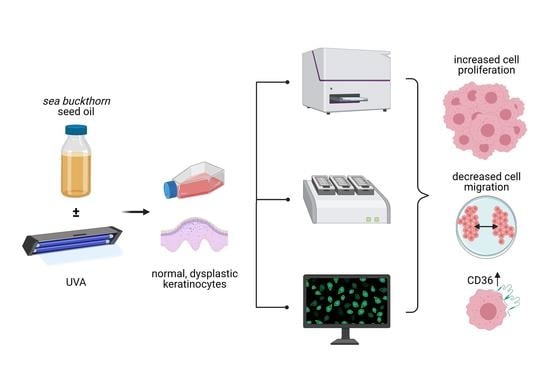
Sea-Buckthorn Seed Oil Induces Proliferation of both Normal and Dysplastic Keratinocytes in Basal Conditions and under UVA Irradiation
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Lines and Cell Treatments
2.2. Sea-Buckthorn Seed Oil Fatty Acid Composition
2.3. Cell Proliferation and Toxicity Assays
2.4. Evaluation of the Expression CD36
3. Results
3.1. Biochemical Composition of Sea-Buckthorn Seed Oil
3.2. The Sea-Buckthorn Seed Oil Showed Pro-Proliferative Effects at Low Concentrations
3.3. Sea-Buckthorn Seed Oil Inhibits Cell Migration of Dysplastic Keratinocytes
3.4. Treatment with Sea-Buckthorn Oil Following UVA Irradiation Does Not Mitigate the Deleterious Effect on Dysplastic Cells
3.5. UVA Irradiation Does Not Affect Uptake of Lipids into Normal and Dysplastic Keratinocytes
3.6. The Fatty Acid Translocator CD36 Is Expressed in Dysplastic, but Not Normal Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Jastrząb, A.; Jarocka-Karpowicz, I.; Muszyńska, M.; Skrzydlewska, E. The Effect of Sea Buckthorn (Hippophae rhamnoides L.) Seed Oil on UV-Induced Changes in Lipid Metabolism of Human Skin Cells. Antioxidants 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olas, B.; Skalski, B.; Ulanowska, K. The Anticancer Activity of Sea Buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front. Pharmacol. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [Green Version]
- Bala, M.; Gupta, M.; Saini, M.; Abdin, M.Z.; Prasad, J. Sea Buckthorn Leaf Extract Protects Jejunum and Bone Marrow of 60 Cobalt-Gamma-Irradiated Mice by Regulating Apoptosis and Tissue Regeneration. Evid. Based Complement Altern Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Kitanaka, S.; Kawata, K.; Goto, K. Anti-tumor promoters phenolics and triterpenoid from Hippophae rhamnoides. Fitoterapia 2009, 80, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nie, F.; Ouyang, J.; Wang, X.; Ma, X. Inhibitory effects of sea buckthorn procyanidins on fatty acid synthase and MDA-MB-231 cells. Tumour Biol. 2014, 35, 9563–9569. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Snyder, C.L.; Schroeder, W.R.; Cram, D.; Datla, R.; Wishart, D.; Weselake, R.J.; Krishna, P. Fatty Acid Composition of Developing Sea Buckthorn (Hippophae rhamnoides L.) Berry and the Transcriptome of the Mature Seed. PLoS ONE 2012, 7, e34099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krouse, R.S.; Alberts, D.S.; Prasad, A.R.; Bartels, H.; Yozwiak, M.; Liu, Y.; Bartels, P.H. Progression of skin lesions from normal skin to squamous cell carcinoma. Anal. Quant. Cytol. Histol. 2009, 31, 17–25. [Google Scholar] [PubMed]
- Berman, B.; Cockerell, C.J. Pathobiology of actinic keratosis: Ultraviolet-dependent keratinocyte proliferation. J. Am. Acad. Dermatol. 2013, 68, S10–S19. [Google Scholar] [CrossRef]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From keratinocyte to cancer: The pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechifor, M.T.; Niculiţe, C.M.; Urs, A.O.; Regalia, T.; Mocanu, M.; Popescu, A.; Manda, G.; Dinu, D.; Leabu, M. UVA Irradiation of Dysplastic Keratinocytes: Oxidative Damage versus Antioxidant Defense. Int. J. Mol. Sci. 2012, 13, 16718–16736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Niculiţe, C.M.; Nechifor, M.T.; Urs, A.O.; Olariu, L.; Ceafalan, L.C.; Leabu, M. Keratinocyte Motility Is Affected by UVA Radiation-A Comparison between Normal and Dysplastic Cells. Int. J. Mol. Sci. 2018, 19, 1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigruener, A.; Tarabin, V.; Paragh, G.; Liebisch, G.; Koehler, T.; Farwick, M.; Schmitz, G. Effects of sphingoid bases on the sphingolipidome in early keratinocyte differentiation. Exp. Dermatol. 2013, 22, 677–679. [Google Scholar] [CrossRef]
- Enciu, A.-M.; Radu, E.; Popescu, I.D.; Hinescu, M.E.; Ceafalan, L.C. Targeting CD36 as Biomarker for Metastasis Prognostic: How Far from Translation into Clinical Practice? Biomed. Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Hanna, A.N.; Sim, A.B.; Chua, P.P.; Chong, M.T.; Tron, V.A. GADD45 Regulates G2/M Arrest, DNA Repair, and Cell Death in Keratinocytes Following Ultraviolet Exposure. J. Investig. Dermatol. 2002, 119, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Farrell, A.W.; Halliday, G.M.; Lyons, J.G. Brahma deficiency in keratinocytes promotes UV carcinogenesis by accelerating the escape from cell cycle arrest and the formation of DNA photolesions. J. Dermatol. Sci. 2018, 92, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. The Role of p21 in Apoptosis, Proliferation, Cell Cycle Arrest, and Antioxidant Activity in UVB-Irradiated Human HaCaT Keratinocytes. Med. Sci. Monit. Basic Res. 2015, 21, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.-H.; Khnykin, D. Fatty acid transporters in skin development, function and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Kuriki, C.; Fukui, Y.; Motojima, K. Dual Promoter Structure of Mouse and Human Fatty Acid Translocase/CD36 Genes and Unique Transcriptional Activation by Peroxisome Proliferator-activated Receptor α and γ Ligands. J. Biol. Chem. 2002, 277, 15703–15711. [Google Scholar] [CrossRef] [Green Version]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martín, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef]
- Simon, M.; Juhász, I.; Herlyn, M.; Hunyadi, J. Thrombospondin receptor (CD36) expression of human keratinocytes during wound healing in a SCID mouse/human skin repair model. J. Dermatol. 1996, 23, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Chéreau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of superoxide anions by keratinocytes initiates, P. acnes-induced inflammation of the skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef]
- Das, D.; Anand, V.; Khandpur, S.; Sharma, V.K.; Sharma, A. T helper type 1 polarizing γδ T cells and Scavenger receptors contribute to the pathogenesis of Pemphigus vulgaris. Immunology 2018, 153, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Bumiller-Bini, V.; Cipolla, G.A.; Spadoni, M.B.; Augusto, D.G.; Petzl-Erler, M.L.; Beltrame, M.H.; Boldt, A.B. Condemned or Not to Die? Gene Polymorphisms Associated with Cell Death in Pemphigus Foliaceus. Front. Immunol. 2019, 10, 2416. [Google Scholar] [CrossRef]
- Allen, M.H.; Barker, J.N.W.N.; MacDonald, D.M. Keratinocyte expression of CD36 antigen in benign and malignant epidermal cell-derived tumours. J. Cutan. Pathol. 1991, 18, 198–203. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudau, M.; Vilceanu, A.C.; Codrici, E.; Mihai, S.; Popescu, I.D.; Albulescu, L.; Tarcomnicu, I.; Moise, G.; Ceafalan, L.C.; Hinescu, M.E.; et al. Sea-Buckthorn Seed Oil Induces Proliferation of both Normal and Dysplastic Keratinocytes in Basal Conditions and under UVA Irradiation. J. Pers. Med. 2021, 11, 278. https://doi.org/10.3390/jpm11040278
Dudau M, Vilceanu AC, Codrici E, Mihai S, Popescu ID, Albulescu L, Tarcomnicu I, Moise G, Ceafalan LC, Hinescu ME, et al. Sea-Buckthorn Seed Oil Induces Proliferation of both Normal and Dysplastic Keratinocytes in Basal Conditions and under UVA Irradiation. Journal of Personalized Medicine. 2021; 11(4):278. https://doi.org/10.3390/jpm11040278
Chicago/Turabian StyleDudau, Maria, Alexandra Catalina Vilceanu, Elena Codrici, Simona Mihai, Ionela Daniela Popescu, Lucian Albulescu, Isabela Tarcomnicu, Georgeta Moise, Laura Cristina Ceafalan, Mihail E. Hinescu, and et al. 2021. "Sea-Buckthorn Seed Oil Induces Proliferation of both Normal and Dysplastic Keratinocytes in Basal Conditions and under UVA Irradiation" Journal of Personalized Medicine 11, no. 4: 278. https://doi.org/10.3390/jpm11040278