Identification of Pneumococcal Serotypes by PCR–Restriction Fragment Length Polymorphism
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Silico Evaluation of Restriction Enzymes
2.2. Pneumococcal Isolates
2.3. PCR Amplification of S. pneumoniae cps Genes
2.4. Analysis of Fragments Obtained from Enzyme Digestions
2.5. Serotyping of Clinical Isolates
3. Results
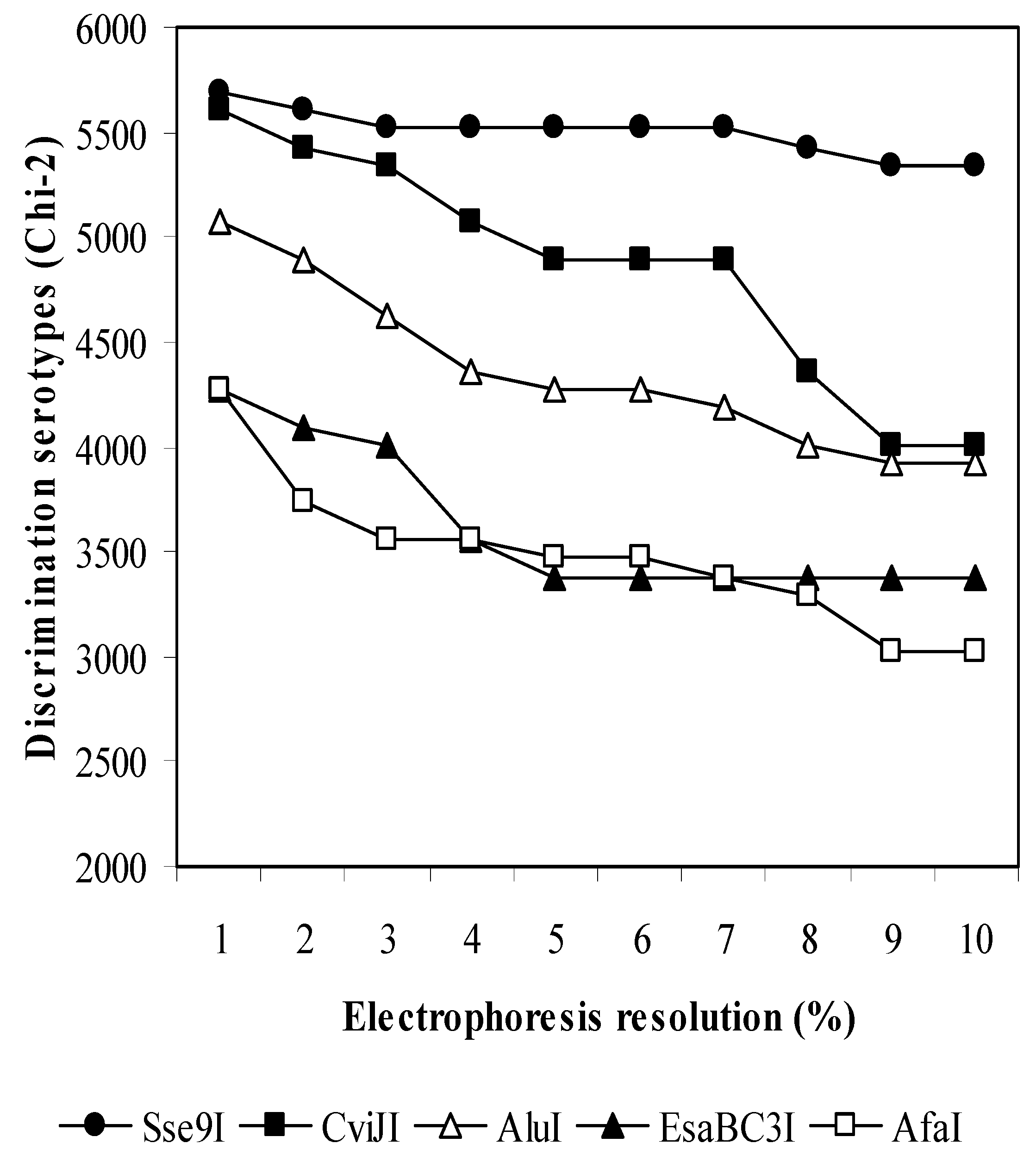
3.1. In Silico Analysis of GenBank Sequences
3.2. Generation of a Preliminary Database of Fragment Patterns.
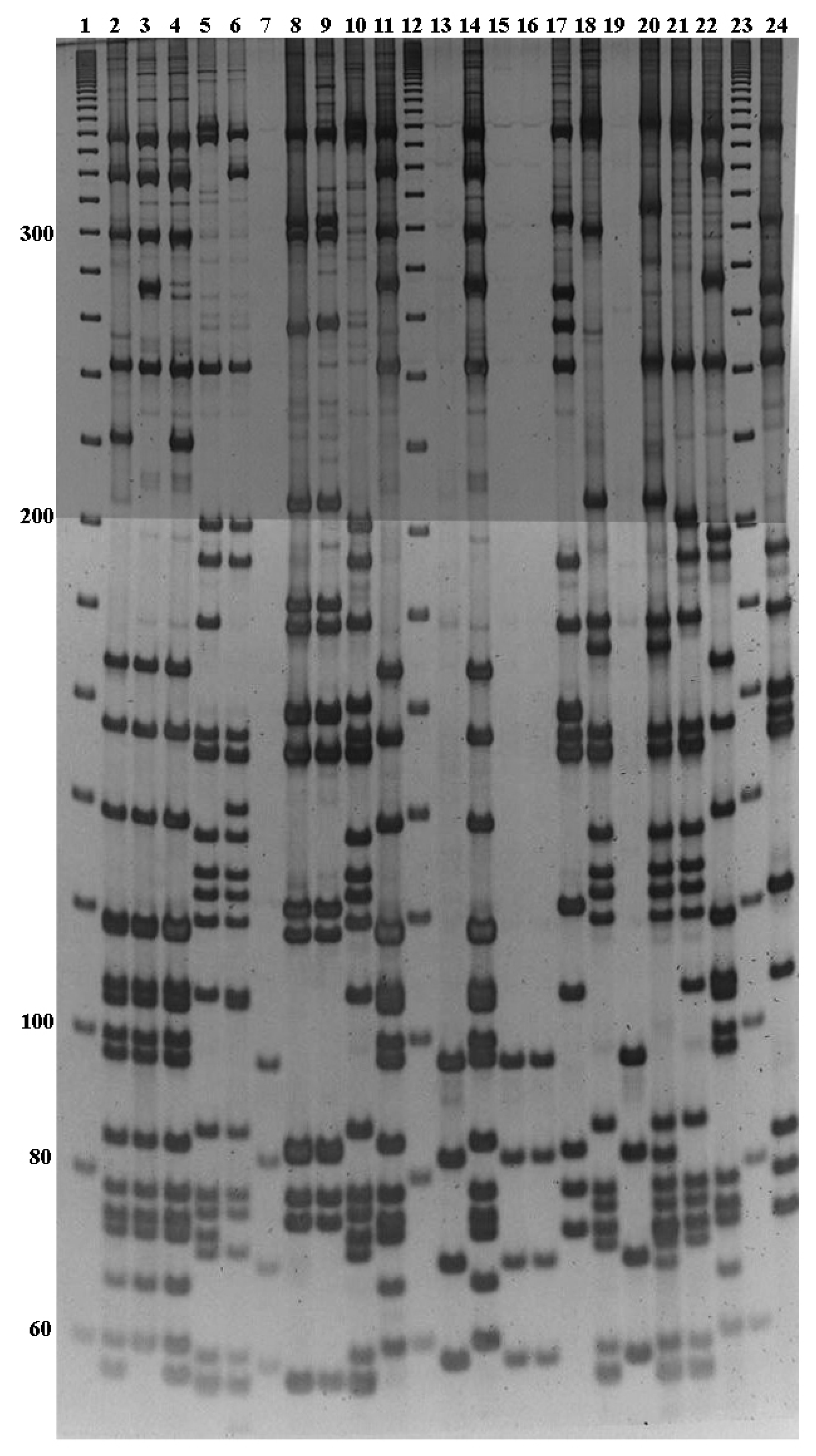
3.3. PCR-RFLP Analysis of S. pneumoniae Collection Type Strains
3.4. Serotype Identification of Clinical Isolates by PCR-RFLP Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Estimated Hib and Pneumococcal Deaths for Children under 5 Years of Age. 2008. Available online: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en (accessed on 18 November 2019).
- Wahl, B.; Sharan, A.; Deloria Knoll, M.; Kumar, R.; Liu, L.; Chu, Y.; McAllister, D.A.; Nair, H.; Campbell, H.; Rudan, I.; et al. National, regional, and state-level burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in India: Modelled estimates for 2000–2015. Lancet Glob. Health 2019, 7, e735–e747. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Malley, R.; Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011, 378, 1962–1973. [Google Scholar] [CrossRef]
- Balsells, E.; Guillot, L.; Nair, H.; Kyaw, M.H. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0177113. [Google Scholar] [CrossRef]
- Van Tonder, A.J.; Bray, J.E.; Quirk, S.J.; Haraldsson, G.; Jolley, K.A.; Maiden, M.C.; Hoffmann, S.; Bentley, S.D.; Haraldsson, Á.; Erlendsdóttir, H.; et al. Putatively novel serotypes and the potential for reduced vaccine effectiveness: Capsular locus diversity revealed among 5405 pneumococcal genomes. Microb. Genom. 2016, 2, 000090. [Google Scholar] [CrossRef]
- Mostowy, R.J.; Croucher, N.J.; De Maio, N.; Chewapreecha, C.; Salter, S.J.; Turner, P.; Aanensen, D.M.; Bentley, S.D.; Didelot, X.; Fraser, C. Pneumococcal capsule synthesis locus cps as evolutionary hotspot with potential to generate novel serotypes by recombination. Mol. Biol. Evol. 2017, 34, 2537–2554. [Google Scholar] [CrossRef] [PubMed]
- Henrichsen, J. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 1995, 33, 2759–2762. [Google Scholar] [PubMed]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–999. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Aanensen, D.M.; Mavroidi, A.; Saunders, D.; Rabbinowitsch, E.; Collins, M.; Donohoe, K.; Harris, D.; Murphy, L.; Quail, M.A.; et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006, 2, e31. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E.; Khan, M.N.; Xu, Q. Next generation protein-based Streptococcus pneumoniae vaccines. Hum. Vaccin. Immunother. 2016, 12, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.; Gertz REJr Park, S.H.; Kim, E.; Moura, I.; Milucky, J.; Rouphael, N.; Farley, M.M.; Harrison, L.H.; Bennett, N.M.; Bigogo, G.; et al. Streptococcus infantis, Streptococcus mitis, and Streptococcus oralis strains with highly similar cps5 loci and antigenic relatedness to serotype 5 pneumococci. Front. Microbiol. 2019, 9, 3199. [Google Scholar] [CrossRef]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2014, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Austrian, R. Pneumococcal polysaccharide vaccines. Rev. Infect. Dis 1989, 11, S598–S602. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.; Gertz, R.E.; Beall, B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 2006, 44, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, S.; Drèze, P.A.; Vandeven, J.; Verhaegen, J.; Van Melderen, L.; Smeesters, P.R. Sequential multiplex PCR assay for determining capsular serotypes of colonizing S. Pneumoniae. BMC Infect. Dis 2011, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Marimón, J.M.; Ercibengoa, M.; Santacatterina, E.; Alonso, M.; Pérez-Trallero, E. Single-Step multiplex PCR assay for determining 92 pneumococcal serotypes. J. Clin. Microbiol. 2016, 54, 2197–2200. [Google Scholar] [CrossRef]
- Marimón, J.M.; Morales, M.; Gamen, S.; Manrique, A.; Ercibengoa, M.; Cilla, G. A reverse-hybridization test for the identification of 76 pneumococcal serotypes, 42 individually and 34 in pairs. J. Microbiol. Methods 2017, 143, 13–16. [Google Scholar] [CrossRef]
- Ercibengoa, M.; Alonso, M.; Vicente, D.; Morales, M.; Garcia, E.; Marimón, J.M. Utility of MALDI-TOF MS as a new tool for Streptococcus pneumoniae serotyping. PLoS ONE 2019, 14, e0212022. [Google Scholar] [CrossRef]
- Lo, S.W.; Gladstone, R.A.; van Tonder, A.J.; Lees, J.A.; du Plessis, M.; Benisty, R.; Givon-Lavi, N.; Hawkins, P.A.; Cornick, J.E.; Kwambana-Adams, B.; et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect. Dis. 2019, 19, 759–769. [Google Scholar] [CrossRef]
- Santiago, E.; Camacho, L.; Junquera, M.L.; Vázquez, F. Full HPV typing by a single restriction enzyme. J. Clin. Virol. 2006, 37, 38–46. [Google Scholar] [CrossRef]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE-restriction enzymes and DNA methyltransferases. Nucl Acids Res. 2005, 33, D230–D232. [Google Scholar] [CrossRef]
- Fenoll, A.; Granizo, J.J.; Giménez, M.J.; Yuste, J.; Aguilar, L. Secular trends (1990–2013) in serotypes and associated non-susceptibility of S. pneumoniae isolates causing invasive disease in the pre-/post-era of pneumococcal conjugate vaccines in Spanish regions without universal paediatric pneumococcal vaccination. Vaccine 2015, 33, 5691–5699. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.R.; Arias, C.A.; Duke, B.; Beste, D.; Broughton, K.; Efstratiou, A.; George, R.C.; Hall, L.M. Evaluation of serotype prediction by cpsA-cpsB gene polymorphism in Streptococcus pneumoniae. J. Clin. Microbiol. 2000, 38, 1319–1323. [Google Scholar] [PubMed]
- Batt, S.L.; Charalambous, B.M.; McHugh, T.D.; Martin, S.; Gillespie, S.H. Novel PCR-restriction fragment length polymorphism method for determining serotypes or serogroups of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 2005, 43, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Camargo, D.R.; Pais, F.S.; Volpini, A.C.; Oliveira, M.A.; Coimbra, R.S. Revisiting molecular serotyping of Streptococcus pneumoniae. BMC Genom. 2015, 16. [Google Scholar] [CrossRef]
- Brito, D.A.; Ramirez, M.; de Lencastre, H. Serotyping Streptococcus pneumoniae by multiplex PCR. J. Clin. Microbiol. 2003, 41, 2378–2384. [Google Scholar] [CrossRef]
- Rubin, L.G.; Rizvi, A. PCR-based assays for detection of Streptococcus pneumoniae serotypes 3, 14, 19F and 23F in respiratory specimens. J. Med. Microbiol. 2004, 53, 595–602. [Google Scholar] [CrossRef]
- Austrian, R. The quellung reaction, a neglected microbiologic technique. Mt. Sinai J. Med. 1976, 43, 699–709. [Google Scholar]
- Lund, E. Laboratory diagnosis of Pneumococcus infections. Bull. World Health Organ. 1960, 23, 5–13. [Google Scholar]
- Sørensen, U.B. Typing of pneumococci by using 12 pooled antisera. J. Clin. Microbiol. 1993, 31, 2097–2100. [Google Scholar]
- Mauffrey, F.; Fournier, É.; Demczuk, W.; Martin, I.; Mulvey, M.; Martineau, C.; Lévesque, S.; Bekal, S.; Domingo, M.C.; Doualla-Bell, F.; et al. Comparison of sequential multiplex PCR, sequetyping and whole genome sequencing for serotyping of Streptococcus pneumoniae. PLoS ONE 2017, 13, e0189163. [Google Scholar] [CrossRef]
- Elberse, K.E.; van de Pol, I.; Witteveen, S.; van der Heide, H.G.; Schot, C.S.; van Dijk, A.; van der Ende, A.; Schouls, L.M. Population structure of invasive Streptococcus pneumoniae in The Netherlands in the pre-vaccination era assessed by MLVA and capsular sequence typing. PLoS ONE 2011, 6, e20390. [Google Scholar] [CrossRef] [PubMed]
- Everett, D.B.; Cornick, J.; Denis, B.; Chewapreecha, C.; Croucher, N.; Harris, S.; Parkhill, J.; Gordon, S.; Carrol, E.D.; French, N.; et al. Genetic characterisation of Malawian pneumococci prior to the roll-out of the PCV13 vaccine using a high-throughput whole genome sequencing approach. PLoS ONE 2012, 7, e44250. [Google Scholar] [CrossRef] [PubMed]
- Dube, F.S.; van Mens, S.P.; Robberts, L.; Wolter, N.; Nicol, P.; Mafofo, J.; Africa, S.; Zar, H.J.; Nicol, M.P. Comparison of a real-time multiplex PCR and sequetyping assay for pneumococcal serotyping. PloS ONE 2015, 10, e0137349. [Google Scholar] [CrossRef] [PubMed]
- Jaunekaite, E.; Tocheva, A.S.; Jefferies, J.M.; Gladstone, R.A.; Faust, S.N.; Christodoulides, M.; Hibberd, M.L.; Clarke, S.C. Current methods for capsular typing of Streptococcus pneumoniae. J. Microbiol. Methods 2015, 113, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Hoover, P.A.; Vesikari, T.; Peltier, C.; Hurley, D.C.; McFetridge, R.D.; Dallas, M.; Hartzel, J.; Marchese, R.D.; Coller, B.G.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine 2018, 36, 6883–6891. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Ezpeleta, C.; Gil-Setas, A.; Torroba, L.; Beristain, X.; Aguinaga, A.; García-Irure, J.J.; Navascués, A.; García-Cenoz, M.; Castilla, J.; et al. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001–2013. Vaccine 2014, 32, 2553–2562. [Google Scholar] [CrossRef]
- Ercibengoa, M.; Arostegi, N.; Marimón, J.M.; Alonso, M.; Pérez-Trallero, E. Dynamics of pneumococcal nasopharyngeal carriage in healthy children attending a day care center in northern Spain. Influence of detection techniques on the results. BMC Infect. Dis. 2012, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, R.; España, P.P.; Pérez-Trallero, E.; Uranga, A.; Méndez, R.; Cilloniz, C.; Marimón, J.M.; Cifuentes, I.; Méndez, C.; Torres, A. The burden of PCV13 serotypes in hospitalized pneumococcal pneumonia in Spain using a novel urinary antigen detection test. CAPA study. Vaccine 2017, 35, 5264–5270. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Cillóniz, C.; Blasi, F.; Chalmers, J.D.; Gaillat, J.; Dartois, N.; Schmitt, H.J.; Welte, T. Burden of pneumococcal community-acquired pneumonia in adults across Europe: A literature review. Respir. Med. 2018, 137, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Marimón, J.M.; Ercibengoa, M.; Tamayo, E.; Alonso, M.; Pérez-Trallero, E. Long-Term epidemiology of Streptococcus pneumoniae serogroup 6 in a region of southern Europe with special reference to serotype 6E. PLoS ONE 2016, 11, e0149047. [Google Scholar] [CrossRef] [Green Version]
- Staples, M.; Graham, R.M.A.; Hicks, V.; Strachan, J.; Gonçalves da Silva, A.; Peverall, J.; Wicks, V.; Jennison, A.V. Discovery of Streptococcus pneumoniae serogroup 35 variants in Australian patients. Clin. Microbiol. Infect. 2017, 23, 476–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.; Ortika, B.D.; Dunne, E.M.; Holt, K.E.; Kama, M.; Russell, F.M.; Hinds, J.; Satzke, C. A novel genetic variant of Streptococcus pneumoniae serotype 11A discovered in Fiji. Clin. Microbiol. Infect. 2018, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strain |
|---|
| S. pneumoniae serotype 1 CCUG 2839A a |
| S. pneumoniae serotype 2 CCUG 8435 a |
| S. pneumoniae serotype 3 GB05 b |
| S. pneumoniae serotype 4 CCUG 2226 a |
| S. pneumoniae serotype 5 CCUG 2541 a |
| S. pneumoniae serotype 6B CCUG 1350 a |
| S. pneumoniae serotype 7A CCUG 8436 a |
| S. pneumoniae serotype 9A CCUG 3506 a |
| S. pneumoniae serotype 10B Sri Lanka b |
| S. pneumoniae serotype 10C/1 Sanger b |
| S. pneumoniae serotype 10F CCUG 5697 a |
| S. pneumoniae serotype 11A CCUG 36617 a |
| S. pneumoniae serotype 11B CCUG 8440 a |
| S. pneumoniae serotype 11D Sanger 70/86 b |
| S. pneumoniae serotype 12A CCUG 8444 a |
| S. pneumoniae serotype 12B Gambia 1/81 b |
| S. pneumoniae serotype 14 CCUG 1086B a |
| S. pneumoniae serotype 18C Sanger 4593/4 b |
| S. pneumoniae serotype 19A Sanger 1773/39 b |
| S. pneumoniae serotype 19C Sanger 408/41 b |
| S. pneumoniae serotype 19F CCUG 1407 a |
| S. pneumoniae serotype 20 CCUG 8451 a |
| S. pneumoniae serotype 21 CCUG 1697 a |
| S. pneumoniae serotype 22F Sanger 1772/40 b |
| S. pneumoniae serotype 24F CCUG 8457 a |
| S. pneumoniae serotype 27 CCUG 5898 a |
| S. pneumoniae serotype 31 CCUG 6956 a |
| S. pneumoniae serotype 32A CCUG 8458 a |
| S. pneumoniae serotype 33D India b |
| S. pneumoniae serotype 33F Sanger 3077/37 b |
| S. pneumoniae serotype 34 CCUG 2399 a |
| S. pneumoniae serotype 35A CCUG 3556 a |
| S. pneumoniae serotype 36 CCUG 5906 a |
| S. pneumoniae serotype 40 CCUG 8468 a |
| S. pneumoniae serotype 42 CCUG 6568 a |
| S. pneumoniae serotype 45 CCUG 8472 a |
| S. pneumoniae serotype 48 CCUG 8476 a |
| Serotype | Nº Isolates | Origin | Nº Concordant Patterns (nº Strains) | Nº New Patterns (nº Strains) |
|---|---|---|---|---|
| 1 | 3 | aSPRL | 3 (3) | |
| 2 | 1 | bHUCA | 1 (1) | |
| 3 | 8 | SPRL | 8 (8) | |
| 4 | 4 | HUCA, SPRL | 4 (4) | |
| 6A | 1 | HUCA | 1 (1) | |
| 6B | 3 | HUCA | 2 (3) | |
| 7F | 8 | cUA, SPRL | 7 (7) | 1(1) |
| 8 | 4 | UA, SPRL | 4 (4) | |
| 9N | 2 | HUCA, SPRL | 2 (2) | |
| 9V | 3 | HUCA, SPRL | 3 (3) | |
| 10A | 1 | SPRL | 1 (1) | |
| 11A | 4 | HUCA, SPRL | 4 (4) | |
| 12F | 3 | dCNM, SPRL | 2 (2) | 1 (1) |
| 13 | 1 | HUCA | 1(1) | |
| 14 | 7 | HUCA, SPRL | 6 (6) | 1 (1) |
| 15A | 2 | SPRL | 1 (1) | 1 (1) |
| 15B | 1 | CNM | 1 (1) | |
| 16F | 2 | SPRL | 2 (2) | |
| 17F | 1 | CNM | 1 (1) | |
| 18C | 1 | SPRL | 1 (1) | |
| 19A | 11 | SPRL | 3 (3) | 3 (8) |
| 19F | 1 | HUCA | 1 (1) | |
| 22F | 3 | HUCA, SPRL | 3 (3) | |
| 23B | 2 | SPRL | 2 (2) | |
| 23F | 7 | HUCA, SPRL | 7 (7) | |
| 24F | 2 | SPRL | 1 (1) | 1 (1) |
| 28A | 1 | SPRL | 1 (1) | |
| 29 | 1 | SPRL | 1 (1) | |
| 33F | 2 | SPRL | 2 (2) | |
| 34 | 2 | HUCA, SPRL | 2 (2) | |
| 35B | 2 | SPRL | 2 (2) | |
| 35F | 2 | HUCA, SPRL | 2 (2) | |
| NT | 2 | SPRL | 2 (2) | |
| Total | 98 | 77 (77) | 15 (21) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Suárez, M.d.M.; González-Rodríguez, I.; Cima-Cabal, M.D.; Yuste, J.E.; Vazquez, F.; Santiago, E. Identification of Pneumococcal Serotypes by PCR–Restriction Fragment Length Polymorphism. Diagnostics 2019, 9, 196. https://doi.org/10.3390/diagnostics9040196
García-Suárez MdM, González-Rodríguez I, Cima-Cabal MD, Yuste JE, Vazquez F, Santiago E. Identification of Pneumococcal Serotypes by PCR–Restriction Fragment Length Polymorphism. Diagnostics. 2019; 9(4):196. https://doi.org/10.3390/diagnostics9040196
Chicago/Turabian StyleGarcía-Suárez, María del Mar, Irene González-Rodríguez, María Dolores Cima-Cabal, Jose Enrique Yuste, Fernando Vazquez, and Enrique Santiago. 2019. "Identification of Pneumococcal Serotypes by PCR–Restriction Fragment Length Polymorphism" Diagnostics 9, no. 4: 196. https://doi.org/10.3390/diagnostics9040196






