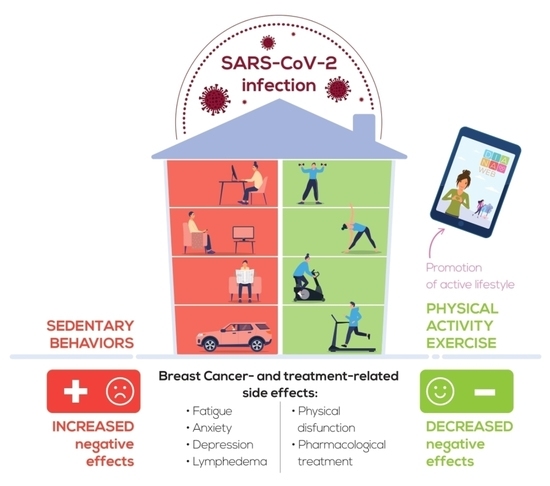
Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Questionnaires
- (i)
- general information, such as sociodemographic characteristics (age, education level, marital status, region of residence, and residential density);
- (ii)
- anthropometric parameters (body weight, body height, and waist circumference);
- (iii)
- information about medical history (lymphedema arms, use of drugs, tumor metastasis, secondary tumor, etc.) and other health issues (from this section we collected information on SARS-CoV-2 positive swab);
- (iv)
- results of the last routine blood tests;
- (v)
- physical activity level, through the IPAQ-SF, whose reliability and validity are documented [18,19]: subjects reported the frequency (days/week) and duration (minutes/day) of different types of activity: vigorous (e.g., intense home or gardening activity, performing intense aerobic exercises, and using bike or treadmill); moderate (e.g., moderate home activity, work out in the garden, carrying light loads, and bicycling at a steady pace); and walking activities, as well as the average time spent sitting on a day; and
- (vi)
- lifestyle habits on QoL, through the question on one-dimension present in EORTC QLQ-C30 questionnaire [20]: global health-status/quality of life. The global health-status/quality of life scale has response options ranging from (1) “very poor” to (7) “excellent”.
2.3. Statistical Analysis
3. Results
Sample Characteristics
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wirtz, P.; Baumann, F.T. Physical Activity, Exercise and Breast Cancer—What is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. Breast Care 2018, 13, 93–101. [Google Scholar]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Re-currence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, Y.; Li, T.; Qin, Q.; Yin, J.; Pang, S.; Nie, S.; Wei, S. Leisure time physical activity and cancer risk: Evaluation of the WHO’s recommendation based on 126 high-quality epidemiological studies. Br. J. Sports Med. 2016, 50, 372–378. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Lima, R.M.; Myers, J. Cardiorespiratory fitness and cancer in women: A prospective pilot study. J. Sport Health Sci. 2019, 8, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Klika, R.J.; Ballard, T.M. How Does Physical Activity Prior to Breast Cancer Diagnosis Effect Rehabilitation Outcomes? Southern California Conferences for Undergraduate Research. November 2016. Available online: https://www.sccur.org/sccur/fall_2016_conference/posters/220/ (accessed on 12 November 2016).
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Coletta, A.M.; Marquez, G.; Thomas, P.; Thoman, W.; Bevers, T.; Brewster, A.M.; Hawk, E.; Basen-Engquist, K.; Gilchrist, S.C. Clinical factors associated with adherence to aerobic and resistance physical activity guidelines among cancer prevention patients and survivors. PLoS ONE. 2019, 14, e0220814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibney, E. Coronavirus lockdowns have changed the way Earth moves. Nature 2020, 580, 176–177. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- American Society of Clinical Oncology (ASCO). COVID-19 Clinical Oncology Frequently Asked Questions (FAQs). 2020. Available online: https://www.asco.org/sites/new-www.asco.org/files/content-files/blog-release/pdf/COVID-19-Clinical%20Oncology-FAQs-3-12-2020.pdf (accessed on 12 March 2021).
- Battershill, P.M. Influenza pandemic planning for cancer patients. Curr. Oncol. 2006, 13, 119–120. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Pandemic. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 10 February 2021).
- Violant-Holz, V.; Gallego-Jiménez, M.G.; González-González, C.S.; Muñoz-Violant, S.; Rodríguez, M.J.; Sansano-Nadal, O.; Guerra-Balic, M. Psychological Health and Physical Activity Levels during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9419. [Google Scholar] [CrossRef]
- Breastcancer.org. Available online: https://www.breastcancer.org/treatment/covid-19-and-breast-cancer-care (accessed on 10 February 2021).
- Brunet, J.; Taran, S.; Burke, S.; Sabiston, C.M. A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disabil. Rehabil. 2013, 35, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Balzarini, M.; Acito, M.; Moretti, M.; Villarini, A.; Villarini, M. E-Coaching: The DianaWeb study to prevent breast cancer recurrences. Clin. Ter. 2020, 170, e59–e65. [Google Scholar] [PubMed]
- Villarini, M.; Lanari, C.; Nucci, D.; Gianfredi, V.; Marzulli, T.; Berrino, F.; Borgo, A.; Bruno, E.; Gargano, G.; Moretti, M.; et al. Community-based participatory research to improve life quality and clinical outcomes of patients with breast cancer (DianaWeb in Umbria pilot study). BMJ Open 2016, 6, e009707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L. A simple, easy-to-use spreadsheet for automatic scoring of the International Physical Activity Questionnaire (IPAQ) Short Form. ResearchGate 2016. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 4 March 2021).
- Villarini, A.; Villarini, M.; Gargano, G.; Moretti, M.; Berrino, F. DianaWeb: Un progetto dimostrativo per migliorare la prognosi in donne con carcinoma mammario attraverso gli stili di vita [DianaWeb: A demonstration project to improve breast cancer prognosis through lifestyles]. Epidemiol. Prev. 2015, 39, 402–405. [Google Scholar]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2020, 12, 1–22. [Google Scholar] [CrossRef]
- Luciano, F.; Cenacchi, V.; Vegro, V.; Pavei, G. COVID-19 lockdown: Physical activity, sedentary behaviour and sleep in Italian medicine students. Eur. J. Sport Sci. 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kanera, I.M.; Bolman, C.A.; Mesters, I.; Willems, R.A.; Beaulen, A.A.; Lechner, L. Prevalence and correlates of healthy lifestyle behaviors among early cancer survivors. BMC Cancer 2016, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Trinh, L.; Amireault, S.; Lacombe, J.; Sabiston, C.M. Physical and psychological health among breast cancer survivors: Interactions with sedentary behavior and physical activity. Psychooncology 2015, 24, 1279–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.U.; Hart, N.H.; Clay, T. Keeping Patients with Cancer Exercising in the Age of COVID-19. JCO Oncol. Pract. 2020, 16, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Meyer, J.; McDowell, C.; Lansing, J.; Brower, C.; Smith, L.; Tully, M.; Herring, M. Changes in Physical Activity and Sedentary Behavior in Response to COVID-19 and Their Associations with Mental Health in 3052 US Adults. Int. J. Environ. Res. Public Health 2020, 17, 6469. [Google Scholar] [CrossRef]
- Gurgel, A.R.B.; Mingroni-Netto, P.; Farah, J.C.; de Brito, C.M.M.; Levin, A.S.; Brum, P.C. Determinants of Health and Physical Activity Levels Among Breast Cancer Survivors During the COVID-19 Pandemic: A Cross-Sectional Study. Front. Physiol. 2021, 12, 624169. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, J.; Suh, S.H.; Ligibel, J.; Courneya, K.S.; Jeon, J.Y. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Am. Soc. Prev. Oncol. 2017, 26, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.U. Overwhelming research and clinical evidence of exercise medicine efficacy in cancer management-translation into practice is the challenge before us. Current oncology 2018, 25, 117–118. [Google Scholar] [CrossRef] [Green Version]
- Patsou, E.D.; Alexias, G.D.; Anagnostopoulos, F.G.; Karamouzis, M.V. Effects of physical activity on depressive symptoms during breast cancer survivorship: A meta-analysis of randomised control trials. ESMO Open 2017, 2, e000271. [Google Scholar] [CrossRef] [Green Version]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.J.; Weaver, K.E. Physical and mental health among cancer survivors: Considerations for long-term care and quality of life. N. C. Med. J. 2014, 75, 283–286. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Whole DianaWeb Cohort (n = 1527) | Group A (n = 781) | Group B (n = 746) | p |
|---|---|---|---|---|
| Age in years a | 54.14 (±8.80) | 54.68 (± 8.75) | 53.58 (±8.83) | 0.014 d |
| Young adults (aged 21–40) b | 85 (5.6) | 37 (4.7) | 48 (6.4) | 0.170 e |
| Adults (aged 41–60) b | 1.108 (72.6) | 562 (72.0) | 546 (73.2) | |
| Over 60 age b | 334 (21.9) | 182 (23.3) | 152 (20.4) | |
| Marital status b | ||||
| Married | 987 (64.6) | 526 (67.3) | 461 (61.8) | 0.053 e |
| Separated/divorced | 177 (11.6) | 92 (11.8) | 85 (11.4) | |
| Widowed | 44 (2.9) | 21 (2.7) | 23 (3.1) | |
| Never married | 319 (20.9) | 142 (18.2) | 177 (23.7) | |
| Level of education b | ||||
| High school or some college (≤13 years) | 810 (53.0) | 392 (50.2) | 418 (56.0) | 0.022 e |
| College graduates or higher (>13 years) | 717 (47.0) | 389 (49.8) | 328 (44.0) | |
| Region of residence b,c | ||||
| Northern Italy | 1033 (67.6) | 576 (73.8) | 457 (61.3) | 0.000 e |
| Central Italy | 331 (21.7) | 128 (16.4) | 203 (27.2) | |
| Southern Italy | 163 (10.7) | 77 (9.9) | 86 (11.5) |
| Facilitators or Barriers | Number of Subjects (%) |
|---|---|
| Residential density | |
| Cities | 373 (57.7) |
| Suburbs | 235 (30.1) |
| Countryside | 173 (22.2) |
| House dwelling floor space | |
| <50 m2 | 45 (5.8) |
| 50–90 m2 | 306 (39.2) |
| >90 m2 | 430 (55.1) |
| Private outdoor spaces | |
| None | 48 (6.1) |
| Balcony | 451 (57.7) |
| Garden | 282 (36.1) |
| Number of family members | |
| 1 | 224 (28.7) |
| 2 | 248 (31.8) |
| 3 or more | 309 (39.6) |
| Working activity during quarantine | |
| Retired or laid off | 215 (27.5) |
| Remote working | 352 (45.1) |
| Normal working activity | 72 (9.2) |
| Other | 142 (18.2) |
| Lymphedema | |
| No | 695 (89.0) |
| Yes | 86 (11.0) |
| SARS-CoV-2 diagnostic test | |
| Positive | 4 (0.5) |
| Negative | 204 (26.1) |
| Not tested | 573 (73.4) |
| Before Quarantine | During Quarantine | p | |
|---|---|---|---|
| Body Weight a | 61.46 ± 11.50 | 61.57 ± 11.03 | 0.525 c |
| Waist circumference a | 80.52 ± 10.33 | 80.91 ± 11.03 | 0.101 c |
| Normal b | 449 (57.5) | 446 (57.1) | 0.459 d |
| Abdominal obesity b | 332 (42.5) | 335 (42.9) | |
| Body mass index (BMI) a | 23.08 ± 4.00 | 23.13 ± 3.87 | 0.390 c |
| Underweight b | 54 (6.9) | 53 (6.8) | |
| Normal weight b | 542 (69.4) | 528 (67.6) | 0.678 d |
| Overweight and obese b | 185 (23.7) | 200 (5.6) |
| Before Quarantine | During Quarantine | p b | |
|---|---|---|---|
| Quality of life a | |||
| Very poor | 10 (1.3) | 27 (3.5) | <0.001 |
| Poor | 44 (5.6) | 146 (18.7) | |
| Neither poor nor good | 238 (30.5) | 306 (39.2) | |
| Good | 421 (53.9) | 275 (35.2) | |
| Very good | 68 (8.7) | 27 (3.5) | |
| Health perception a | |||
| Very poor | 5 (0.6) | 8 (1.0) | <0.001 |
| Poor | 37 (4.7) | 113 (14.5) | |
| Neither poor nor good | 253 (32.4) | 273 (35.0) | |
| Good | 423 (54.2) | 341 (43.7) | |
| Very good | 63 (8.1) | 46 (5.9) | |
| Psychotropic drugs a | 123 (15.7) | 128 (16.4) | 0.391 |
| Before Quarantine | During Quarantine | Δ a | p | |
|---|---|---|---|---|
| Vigorous PA a | 361.95 ± 793.62 | 117.70 ± 468.78 | −244.25 ± 685.82 | <0.001 b |
| Moderate PA a | 909.71 ± 902.68 | 888.53 ± 940.88 | −21.18 ± 754.87 | 0.433 b |
| Walking a | 941.22 ± 841.80 | 331.44 ± 590.33 | −609.78 ± 801.77 | <0.001 b |
| Total PA a | 2212.87 ± 1696.11 | 1337.66 ± 1305.51 | −875.20 ± 1361.51 | <0.001 b |
| Sitting time ≤ 6 h/day c | 480 (61.5) | 358 (45.8) | <0.001 d | |
| Sitting time > 6 h/day c | 301 (38.5) | 423 (54.2) |
| Vigorous PA | Moderate PA | Walking | Total PA | Sitting/Lying | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | |
| Block 1 | ||||||||||
| Region of residence | −0.032 | 0.380 | 0.076 | 0.032 | 0.031 | 0.392 | 0.057 | 0.107 | 0.004 | 0.905 |
| Residential density | 0.042 | 0.294 | 0.035 | 0.381 | 0.074 | 0.064 | 0.074 | 0.064 | −0.043 | 0.280 |
| Dwelling floor space | −0.011 | 0.780 | 0.092 | 0.015 | −0.032 | 0.397 | 0.048 | 0.209 | −0.060 | 0.115 |
| Private outdoor spaces | −0.024 | 0.574 | 0.073 | 0.083 | 0.031 | 0.463 | 0.058 | 0.169 | −0.068 | 0.105 |
| Block 2 | ||||||||||
| Age | −0.074 | 0.070 | 0.097 | 0.017 | 0.058 | 0.155 | 0.070 | 0.090 | −0.173 | 0.000 |
| Marital status | −0.022 | 0.587 | 0.071 | 0.079 | −0.008 | 0.837 | 0.039 | 0.334 | 0.038 | 0.349 |
| Level of education | 0.013 | 0.735 | −0.031 | 0.407 | 0.073 | 0.052 | 0.015 | 0.681 | 0.045 | 0.221 |
| Working activity | 0.023 | 0.543 | 0.086 | 0.024 | −0.024 | 0.523 | 0.059 | 0.122 | −0.016 | 0.676 |
| Family members | −0.011 | 0.788 | 0.072 | 0.082 | 0.008 | 0.846 | 0.052 | 0.218 | −0.093 | 0.025 |
| Block 3 | ||||||||||
| Body Mass Index | −0.046 | 0.402 | 0.039 | 0.484 | −0.146 | 0.008 | −0.055 | 0.322 | 0.067 | 0.227 |
| Waist circumference | −0.024 | 0.666 | 0.010 | 0.860 | 0.074 | 0.174 | 0.032 | 0.561 | −0.050 | 0.363 |
| Lymphedema | 0.012 | 0.731 | −0.021 | 0.554 | −0.030 | 0.401 | −0.024 | 0.495 | −0.037 | 0.302 |
| Quality of life | 0.098 | 0.034 | 0.070 | 0.135 | 0.217 | 0.000 | 0.184 | 0.000 | −0.136 | 0.003 |
| Health perception | −0.012 | 0.792 | −0.017 | 0.715 | −0.059 | 0.205 | −0.044 | 0.352 | 0.028 | 0.559 |
| Psychotropic drugs | −0.031 | 0.394 | 0.033 | 0.372 | 0.005 | 0.885 | 0.015 | 0.685 | −0.011 | 0.772 |
| Physical activity strategies | −0.003 | 0.931 | −0.011 | 0.759 | −0.025 | 0.486 | −0.020 | 0.571 | −0.004 | 0.912 |
| B | p | OR | 95% CI | |
|---|---|---|---|---|
| Vigorous PA | ||||
| Region of residence | −0.176 | 0.335 | 0.839 | 0.587–1.199 |
| Dwelling floor space | 0.201 | 0.336 | 1.222 | 0.812–1.840 |
| Age | −0.045 | 0.002 | 0.956 | 0.929–0.983 |
| Working activity | −0.125 | 0.302 | 0.883 | 0.696–1.119 |
| Family members | −0.040 | 0.796 | 0.961 | 0.710–1.301 |
| Quality of life | 0.357 | 0.009 | 1.429 | 1.092–1.870 |
| Moderate PA | ||||
| Region of residence | 0.033 | 0.861 | 1.033 | 0.714–1.495 |
| Dwelling floor space | −0.010 | 0.962 | 0.990 | 0.655–1.496 |
| Age | −0.006 | 0.674 | 0.994 | 0.965–1.024 |
| Working activity | 0.064 | 0.607 | 1.066 | 0.836–1.359 |
| Family members | −0.138 | 0.392 | 0.871 | 0.636–1.194 |
| Quality of life | 0.347 | 0.008 | 1.415 | 1.093–1.831 |
| Walking | ||||
| Region of residence | 0.031 | 0.789 | 1.032 | 0.822–1.294 |
| Dwelling floor space | −0.137 | 0.300 | 0.872 | 0.673–1.130 |
| Age | 0.017 | 0.081 | 1.017 | 0.998–1.036 |
| Working activity | 0.034 | 0.661 | 1.034 | 0.890–1.201 |
| Family members | −0.032 | 0.747 | 0.968 | 0.796–1.178 |
| Quality of life | 0.359 | 0.000 | 1.432 | 1.211–1.693 |
| Total PA | ||||
| Region of residence | 0.017 | 0.945 | 1.017 | 0.637–1.624 |
| Dwelling floor space | 0.227 | 0.384 | 1.255 | 0.753–2.093 |
| Age | −0.008 | 0.699 | 0.993 | 0.955–1.031 |
| Working activity | −0.009 | 0.954 | 0.991 | 0.733–1.340 |
| Family members | −0.255 | 0.217 | 0.775 | 0.517–1.162 |
| Quality of life | 0.500 | 0.003 | 1.649 | 1.191–2.284 |
| Sitting/lying time | ||||
| Region of residence | −0.059 | 0.609 | 0.943 | 0.753–1.181 |
| Dwelling floor space | −0.230 | 0.078 | 0.794 | 0.615–1.026 |
| Age | −0.040 | 0.001 | 0.961 | 0.943–0.979 |
| Working activity | 0.029 | 0.704 | 1.029 | 0.887–1.193 |
| Family members | −0.047 | 0.636 | 0.954 | 0.786–1.159 |
| Quality of life | −0.250 | 0.003 | 0.779 | 0.659–0.920 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natalucci, V.; Villarini, M.; Emili, R.; Acito, M.; Vallorani, L.; Barbieri, E.; Villarini, A. Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort. J. Pers. Med. 2021, 11, 381. https://doi.org/10.3390/jpm11050381
Natalucci V, Villarini M, Emili R, Acito M, Vallorani L, Barbieri E, Villarini A. Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort. Journal of Personalized Medicine. 2021; 11(5):381. https://doi.org/10.3390/jpm11050381
Chicago/Turabian StyleNatalucci, Valentina, Milena Villarini, Rita Emili, Mattia Acito, Luciana Vallorani, Elena Barbieri, and Anna Villarini. 2021. "Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort" Journal of Personalized Medicine 11, no. 5: 381. https://doi.org/10.3390/jpm11050381