Cardiac Sympathetic Activity and Rhythm Control Following Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation—A Prospective 123I-mIBG-SPECT/CT Imaging Study
Abstract
:1. Background
2. Methods
2.1. Patients
2.2. Radionuclide (123I-mIBG) Imaging of Cardiac Sympathetic Innervation
2.3. Analysis
2.4. Pulmonary Vein Isolation
2.5. Statistics
3. Results
3.1. Cardiac Sympathetic Activity before and after PVI
3.1.1. Analysis of Global Sympathetic Cardiac Innervation
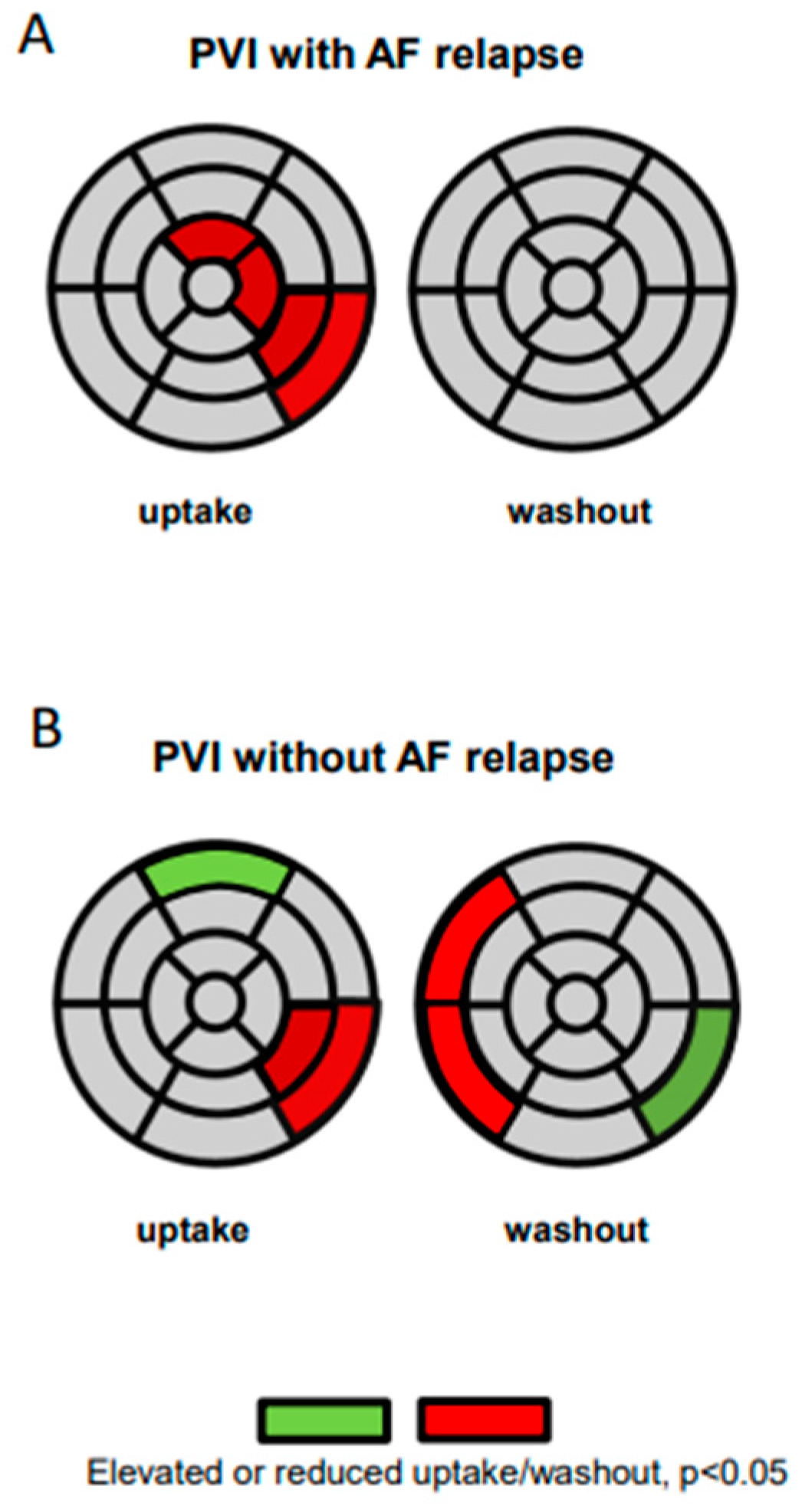
3.1.2. Semiquantitative Analysis of Regional Sympathetic Cardiac Innervation
3.1.3. Quantitative Analysis of Regional Sympathetic Cardiac Innervation
3.2. Innervation Defects and AF Relapses
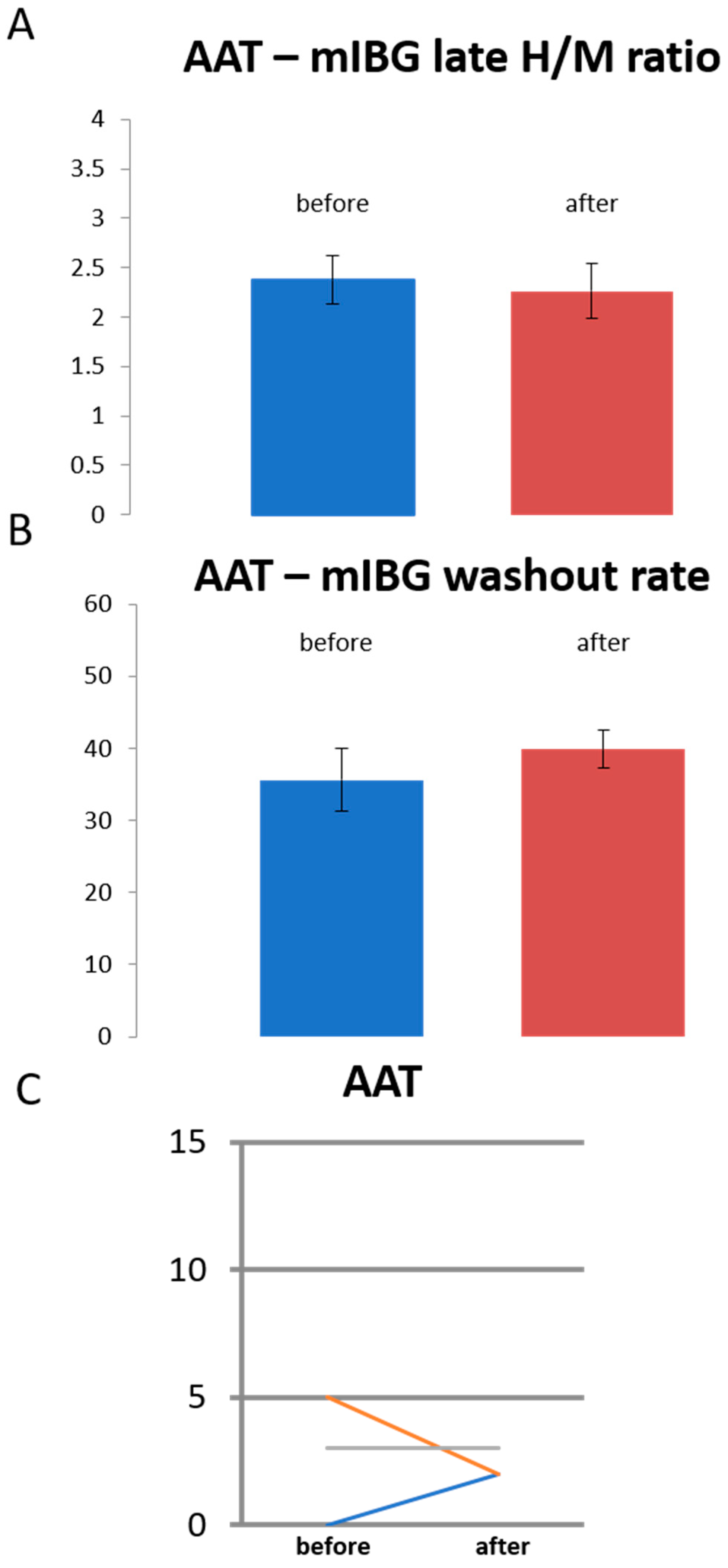
3.3. Sympathetic Innervation in Patients Treated with Antiarrhythmic Drugs Only
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Maurer, T.; Kuck, K.H. The quest for durable lesions in catheter ablation of atrial fibrillation—Technological advances in radiofrequency catheters and balloon devices. Expert Rev. Med. Devices 2017, 14, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Datino, T.; Macle, L.; Nattel, S. Atrial fibrillation ablation: Translating basic mechanistic insights to the patient. J. Am. Coll. Cardiol. 2014, 64, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driessen, A.H.G.; Berger, W.R.; Krul, S.P.J.; van den Berg, N.W.E.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.; de Jong, J.; van Boven, W.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-H.; Hu, G.-S.; Liu, Q.; Sheng, X.; Sun, Y.-X.; Yu, L.U.; Zhang, P.; Zhang, Z.-W.; Chen, S.-Q.; Ye, Y.; et al. Impact of Anatomically Guided Ganglionated Plexus Ablation on Electrical Firing from Isolated Pulmonary Veins. Pacing Clin. Electrophysiol. 2016, 39, 1351–1358. [Google Scholar] [CrossRef]
- Pokushalov, E.; Romanov, A.; Katritsis, D.G.; Artyomenko, S.; Shirokova, N.; Karaskov, A.; Mittal, S.; Steinberg, J.S. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: A randomized comparison. Heart Rhythm 2013, 10, 1280–1286. [Google Scholar] [CrossRef]
- Watanabe, M.; Kohno, H.; Kondo, Y.; Ueda, H.; Ishida, K.; Tamura, Y.; Abe, S.; Sato, Y.; Kobayashi, Y.; Matsumiya, G. Is ganglionated plexus ablation effective for treating atrial fibrillation? Surg. Today 2018, 48, 875–882. [Google Scholar] [CrossRef]
- Bengel, F.M.; Barthel, P.; Matsunari, I.; Schmidt, G.; Schwaiger, M. Kinetics of 123I-MIBG after acute myocardial infarction and reperfusion therapy. J. Nucl. Med. 1999, 40, 904–910. [Google Scholar]
- Herring, N.; Kalla, M.; Paterson, D.J. The autonomic nervous system and cardiac arrhythmias: Current concepts and emerging therapies. Nat. Rev. Cardiol. 2019, 16, 707–726. [Google Scholar] [CrossRef]
- Arimoto, T.; Tada, H.; Igarashi, M.; Sekiguchi, Y.; Sato, A.; Koyama, T.; Yamasaki, H.; Machino, T.; Kuroki, K.; Kuga, K.; et al. High washout rate of iodine-123-metaiodobenzylguanidine imaging predicts the outcome of catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011, 22, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Yamada, T.; Mizuno, H.; Minamiguchi, H.; Konishi, S.; Ohtani, T.; Yamaguchi, O.; Okuyama, Y.; Uematsu, M.; Sakata, Y. Impact of atrial fibrillation ablation on cardiac sympathetic nervous system in patients with and without heart failure. Int. J. Cardiol. 2015, 199, 65–70. [Google Scholar] [CrossRef]
- Wenning, C.; Lange, P.S.; Schülke, C.; Vrachimis, A.; Mönnig, G.; Schober, O.; Eckardt, L.; Schäfers, M. Pulmonary vein isolation in patients with paroxysmal atrial fibrillation is associated with regional cardiac sympathetic denervation. EJNMMI Res. 2013, 3, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flotats, A.; Carrio, I.; Agostini, D.; Le Guludec, D.; Marcassa, C.; Schafers, M.; Somsen, G.A.; Unlu, M.; Verberne, H.J. Committee EC and European Council of Nuclear, C. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Germano, G.; Kavanagh, P.B.; Waechter, P.; Areeda, J.; Van Kriekinge, S.; Sharir, T.; Lewin, H.C.; Berman, D.S. A new algorithm for the quantitation of myocardial perfusion SPECT. I: Technical principles and reproducibility. J. Nucl. Med. 2000, 41, 712–719. [Google Scholar]
- Sharir, T.; Germano, G.; Waechter, P.B.; Kavanagh, P.B.; Areeda, J.S.; Gerlach, J.; Kang, X.; Lewin, H.C.; Berman, D.S. A new algorithm for the quantitation of myocardial perfusion SPECT. II: Validation and diagnostic yield. J. Nucl. Med. 2000, 41, 720–727. [Google Scholar]
- Stegger, L.; Lipke, C.S.; Kies, P.; Nowak, B.; Schober, O.; Buell, U.; Schafers, M.; Schaefer, W.M. Quantification of left ventricular volumes and ejection fraction from gated 99mTc-MIBI SPECT: Validation of an elastic surface model approach in comparison to cardiac magnetic resonance imaging, 4D-MSPECT and QGS. Eur J. Nucl. Med. Mol. Imaging 2007, 34, 900–909. [Google Scholar] [CrossRef]
- Chen, P.S.; Tan, A.Y. Autonomic nerve activity and atrial fibrillation. Heart Rhythm 2007, 4, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, J.; Burton, R.A.B.; Bub, G.; Smaill, B.H.; Montgomery, J.M. Synaptic Plasticity in Cardiac Innervation and Its Potential Role in Atrial Fibrillation. Front. Physiol. 2018, 9, 240. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Friberg, L.; Tabrizi, F.; Englund, A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: Data from Swedish health registries. Eur. Hear. J. 2016, 37, 2478–2487. [Google Scholar] [CrossRef] [Green Version]
- Soman, P.; Travin, M.I.; Gerson, M.; Cullom, S.J.; Thompson, R. I-123 MIBG Cardiac Imaging. J. Nucl. Cardiol. 2015, 22, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Kossyvakis, C.; Angelidis, C.; Panagopoulou, V.; Tsiachris, D.; Vrachatis, D.A.; Doudoumis, K.; Letsas, K.; Pagoni, S.; Stefanadis, C.; et al. Coincidental ganglionated plexus modification during radiofrequency pulmonary vein isolation and post-ablation arrhythmia recurrence. Europace 2016, 19, 1967–1972. [Google Scholar] [CrossRef]
- Lemery, R.; Ben-Haim, S.; Wells, G.; Ruddy, T.D. I-123-Metaiodobenzylguanidine imaging in patients with atrial fibrillation undergoing cardiac mapping and ablation of autonomic ganglia. Hearth Rhythm. 2017, 14, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.; Wong, T.C.; Soman, P. Direct visualization of regional cardiac sympathetic dysfunction in stress-induced cardiomyopathy. J. Nucl. Cardiol. 2015, 22, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Verberne, H.J.; Burchert, W.; Knuuti, J.; Povinec, P.; Sambuceti, G.; Unlu, M.; Estorch, M.; Banerjee, G.; Jacobson, A.F. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: Insights from a retrospective European multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Carrió, I.; Cowie, M.; Yamazaki, J.; Udelson, J.; Camici, P.G. Cardiac Sympathetic Imaging With mIBG in Heart Failure. JACC Cardiovasc. Imaging 2010, 3, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamatsu, H.; Momose, M.; Kobayashi, H.; Kusakabe, K.; Kasanuki, H. Prognostic value of 123I-metaiodobenzylguanidine in patients with various heart diseases. Ann. Nucl. Med. 2007, 21, 513–520. [Google Scholar] [CrossRef]



| Individual # | Treatment | Sex | Age | LA Volume (mL) | Body Mass Index | AA Drugs before Treatment (PVI or AAT Only) | Relapse after Treatment (PVI or AAT Only) | AA Drugs after Treatment (PVI or AAT Only) |
|---|---|---|---|---|---|---|---|---|
| 1 | PVI | M | 55 | 44 | 25 | sotalol | Yes | sotalol |
| 2 | PVI | F | 58 | 40 | 21 | beta blocker | Yes | beta blocker |
| 3 | AAT only | F | 70 | 38 | 27 | None | Yes | beta blocker, propafenone |
| 5 | PVI | M | 63 | 119 | 30 | beta blocker, flecainide | Yes | beta blocker, flecainide |
| 6 | PVI | M | 67 | 87 | 29 | beta blocker, flecainide | Yes | beta blocker, flecainide |
| 7 | PVI | F | 58 | 60 | 27 | beta blocker, dronedarone | None | beta blocker, dronedarone |
| 8 | AAT only | F | 52 | 35 | 27 | beta blocker | None | beta blocker, dronedarone |
| 9 | PVI | F | 53 | 85 | 35 | beta blocker, flecainide | Yes | beta blocker, propafenone |
| 10 | PVI | M | 45 | 56 | 28 | beta blocker, flecainide | Yes | beta blocker, flecainide |
| 11 | PVI | F | 61 | 34 | 28 | beta blocker, dronedarone | Yes | dronedarone |
| 12 | PVI | M | 53 | 52 | 26 | beta blocker, dronedarone | None | beta blocker, flecainide |
| 13 | PVI | M | 62 | 63 | 31 | beta blocker, flecainide | None | beta blocker, flecainide |
| 14 | PVI | M | 60 | 39 | 27 | beta blocker | None | beta blocker, flecainide |
| 15 | PVI | M | 57 | 41 | 26 | beta blocker, flecainide | Yes | beta blocker, flecainide |
| 16 | PVI | M | 54 | 56 | 33 | beta blocker, flecainide | None | beta blocker, flecainide |
| 17 | PVI | F | 68 | 57 | 33 | beta blocker, amiodarone | None | beta blocker, amiodarone |
| 18 | PVI | M | 44 | 58 | 26 | Beta blocker, flecainide | None | beta blocker, flecainide |
| 19 | PVI | M | 48 | 36 | 29 | Beta blocker, flecainide | None | Beta blocker, flecainide |
| 20 | PVI | M | 51 | 56 | 34 | Beta blocker, flecainide | Yes | Beta blocker, flecainide |
| 21 | PVI | M | 58 | 71 | 25 | flecainide | Yes | flecainide |
| 22 | PVI | M | 55 | 72 | 26 | Beta blocker, flecainide | Yes | Beta blocker, flecainide |
| 23 | PVI | F | 58 | 44 | 22 | Beta blocker, flecainide | None | none |
| 24 | PVI | F | 58 | 49 | 26 | Beta blocker, flecainide | None | Beta blocker, flecainide |
| 25 | AAT only | M | 61 | 53 | 31 | Beta blocker | None | Beta blocker, flecainide |
| 26 | PVI | M | 55 | 42 | 25 | Propafenone | None | Propafenone |
| 27 | PVI | M | 66 | 152 | 28 | Beta blocker, flecainide | Yes | Beta blocker, flecainide |
| 28 | PVI | M | 53 | 52 | 24 | Beta blocker | None | Beta blocker |
| 29 | PVI | M | 67 | 87 | 25 | Beta blocker, amiodarone | None | Beta blocker |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, P.S.; Wenning, C.; Avramovic, N.; Leitz, P.; Larbig, R.; Frommeyer, G.; Schäfers, M.; Eckardt, L. Cardiac Sympathetic Activity and Rhythm Control Following Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation—A Prospective 123I-mIBG-SPECT/CT Imaging Study. J. Pers. Med. 2021, 11, 995. https://doi.org/10.3390/jpm11100995
Lange PS, Wenning C, Avramovic N, Leitz P, Larbig R, Frommeyer G, Schäfers M, Eckardt L. Cardiac Sympathetic Activity and Rhythm Control Following Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation—A Prospective 123I-mIBG-SPECT/CT Imaging Study. Journal of Personalized Medicine. 2021; 11(10):995. https://doi.org/10.3390/jpm11100995
Chicago/Turabian StyleLange, Philipp S., Christian Wenning, Nemanja Avramovic, Patrick Leitz, Robert Larbig, Gerrit Frommeyer, Michael Schäfers, and Lars Eckardt. 2021. "Cardiac Sympathetic Activity and Rhythm Control Following Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation—A Prospective 123I-mIBG-SPECT/CT Imaging Study" Journal of Personalized Medicine 11, no. 10: 995. https://doi.org/10.3390/jpm11100995






