Customization of Diet May Promote Exercise and Improve Mental Wellbeing in Mature Adults: The Role of Exercise as a Mediator
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Classification of Dietary Patterns
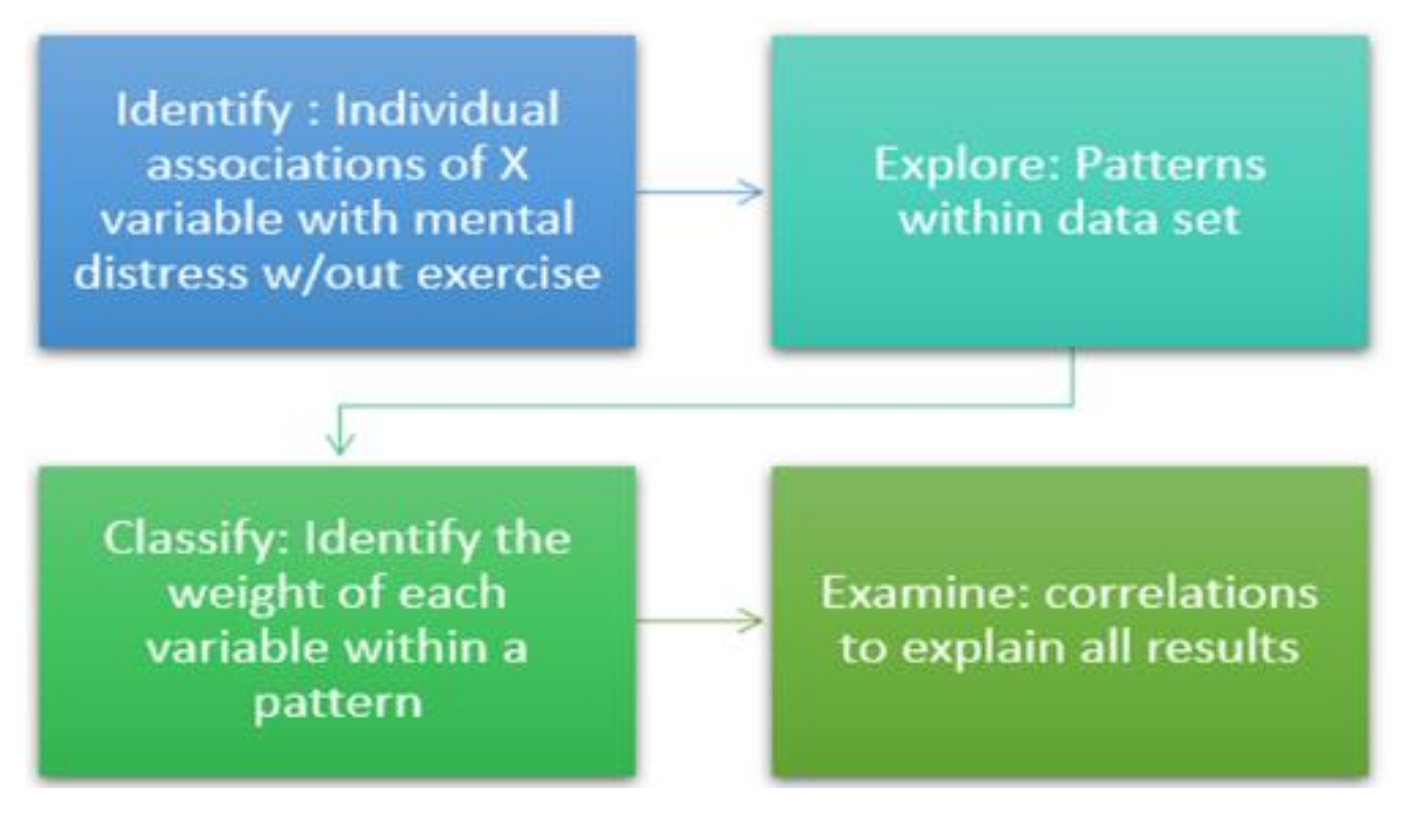
2.4. Data Partitioning
2.5. Statistical Analysis
2.5.1. Data Pre-Processing
2.5.2. Rationale of the Study Design
2.5.3. Mediation Analysis
2.5.4. Cluster Analysis
2.5.5. Principal Component Analysis
2.5.6. Spearman’s Rank–Order Analysis
3. Results
3.1. Mediation Analysis
3.1.1. The c and c’ Paths
3.1.2. The a Path
3.1.3. Cluster Analysis
3.1.4. PCA
3.1.5. Spearman’s Rank–Order Correlation
4. Discussion
4.1. Mediation Regression Analysis (MA)
4.2. Cluster Analysis (CA)
4.3. PCA
4.4. Spearman’s Rank–Order Correlation Analysis
4.5. Summary of Findings
4.6. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Begdache, L.; Chaar, M.; Sabounchi, N.; Kianmehr, H. Assessment of Dietary Factors, Dietary Practices and Exercise on Mental Distress in Young Adults versus Matured Adults: A Cross-Sectional Study. Nutr. Neurosci. 2019, 22, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. Jpn. 2015, 40, 219. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.C.; Turetsky, B.I.; Matsui, M.; Yan, M.; Bilker, W.; Hughett, P.; Gur, R.E. Sex Differences in Brain Gray and White Matter in Healthy Young Adults: Correlations with Cognitive Performance. J. Neurosci. 1999, 19, 4065. [Google Scholar] [CrossRef] [PubMed]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex Differences in the Structural Connectome of the Human Brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; Harris, M.A.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975. [Google Scholar] [CrossRef]
- Zaidi, Z.F. Gender Differences in Human Brain: A Review. Open Anat. J. 2010, 2, 37–55. [Google Scholar] [CrossRef]
- Wager, T.D.; Phan, K.L.; Liberzon, I.; Taylor, S.F. Valence, Gender, and Lateralization of Functional Brain Anatomy in Emotion: A Meta-Analysis of Findings from Neuroimaging. Neuroimage 2003, 19, 513–531. [Google Scholar] [CrossRef]
- Toga, A.W.; Sowell, E.R.; Peterson, B.S.; Welcome, S.E.; Thompson, P.M.; Henkenius, A.L. Mapping Cortical Change across the Human Life Span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Viboolvorakul, S.; Patumraj, S. Exercise Training Could Improve Age-Related Changes in Cerebral Blood Flow and Capillary Vascularity through the Upregulation of VEGF and ENOS. BioMed Res. Int. 2014, 2014, 230791. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The Role of Insulin-Like Growth Factor 1 (IGF-1) in Brain Development, Maturation and Neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef]
- Ingold, M.; Tulliani, N.; Chan, C.C.H.; Liu, K.P.Y. Cognitive Function of Older Adults Engaging in Physical Activity. BMC Geriatr. 2020, 20, 229. [Google Scholar] [CrossRef]
- Begdache, L.; Marhaba, R.; Chaar, M. Validity and Reliability of Food–Mood Questionnaire (FMQ). Nutr. Health 2019, 25, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Craft, L.L.; Perna, F.M. The Benefits of Exercise for the Clinically Depressed. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.B.; Sallis, J.F.; Needle, R. The Relation of Physical Activity and Exercise to Mental Health. Public Health Rep. 1985, 100, 195–202. [Google Scholar] [PubMed]
- Ströhle, A. Physical Activity, Exercise, Depression and Anxiety Disorders. J. Neural Transm. 2009, 116, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.A.; Kessler, R.C.; Slade, T.; Andrews, G. The Performance of the K6 and K10 Screening Scales for Psychological Distress in the Australian National Survey of Mental Health and Well-Being. Psychol. Med. 2003, 33, 357–362. [Google Scholar] [CrossRef]
- Krynen, A.M.; Osborne, D.; Duck, I.M.; Houkamau, C.A.; Sibley, C.G. Measuring Psychological Distress in New Zealand: Item Response Properties and Demographic Differences in the Kessler-6 Screening Measure. N. Zeal. J. Psychol. 2013, 42, 69. [Google Scholar]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020.
- Analysis of Total Food Intake and Composition of Individual’s Diet Based on the U.S. Department of Agriculture’s 1994–96, 1998 Continuing Survey of Food Intakes by Individuals (CSFII) (2005, Final Report). Available online: https://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=461341 (accessed on 18 May 2021).
- Somerville, L.H. Searching for Signatures of Brain Maturity: What Are We Searching for? Neuron 2016, 92, 1164–1167. [Google Scholar] [CrossRef]
- Kessler, R.C.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Bromet, E.; Cuitan, M.; Furukawa, T.A.; Gureje, O.; Hinkov, H.; Hu, C.-Y.; et al. Screening for Serious Mental Illness in the General Population with the K6 Screening Scale: Results from the WHO World Mental Health (WMH) Survey Initiative. Int. J. Methods Psychiatr. Res. 2011, 20, 62. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. Asymptotic and Resampling Strategies for Assessing and Comparing Indirect Effects in Multiple Mediator Models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Abdi, H.; Lynne, W.J. Principal Component Analysis & NBSP. Wires Comput. Stat. 2010, 2, 433–459. [Google Scholar]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. An Approach to Construct Simplified Measures of Dietary Patterns from Exploratory Factor Analysis. Br. J. Nutr. 2003, 89, 409–418. [Google Scholar] [CrossRef]
- Begdache, L.; Kianmehr, H.; Sabounchi, N.; Chaar, M.; Marhaba, J. Principal Component Analysis Identifies Differential Gender-Specific Dietary Patterns That May Be Linked to Mental Distress in Human Adults. Nutr. Neurosci. 2018, 23, 295–308. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S.A. Vitamins, Minerals, and Mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef]
- Chalon, S. Omega-3 Fatty Acids and Monoamine Neurotransmission. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 259–269. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, J.T.; Santos, B.L.R.; Dias, F.C.; Martinez, A.M.B.; Lima, C.K.F.; Miranda, A.L.P. Long-Chain Omega-3 Fatty Acids Supplementation Accelerates Nerve Regeneration and Prevents Neuropathic Pain Behavior in Mice. Front. Pharmacol. 2017, 8, 723. [Google Scholar] [CrossRef]
- Atlantis, E.; Chow, C.-M.; Kirby, A.; Singh, M.F. An Effective Exercise-Based Intervention for Improving Mental Health and Quality of Life Measures: A Randomized Controlled Trial. Prev. Med. 2004, 39, 424–434. [Google Scholar] [CrossRef]
- Benton, D. Carbohydrate Ingestion, Blood Glucose and Mood. Neurosc. Biobehav. Rev. 2002, 26, 293–308. [Google Scholar] [CrossRef]
- Bourre, J.M. Dietary Omega-3 Fatty Acids and Psychiatry: Mood, Behaviour, Stress, Depression, Dementia and Aging. J. Nutr. Health Aging 2005, 9, 31. [Google Scholar] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.A. Stimulatory Effect of Caffeine on the Hypothalamo-Pituitary-Adrenocortical Axis in the Rat. J. Endocrinol. 1989, 122, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kaster, M.P.; Machado, N.J.; Silva, H.B.; Nunes, A.; Ardais, A.P.; Santana, M.; Baqi, Y.; Müller, C.E.; Rodrigues, A.L.S.; Porciúncula, L.O.; et al. Caffeine Acts through Neuronal Adenosine A2A Receptors to Prevent Mood and Memory Dysfunction Triggered by Chronic Stress. Proc. Natl. Acad. Sci. USA 2015, 112, 7833. [Google Scholar] [CrossRef]
- Richards, G.; Smith, A. Caffeine Consumption and Self-Assessed Stress, Anxiety, and Depression in Secondary School Children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef]
- Daniel, P.M.; Love, E.R.; Moorhouse, S.R.; Pratt, O.E. The Effect of Insulin upon the Influx of Tryptophan into the Brain of the Rabbit. J. Physiol. 1981, 312, 551–562. [Google Scholar] [CrossRef]
- Tagliamonte, A.; DeMontis, M.G.; Olianas, M.; Onali, P.L.; Gessa, G.L. Possible Role of Insulin in the Transport of Tyrosine and Tryptophan from Blood to Brain. Adv. Exp. Med. Biol. 1976, 69, 89–94. [Google Scholar] [CrossRef]
- Nishizawa, S.; Benkelfat, C.; Young, S.N.; Leyton, M.; Mzengeza, S.; Montigny, C.D.; Blier, P.; Diksic, M. Differences between Males and Females in Rates of Serotonin Synthesis in Human Brain. Proc. Natl. Acad. Sci. USA 1997, 94, 5308–5313. [Google Scholar] [CrossRef]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and Traditional Diets with Depression and Anxiety in Women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef]
- Wurtman, R.J.; O’Rourke, D.; Wurtman, J.J. Nutrient Imbalances in Depressive Disorders. Possible Brain Mechanisms. Ann. N. Y. Acad. Sci. 1989, 575, 75–82. [Google Scholar] [CrossRef]
- Hariri, N.; Gougeon, R.; Thibault, L. A Highly Saturated Fat-Rich Diet Is More Obesogenic than Diets with Lower Saturated Fat Content. Nutr. Res. 2010, 30, 632–643. [Google Scholar] [CrossRef]
- Begdache, L.; Sadeghzadeh, S.; Derose, G.; Abrams, C. Diet, Exercise, Lifestyle, and Mental Distress among Young and Mature Men and Women: A Repeated Cross-Sectional Study. Nutrients 2021, 13, 24. [Google Scholar] [CrossRef]
- Lautenschlager, N.T.; Almeida, O.P.; Flicker, L.; Janca, A. Can Physical Activity Improve the Mental Health of Older Adults? Ann. Gen. Hosp. Psychiatry 2004, 3, 12. [Google Scholar] [CrossRef][Green Version]
- Gariballa, S.; Alessa, A. Associations between Low Muscle Mass, Blood-Borne Nutritional Status and Mental Health in Older Patients. BMC Nutr. 2020, 6, 6. [Google Scholar] [CrossRef]

| Category | Frequency | Percent | |
|---|---|---|---|
| Gender | |||
| Males | 329 | 27.2 | |
| Females | 880 | 72.8 | |
| Age | |||
| 30–39 | 321 | 26.5 | |
| 40–49 | 426 | 35.3 | |
| 50–above | 461 | 38.2 | |
| Region | |||
| Western countries | 704 | 58.5 | |
| MENA and Asian countries | 499 | 41.5 | |
| Education | |||
| Less than high school | 119 | 10 | |
| High School | 576 | 48.2 | |
| 2–4 years college | 372 | 31.1 | |
| Graduate degree | 128 | 10.7 |
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| Independent | Direct Effect of X on Mental Distress | Association between X and Mediator | Mediation or Indirect Effect | Direct Effect of X on Mental Distress | Association between X and Mediator | Mediation or Indirect Effect | |
| Variables (X) | C path (SE) p | A path (SE) P | C’ path (SE) CI | C path (SE) P | A path (SE) p | C’ path (SE) CI | |
| Caffeine | 0.1468 0.1134 0.1965 | 0.0747 0.0576 0.1957 | −0.0266 0.0233 [−0.0788–0.0133] | 0.2371 0.0839 0.0048 | 0.0652 0.0331 0.0493 | −0.0298 0.0170 [−0.0671–−0.0002] | |
| Breakfast | 0.2222 0.2108 0.2926 | 0.4550 0.1024 0.0000 | −0.1702 0.0657 [−0.3167–−0.0567] | −0.3819 0.1730 0.0275 | 0.2270 0.0626 0.003 | −0.0943 0.0345 [−0.1719–−0.0340] | |
| Whole Grain | −0.0717 0.1062 0.5000 | 0.2651 0.0544 0.0000 | −0.0869 0.0339 [−0.1611–−0.0275] | 0.0372 0.0858 0.6649 | 0.1927 0.0340 <0.001 | −0.0874 0.0230 [−0.1368–−0.0479] | |
| Dairy | −0.0253 0.1144 0.8252 | 0.1782 0.0578 0.0022 | −0.0610 0.0293 [−0.1272–−0.0149] | 0.1583 0.0874 0.0704 | −0.0202 0.0340 0.5527 | 0.090 0.0156 [−0.0205–0.0412] | |
| Fruits | −0.1673 0.1202 0.1645 | 0.2614 0.0602 0.0000 | −0.0809 0.0355 [−0.1602–−0.0212] | −0.2532 0.0921 0.0061 | 0.1420 0.0351 0.0001 | −0.0581 0.0192 [−0.0999–−0.0253] | |
| Nuts | −0.4347 0.1122 0.0001 | 0.3096 0.0619 0.0000 | −0.0765 0.0374 [−0.1594–−0.0116] | −0.0988 0.0877 0.2602 | 0.2031 0.0346 0.0000 | −0.0859 0.0231 [−0.1356–−0.0459] | |
| HGI Food | 0.1224 0.1335 0.3600 | −0.2044 0.0662 0.0022 | 0.0676 0.0311 [0.0159–0.1385] | 0.3444 0.1043 0.0010 | −0.1828 0.0424 0.0000 | 0.0728 0.0231 [0.0330–0.1227] | |
| Meat | 0.0559 0.1257 0.6569 | 0.1164 0.0702 0.0981 | −0.0408 0.0286 [−0.1042–0.0071] | −0.0644 0.0958 0.5017 | −0.0861 0.0383 0.0247 | 0.0384 0.0190 [0.0041–0.0798] | |
| DGLV | −0.0913 0.1259 0.4691 | 0.2773 0.0653 0.0000 | −0.0910 0.0356 [−0.1959–−0.0279] | −0.3511 0.1294 0.0068 | 0.3329 0.0430 0.0000 | −0.1208 0.0350 [−0.1959–−0.0573] | |
| Beans | −0.0944 0.1722 0.5838 | 0.1746 0.0794 0.0285 | −0.0593 0.0337 [−0.1356–−0.0044] | 0.0555 0.1148 0.6291 | 0.1783 0.0460 0.0001 | −0.0797 0.0257 [−0.1351–−0.0351] | |
| Fish | −0.5430 0.1626 0.0009 | 0.3753 0.0874 0.0000 | −0.1038 0.0468 [−0.2060–−0.0245] | −0.2694 0.1319 0.0415 | 0.3926 0.0476 0.0000 | −0.1544 0.0396 [−0.2385–−0.0815] | |
| Fast Food | 0.1709 0.1739 0.3264 | −0.2168 0.0838 0.0101 | 0.0714 0.0363 [0.0123–0.1546] | 0.4186 0.1505 0.0055 | −0.3646 0.0512 0.0000 | 0.1362 0.0389 [0.0668–0.2220] | |
| MV | −0.1700 0.0976 0.0825 | 0.1544 0.0478 0.0014 | −0.0485 0.0232 [−0.1002–−0.0115] | −0.1126 0.0636 0.0768 | 0.0725 0.0260 0.0043 | −0.0319 0.0131 [−0.0606–−0.0900] | |
| Fish Oil | 0.1903 0.1044 0.8536 | 0.2569 0.0533 0.0000 | −0.0901 0.0335 [−0.1610–−0.0302] | −0.0377 0.0831 0.6847 | 0.1293 0.0334 0.0001 | −0.0566 0.0182 [−0.0954–−0.0242] | |
| B path = −0.1682 SE = 0.0386 p < 0.001 | B path = −0.4268 SE = 0.829 p = 0.0000 | ||||||
| Men | Women | ||||
|---|---|---|---|---|---|
| Western Diet | Healthy Diet and Practices | Healthy Diet | Western Diet | Dietary Practices | |
| Exercise | −0.40543 | 0.55127 | 0.19260 | −0.73570 | 0.29183 |
| Breakfast | −0.224 | 0.30457 | 0.22082 | −0.45985 | 0.05524 |
| Whole Grain | −0.47105 | 0.64049 | 0.50487 | −0.48263 | −0.29798 |
| Dairy | −0.33714 | 0.45842 | 0.54331 | −0.02677 | −0.67831 |
| Caffeine | −0.17636 | 0.2398 | 0.63630 | −0.18214 | −0.68236 |
| Fruits | −0.46929 | 0.6381 | 0.87216 | −0.66765 | −0.63118 |
| Nuts | −0.4573 | 0.62179 | 0.71417 | −0.60315 | −0.47692 |
| HGI Food | −0.01735 | 0.0236 | 0.16037 | 0.25840 | −0.39005 |
| Meat | −0.04312 | 0.05864 | 0.09057 | 0.08212 | −0.17443 |
| DGLV | −0.27635 | 0.37576 | 0.33433 | −0.86512 | 0.19765 |
| Beans | −0.33734 | 0.45869 | 0.23259 | −0.34981 | −0.04267 |
| Fish | −0.25258 | 0.34343 | 0.21341 | −0.54537 | 0.12310 |
| Fast Food | 0.22267 | −0.30276 | −0.23469 | 0.87862 | −0.33358 |
| MV | −0.19725 | 0.2682 | −0.01079 | −0.34881 | 0.26274 |
| Fish Oil | −0.20923 | 0.28449 | 0.09741 | −0.27796 | 0.06827 |
| Mental Distress | 0.16357 | −0.22241 | −0.11843 | 0.64137 | −0.31067 |
| Men | HD | DP | WD | Women | HD | SU | WD |
|---|---|---|---|---|---|---|---|
| Variance explained (%) | 14.812 | 13.968 | 9.118 | Variance explained (%) | 17.477 | 10.775 | 10.364 |
| Fruits | 0.746 | Fruits | 0.77 | ||||
| Nuts | 0.702 | Nuts | 0.694 | ||||
| Whole grain | 0.612 | Caffeine | 0.656 | −0.303 | |||
| Dairy | 0.587 | Dairy | 0.59 | −0.492 | |||
| Caffeine | 0.422 | 0.383 | Whole grain | 0.586 | |||
| Fish oil | 0.702 | Breakfast | 0.31 | ||||
| MV | 0.679 | Beans | 0.332 | 0.677 | 0.274 | ||
| Fish | 0.535 | DGLV | 0.368 | 0.421 | −0.225 | ||
| Exercise | 0.388 | 0.509 | Meat | −0.421 | |||
| Fast food | −0.39 | Fish oil | 0.417 | ||||
| Breakfast | 0.241 | 0.335 | MV | 0.347 | |||
| Mental distress | −0.214 | −0.288 | HGI food | 0.212 | 0.685 | ||
| Meat | 0.695 | Fast food | −0.209 | 0.583 | |||
| HGI food | −0.282 | 0.657 | Mental distress | 0.523 | |||
| DGLV | 0.417 | 0.418 | Exercise | 0.241 | 0.304 | −0.507 | |
| Beans | 0.273 | 0.353 | Fish | 0.299 | 0.311 | −0.336 |
| Exercise | Breakfast | Whole Grain | Dairy | Caffeine | Fruits | Nuts | HGI Food | Meat | DGLV | Beans | Fish | Fast Food | MV | Fish Oil | Mental Distress | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | 1 | 0.118 ** | 0.200 ** | −0.016 | 0.061 | 0.142 ** | 0.203 ** | −0.151 ** | −0.049 | 0.251 ** | 0.119 ** | 0.269 ** | −0.231 ** | 0.095 ** | 0.132 ** | −0.167 ** |
| Breakfast | 1 | 0.204 ** | 0.118 ** | 0.066 | 0.140 ** | 0.152 ** | 0.059 | 0.003 | 0.102 ** | 0.029 | 0.087 ** | −0.099 ** | 0.043 | 0.021 | −0.087 ** | |
| Whole grain | 1 | 0.209 ** | 0.253 ** | 0.302 ** | 0.300 ** | 0.079 * | −0.027 | 0.167 ** | 0.269 ** | 0.164 ** | −0.116 ** | 0.035 | 0.025 | −0.028 | ||
| Dairy | 1 | 0.433 ** | 0.398 ** | 0.229 ** | 0.130 ** | 0.128 ** | −0.043 | −0.054 | −0.044 | 0.022 | −0.089 ** | −0.076 * | 0.061 | |||
| Caffeine | 1 | 0.430 ** | 0.347 ** | 0.034 | 0.110 ** | 0.063 | 0.048 | 0.119 ** | −0.025 | −0.023 | 0.011 | 0.076 * | ||||
| Fruits | 1 | 0.508 ** | 0.101 ** | 0.04 | 0.211 ** | 0.195 ** | 0.181 ** | −0.129 ** | 0.008 | 0.042 | −0.095 ** | |||||
| Nuts | 1 | 0.06 | −0.01 | 0.292 ** | 0.266 ** | 0.245 ** | −0.181 ** | 0.042 | 0.080 * | −0.058 | ||||||
| HGI food | 1 | 0.136 ** | −0.018 | 0.172 ** | −0.097 ** | 0.217 ** | −0.071 * | −0.04 | 0.151 ** | |||||||
| Meat | 1 | 0.074 * | −0.149 ** | 0.003 | 0.126 ** | 0.004 | −0.104 ** | −0.02 | ||||||||
| DGLV | 1 | 0.283 ** | 0.287 ** | −0.212 ** | 0.033 | 0.052 | −0.117 ** | |||||||||
| Beans | 1 | 0.149 ** | −0.045 | 0.048 | 0.087 ** | 0.004 | ||||||||||
| Fish | 1 | −0.124 ** | 0.101 ** | 0.064 | −0.116 ** | |||||||||||
| Fast food | 1 | −0.053 | −0.089 ** | 0.160 ** | ||||||||||||
| MV | 1 | 0.302 ** | −0.049 | |||||||||||||
| Fish oil | 1 | −0.045 | ||||||||||||||
| Mental distress | 1 |
| Exercise | Breakfast | Whole Grain | Dairy | Caffeine | Fruits | Nuts | HGI Food | Meat | DGLV | Beans | Fish | Fast Food | MV | Fish Oil | Mental Distress | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | 1 | 0.259 ** | 0.256 ** | 0.161 ** | 0.075 | 0.242 ** | 0.248 ** | −0.165 ** | 0.081 | 0.245 ** | 0.095 | 0.226 ** | −0.201 ** | 0.135 * | 0.196 ** | −0.172 ** |
| Breakfast | 1 | 0.227 ** | 0.179 ** | −0.068 | 0.144 ** | 0.051 | −0.078 | −0.065 | 0.084 | 0.133 * | 0.112 * | −0.191 ** | 0.134 * | 0.097 | 0.009 | |
| Whole grain | 1 | 0.205 ** | 0.185 ** | 0.305 ** | 0.350 ** | 0.081 | −0.019 | 0.207 ** | 0.223 ** | 0.132 * | −0.043 | 0.060 | 0.048 | −0.098 | ||
| Dairy | 1 | 0.272 ** | 0.348 ** | 0.315 ** | 0.096 | 0.099 | 0.048 | −0.029 | 0.085 | −0.003 | 0.051 | 0.008 | −0.028 | |||
| Caffeine | 1 | 0.277 ** | 0.199 ** | 0.136 * | 0.148 ** | 0.080 | 0.099 | 0.013 | 0.025 | −0.059 | −0.043 | 0.065 | ||||
| Fruits | 1 | 0.470 ** | 0.074 | −0.020 | 0.118 * | 0.149 ** | 0.077 | −0.135 * | 0.042 | 0.067 | −0.123 * | |||||
| Nuts | 1 | −0.046 | 0.029 | 0.163 ** | 0.193 ** | 0.141 * | −0.046 | 0.032 | 0.102 | −0.227 ** | ||||||
| HGI food | 1 | 0.249 ** | 0.032 | 0.159 ** | −0.089 | 0.090 | −0.061 | −0.148 ** | 0.075 | |||||||
| Meat | 1 | 0.175 ** | 0.020 | 0.067 | 0.072 | 0.039 | 0.034 | −0.023 | ||||||||
| DGLV | 1 | 0.317 ** | 0.220 ** | −0.156 ** | 0.076 | 0.100 | −0.086 | |||||||||
| Beans | 1 | 0.137 * | −0.081 | −0.046 | 0.035 | −0.040 | ||||||||||
| Fish | 1 | −0.197 ** | 0.123 * | 0.128 * | −0.189 ** | |||||||||||
| Fast food | 1 | −0.107 | −0.137 * | 0.070 | ||||||||||||
| MV | 1 | 0.472 ** | −0.125 * | |||||||||||||
| Fish Oil | 1 | −0.026 | ||||||||||||||
| Mental distress | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begdache, L.; Patrissy, C.M. Customization of Diet May Promote Exercise and Improve Mental Wellbeing in Mature Adults: The Role of Exercise as a Mediator. J. Pers. Med. 2021, 11, 435. https://doi.org/10.3390/jpm11050435
Begdache L, Patrissy CM. Customization of Diet May Promote Exercise and Improve Mental Wellbeing in Mature Adults: The Role of Exercise as a Mediator. Journal of Personalized Medicine. 2021; 11(5):435. https://doi.org/10.3390/jpm11050435
Chicago/Turabian StyleBegdache, Lina, and Cara M. Patrissy. 2021. "Customization of Diet May Promote Exercise and Improve Mental Wellbeing in Mature Adults: The Role of Exercise as a Mediator" Journal of Personalized Medicine 11, no. 5: 435. https://doi.org/10.3390/jpm11050435
APA StyleBegdache, L., & Patrissy, C. M. (2021). Customization of Diet May Promote Exercise and Improve Mental Wellbeing in Mature Adults: The Role of Exercise as a Mediator. Journal of Personalized Medicine, 11(5), 435. https://doi.org/10.3390/jpm11050435







