positive | 11 | 10 | 1 | 9 | 2 | 6 | 5 | 10 | 1 | 5 | 6 |
| CIS-negative | 34 | 6 | 28 | 12 | 22 | 7 | 27 | 14 | 20 | 5 | 29 |
| Sensitivity | 90.9% (62.3–99.5) | 81.8% (52.3–96.8) | 54.6% (28.0–78.7) | 90.9% (62.3–99.5) | 45.5% (21.3–72.0) | ||||||
| Specificity | 82.4% (66.5–91.7) | 64.7% (47.9–78.5) | 79.4% (63.2–89.7) | 58.8% (42.2–73.6) | 85.3% (69.9–93.6) | ||||||
| PPV | 62.6% (38.6–81.5) | 42.9% (24.5–63.5) | 46.2% (23.2–70.7) | 41.7% (24.5–61.2) | 50.0% (23.7–76.3) | ||||||
| NPV | 96.6% (82.8–99.8) | 91.7% (74.2–98.5) | 84.4% (68.3–93.1) | 95.2% (77.3–99.8) | 82.9% (67.3–91.9) | ||||||
| 4. Right | |||||||||||
| CIS-positive | 9 | 6 | 3 | 6 | 3 | 5 | 4 | 7 | 2 | 4 | 5 |
| CIS-negative | 36 | 3 | 33 | 3 | 33 | 5 | 31 | 5 | 31 | 3 | 33 |
| Sensitivity | 66.7% (35.4–87.9) | 66.7% (35.4–87.9) | 55.6% (26.7–81.1) | 77.8% (45.3–96.1) | 44.4% (18.9–73.3) | ||||||
| Specificity | 91.7% (78.2–97.1) | 91.7% (78.2–97.1) | 86.1% (71.3–93.9) | 86.1% (71.3–93.9) | 91.7% (78.2–97.1) | ||||||
| PPV | 66.7% (35.4–87.9) | 66.7% (35.4–87.9) | 50.0% (23.7–76.3) | 58.3% (32.0–80.7) | 57.1% (25.1–84.2) | ||||||
| NPV | 91.7% (78.2–97.1) | 91.7% (78.2–97.1) | 88.6% (74.1–95.5) | 93.9% (80.4–98.9) | 86.8% (72.7–94.3) | ||||||
| 5. Left | |||||||||||
| CIS-positive | 8 | 1 | 7 | 4 | 4 | 2 | 6 | 4 | 4 | 2 | 6 |
| CIS-negative | 37 | 4 | 33 | 5 | 32 | 5 | 32 | 8 | 29 | 2 | 35 |
| Sensitivity | 12.5% (0.6–47.1) | 50.0% (21.5–78.5) | 25.0% (44.4–59.1) | 50.0% (21.5–78.5) | 25.0% (4.4–59.1) | ||||||
| Specificity | 89.2% (75.3–95.7) | 86.5% (72.0–94.1) | 86.5% (72.0–94.1) | 78.4% (62.8–88.6) | 94.6% (82.3–99.0) | ||||||
| PPV | 20.0% (1.0–62.5) | 44.4% (18.9–73.3) | 28.6% (50.8–64.1) | 33.3% (13.8–60.9) | 50.0% (8.9–91.1) | ||||||
| NPV | 82.5% (68.1–91.3) | 88.9% (74.7–95.6) | 84.2% (69.6–92.6) | 87.9% (72.7–95.2) | 85.4% (71.6–93.1) | ||||||
| 6. Dome | |||||||||||
| CIS-positive | 5 | 3 | 2 | 4 | 1 | 1 | 4 | 4 | 1 | 1 | 4 |
| CIS-negative | 40 | 3 | 37 | 3 | 37 | 4 | 36 | 5 | 35 | 2 | 38 |
| Sensitivity | 60.0% (23.1–92.9) | 80.0% (37.6–99.0) | 20.0% (1.0–62.5) | 80.0% (37.6–99.0) | 20.0% (10.3–62.5) | ||||||
| Specificity | 92.5% (80.1–97.4) | 92.5% (80.1–97.4) | 90.0% (77.0–96.0) | 82.5% (73.9–94.5) | 95.0% (83.5–99.1) | ||||||
| PPV | 50.0% (18.8–81.2) | 57.1% (25.1–84.2) | 20.0% (0.10–62.5) | 44.4% (18.9–73.3) | 33.3% (1.7–88.2) | ||||||
| NPV | 94.9% (83.1–99.1) | 97.4% (86.5–99.9) | 90.0% (77.0–96.0) | 97.2% (85.8–99.9) | 90.5% (77.9–96.2) | ||||||
| 7. Anterior wall | |||||||||||
| CIS-positive | 7 | 1 | 6 | 3 | 4 | 4 | 3 | 4 | 3 | 3 | 4 |
| CIS-negative | 38 | 3 | 35 | 4 | 34 | 2 | 36 | 5 | 33 | 1 | 37 |
| Sensitivity | 14.3% (0.7–51.4) | 42.9% (15.8–75.0) | 57.1% (25.1–84.2) | 57.1% (25.1–84.2) | 42.9% (15.8–75.0) | ||||||
| Specificity | 92.1% (79.2–97.3) | 89.5% (75.9–95.8) | 94.7% (82.7–99.1) | 86.8% (72.7–94.3) | 97.4% (86.5–99.9) | ||||||
| PPV | 25.0% (1.3–69.9) | 42.9% (15.8–75.0) | 66.8% (30.0–94.1) | 44.4% (18.9–73.3) | 75.0% (30.1–98.7) | ||||||
| NPV | 85.4% (71.6–93.1) | 89.5% (75.9–95.8) | 92.3% (79.7–97.4) | 91.7% (78.2–97.1) | 90.2% (77.5–96.1) | ||||||
| 8. Prostatic urethra (opitional, n = 29) | |||||||||||
| CIS-positive | 5 | 1 | 4 | 1 | 4 | 2 | 3 | 3 | 2 | 0 | 5 |
| CIS-negative | 24 | 1 | 23 | 3 | 21 | 6 | 18 | 7 | 17 | 2 | 22 |
| Sensitivity | 20.0% (1.0–62.5) | 20.0% (1.0–62.5) | 40.0% (7.1–76.9) | 60.0% (23.1–92.9) | 0.0% (0.0–43.5) | ||||||
| Specificity | 95.8% (79.8–99.8) | 87.5% (69.0–95.7 | 75.0% (5.5–88.0) | 70.8% (50.8–85.1) | 91.7% (74.2–98.5) | ||||||
| PPV | 50.0% (2.6–97.4) | 25.0% (1.3–69.9) | 25.0% (4.4–59.1) | 30.0% (10.8–60.3) | 0.0% (0.0–82.2) | ||||||
| NPV | 85.2% (67.5–94.1) | 84.0% (65.4–93.6) | 85.7% (65.4–95.0) | 89.5% (68.6–98.1) | 81.5% (63.3–91.8) | ||||||
| Patohological Finding of Biopsy Specimen | Total n = 344 | WL Source | FL Source (ALA-PDD) | DCE-MRI | FL and DCE-MRI Combination 1 † | FL and DCE-MRI Combination 2 ‡ | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | WL Source vs FL Source | FL Source vs Combination 1 | FL Source vs Combination 2 | ||
| CIS-positive | 56 | 30 | 26 | 36 | 20 | 27 | 29 | 42 | 14 | 21 | 35 | |||
| CIS-negative | 288 | 33 | 255 | 47 | 241 | 52 | 236 | 75 | 213 | 24 | 264 | |||
| Sensitivity | 53.6% (40.7–66.0) | 64.3% (51.2–75.5) | 48.2% (35.7–61.0) | 75.0% (62.3–84.5) | 37.5% (26.0–50.6) | 0.15 # | 0.041 # | < 0.001 # | ||||||
| Specificity | 88.5% (84.3–91.7) | 83.7% (79.0–87.5) | 81.9% (77.1–86.0) | 74.0% (68.6–78.7) | 91.7% (87.9–94.3) | 0.002 # | < 0.001 # | < 0.001 # | ||||||
| PPV | 47.6% (35.8–59.7) | 43.4% (33.2–54.1) | 34.2% (24.5–45.2) | 35.9% (27.8–44.9) | 46.7% (32.9–60.9) | 0.70 ## | 0.74 ## | 0.59 ## | ||||||
| NPV | 90.8% (86.8–93.6) | 92.3% (88.5–95.0) | 89.1% (84.7–92.3) | 93.8% (89.9–96.3) | 88.3% (84.2–91.5) | 0.48 ## | 0.41 ## | 0.08 ## | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, M.; Maesaka, F.; Marugami, N.; Miyamoto, T.; Nakai, Y.; Ohnishi, S.; Gotoh, D.; Owari, T.; Hori, S.; Morizawa, Y.; et al. A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’. Diagnostics 2019, 9, 112. https://doi.org/10.3390/diagnostics9030112
Miyake M, Maesaka F, Marugami N, Miyamoto T, Nakai Y, Ohnishi S, Gotoh D, Owari T, Hori S, Morizawa Y, et al. A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’. Diagnostics. 2019; 9(3):112. https://doi.org/10.3390/diagnostics9030112
Chicago/Turabian StyleMiyake, Makito, Fumisato Maesaka, Nagaaki Marugami, Tatsuki Miyamoto, Yasushi Nakai, Sayuri Ohnishi, Daisuke Gotoh, Takuya Owari, Shunta Hori, Yosuke Morizawa, and et al. 2019. "A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’" Diagnostics 9, no. 3: 112. https://doi.org/10.3390/diagnostics9030112
APA StyleMiyake, M., Maesaka, F., Marugami, N., Miyamoto, T., Nakai, Y., Ohnishi, S., Gotoh, D., Owari, T., Hori, S., Morizawa, Y., Itami, Y., Inoue, T., Anai, S., Torimoto, K., Fujii, T., Shimada, K., Tanaka, N., & Fujimoto, K. (2019). A Potential Application of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Combined with Photodynamic Diagnosis for the Detection of Bladder Carcinoma in Situ: Toward the Future ‘MRI-PDD Fusion TURBT’. Diagnostics, 9(3), 112. https://doi.org/10.3390/diagnostics9030112