Photobiological Neuromodulation of Resting-State EEG and Steady-State Visual-Evoked Potentials by 40 Hz Violet Light Optical Stimulation in Healthy Individuals
Abstract
:1. Introduction
1.1. Photobiological Neuromodulation
1.2. Melanopsin/Neuropsin Expressed in Retinal Ganglion Cells and Violet Light (VL)
1.3. Clinical Applicability of Non-Visual Stimulation of Light
1.4. Potential Roles of Gamma Oscillations in Visual Stimulation and Visual Pathways
1.5. EEG as a Means of Detecting Representations in the Brain
1.6. Visual-Evoked Potentials (VEPs) and Cross-Frequency Coupling (CFC) of Alpha-Phase and Gamma-Amplitude
1.7. Objectives and Hypotheses of the Present Study
2. Materials and Methods
2.1. Participants
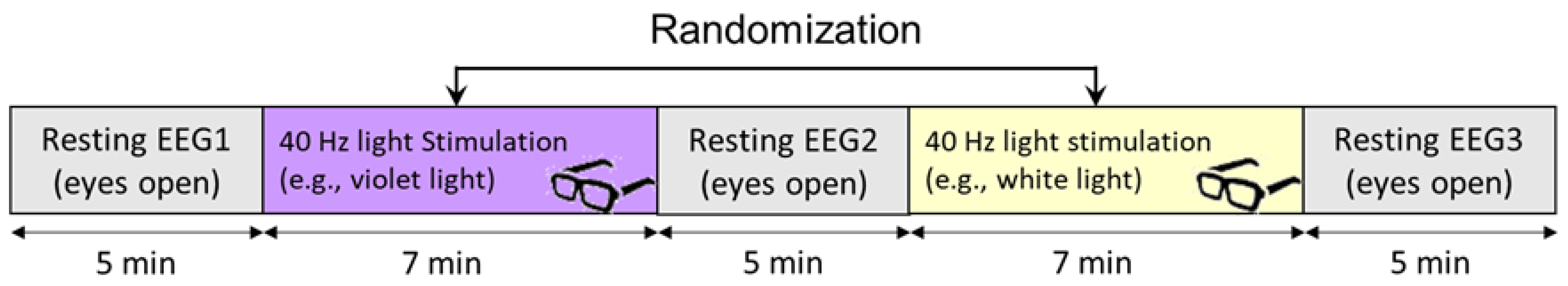
2.2. Experimental Design
2.3. Violet Light (VL) and White Light (WL) Stimulation
2.4. EEG Recording
2.5. EEG Signal Preprocessing
2.6. Power Spectrum Density (PSD) Analysis
2.7. Visual-Evoked Potential (VEP) Analysis
2.8. Phase-Amplitude Coupling (PAC) Analysis during the VEP
2.9. Statistical Analysis
3. Results
3.1. Demographic Data of the Participants
3.2. PSD Analysis
3.2.1. Averaged PSDs over All Electrodes for Baseline EEG, during, and after the Light Stimulations
3.2.2. Topographical Plots of PSDs during the Light Stimulation
PSD of Baseline EEG vs. PSD of VEP with 40 Hz-WL Stimulation
PSD of Baseline EEG vs. PSD of VEP with 40 Hz-VL Stimulation
PSD of VEP with 40 Hz-WL vs. PSD of VEP with 40 Hz-VL
3.2.3. Topographical Plots of PSD after the Light Stimulation
PSD of Baseline EEG vs. PSD of Post-EEG with 40 Hz-WL
PSD of Baseline EEG vs. PSD of Post-EEG with 40 Hz-VL
PSD of Post-EEG with 40 Hz-WL vs. PSD of Post-EEG with 40 Hz-VL
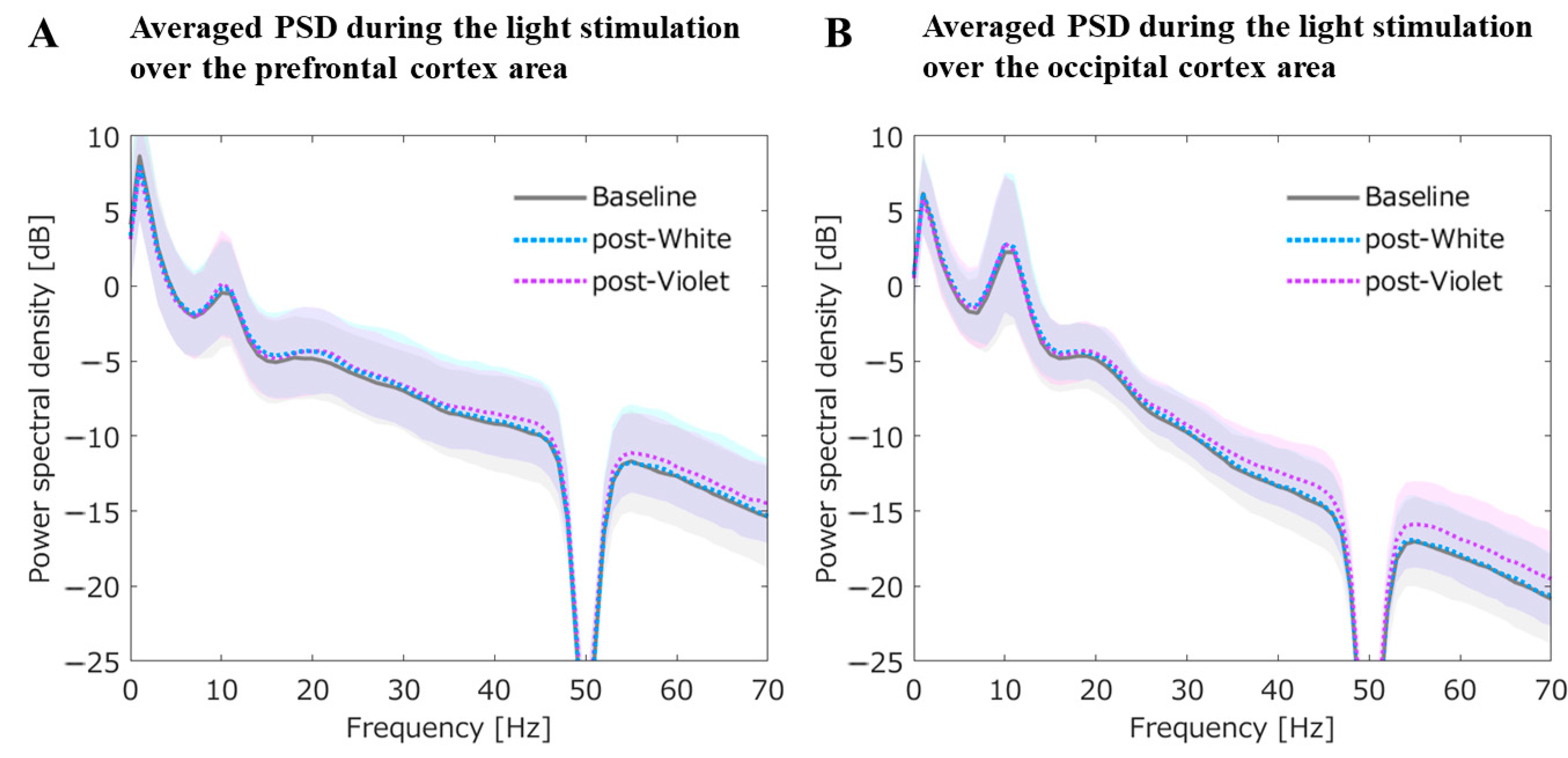
3.2.4. Averaged PSD for the Prefrontal and Occipital Cortex during the Light Stimulation
Averaged PSDs for the Prefrontal ROI during the Light Stimulation
Averaged PSDs for the Occipital ROI during the Light Stimulation
3.2.5. Averaged PSDs for the Prefrontal and Occipital ROIs for Post-EEG with the Light Stimulation
Averaged PSDs for the Prefrontal ROI after the Light Stimulation
Averaged PSDs for the Occipital ROI after the Light Stimulation
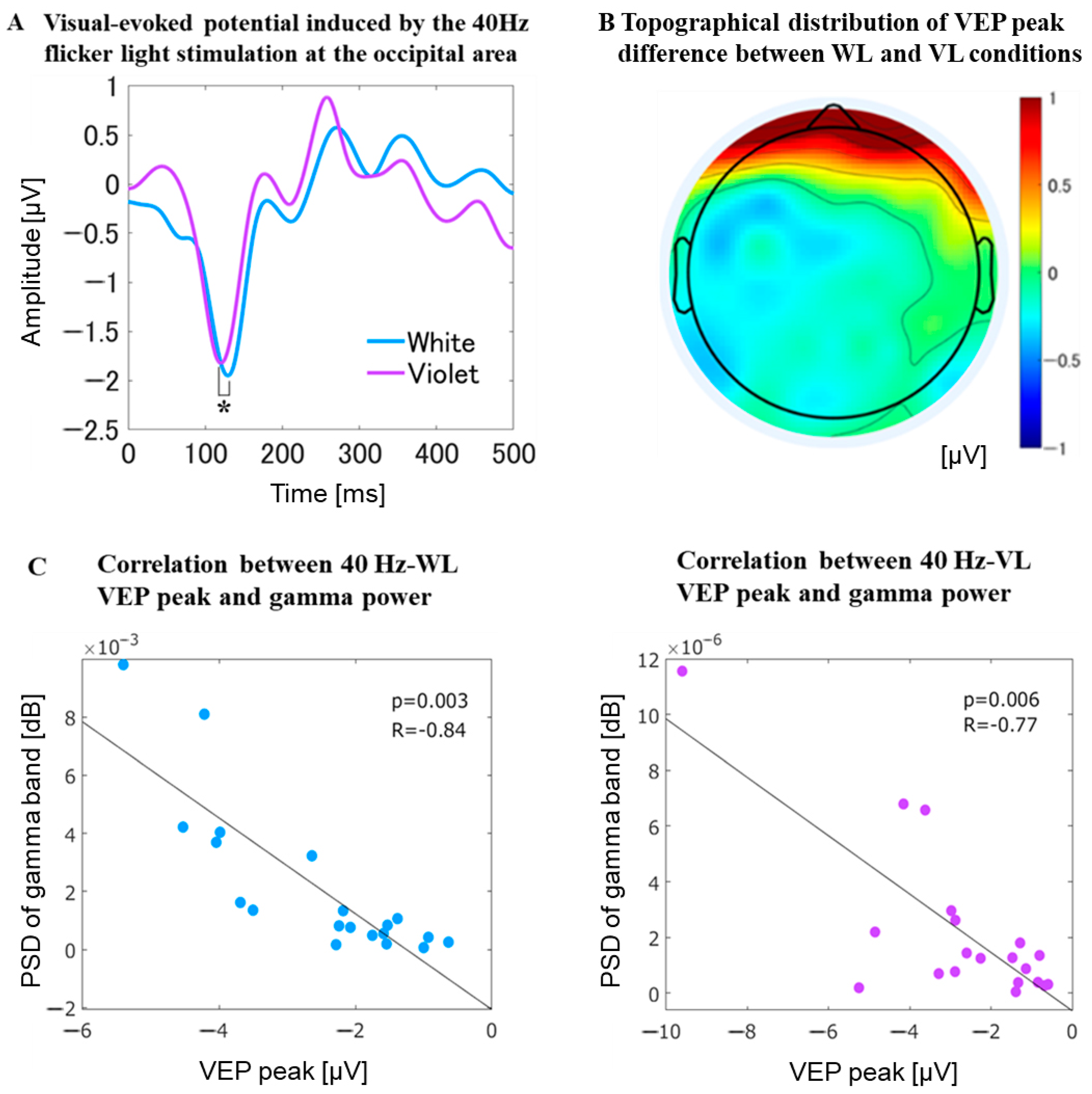
3.2.6. VEP at the Occipital Area
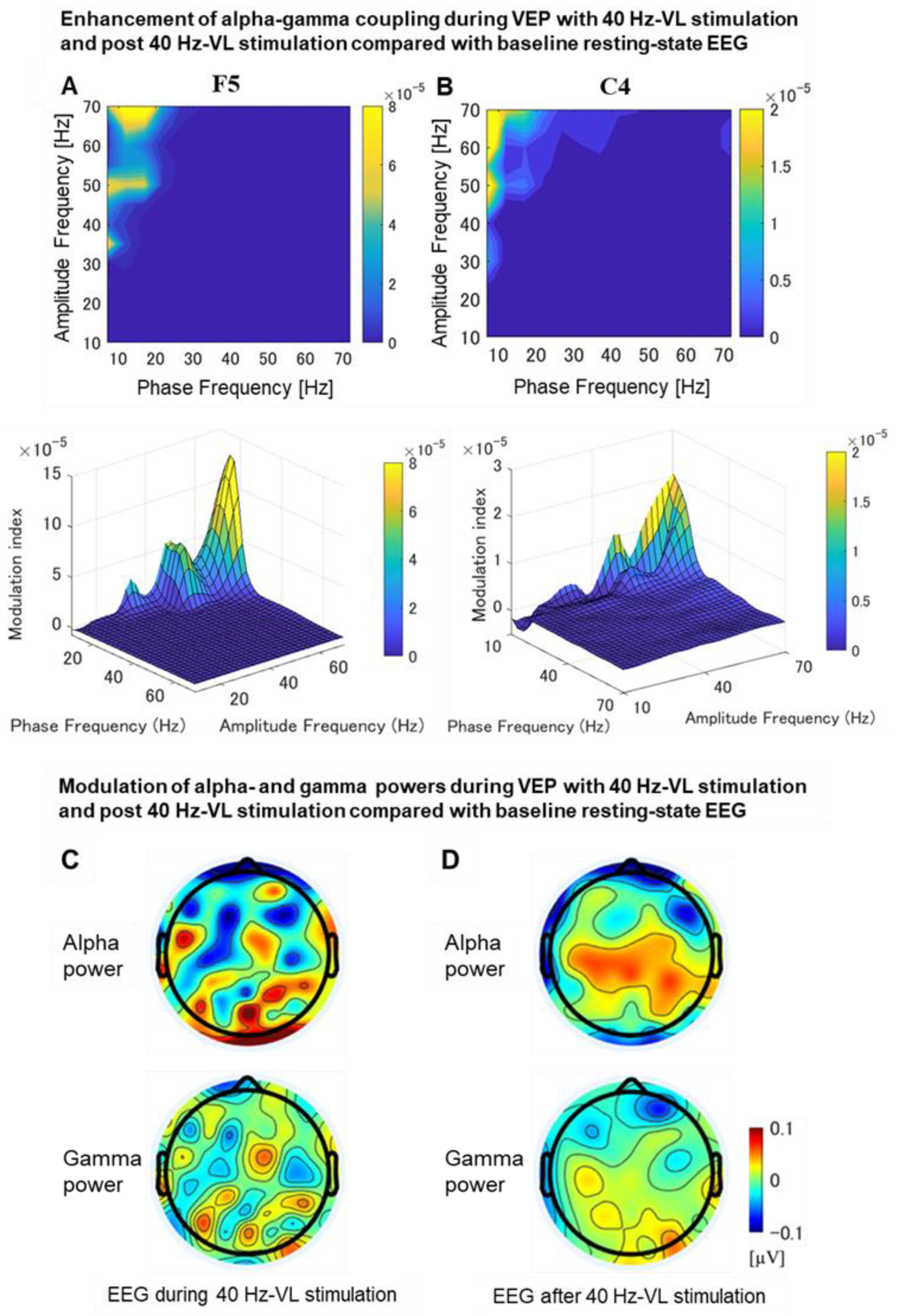
3.2.7. Phase-Amplitude Coupling Analysis during VEP
Differences in Phase-Amplitude Coupling between EEG with 40 Hz-WL Stimulation and Baseline EEG as Well as between Post-EEG for 40 Hz-WL Stimulation and Baseline EEG
Differences in Phase-Amplitude Coupling between EEG with 40 Hz-VL Stimulation and Baseline EEG as Well as between Post-EEG for 40 Hz-VL Stimulation and Baseline EEG
4. Discussion
4.1. Summary of Findings
4.2. Photobiological Neuromodulation of EEG during the 40 Hz-VL Stimulation Compared with 40 Hz-WL Stimulation
4.3. Significant Difference in the Negative Peak of the VEPs between 40 Hz-VL and 40 Hz-WL
4.4. Significant Enhancement of Alpha-Gamma Coupling during and after 40 Hz-VL Stimulation
4.5. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P.; Petrie, S.R.; Hamblin, M.R.; Henderson, T.A.; Iosifescu, D.V. Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 2016, 3, 031404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.T.H.; Yau, K.-W. Intrinsically Photosensitive Retinal Ganglion Cells. Physiol. Rev. 2010, 90, 1547–1581. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Fernandez, E.; Nelson, R. Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Do, M.T.H.; Kang, S.H.; Xue, T.; Zhong, H.; Liao, H.-W.; Bergles, D.; Yau, K.-W. Photon capture and signalling by melanopsin retinal ganglion cells. Nat. Cell Biol. 2008, 457, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Tarttelin, E.E.; Bellingham, J.; Hankins, M.W.; Foster, R.G.; Lucas, R. Neuropsin (Opn5): A novel opsin identified in mammalian neural tissue1. FEBS Lett. 2003, 554, 410–416. [Google Scholar] [CrossRef]
- Fredriksson, R.; Höglund, P.J.; Gloriam, D.E.; Lagerström, M.C.; Schiöth, H.B. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003, 554, 381–388. [Google Scholar] [CrossRef]
- Kojima, D.; Mori, S.; Torii, M.; Wada, A.; Morishita, R.; Fukada, Y. UV-Sensitive Photoreceptor Protein OPN5 in Humans and Mice. PLoS ONE 2011, 6, e26388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, T.; Ohuchi, H.; Tomonari, S.; Ikeda, K.; Sakai, K.; Shichida, Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. USA 2010, 107, 22084–22089. [Google Scholar] [CrossRef] [Green Version]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.-K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Estevez, M.E.; Fogerson, P.M.; Ilardi, M.C.; Borghuis, B.G.; Chan, E.; Weng, S.; Auferkorte, O.N.; Demb, J.; Berson, D.M. Form and Function of the M4 Cell, an Intrinsically Photosensitive Retinal Ganglion Cell Type Contributing to Geniculocortical Vision. J. Neurosci. 2012, 32, 13608–13620. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.M.; Kofuji, P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J. Neurosci. 2009, 29, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.M.; Kofuji, P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J. Neurosci. 2010, 30, 16262–16271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.X.; D’Souza, S.; Upton, B.A.; Kernodle, S.; Vemaraju, S.; Nayak, G.; Gaitonde, K.D.; Holt, A.L.; Linne, C.D.; Smith, A.N.; et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nat. Cell Biol. 2020, 585, 420–425. [Google Scholar] [CrossRef]
- Torii, H.; Kurihara, T.; Seko, Y.; Negishi, K.; Ohnuma, K.; Inaba, T.; Kawashima, M.; Jiang, X.; Kondo, S.; Miyauchi, M.; et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine 2017, 15, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapska, E.; Kaczmarek, L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004, 74, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; von Schantz, M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J. Biol. Rhythms 2005, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nat. Cell Biol. 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, V.S. How Close Are We to Understanding What (if Anything) gamma Oscillations Do in Cortical Circuits? J. Neurosci. 2016, 36, 10489–10495. [Google Scholar] [CrossRef]
- Murty, D.V.; Shirhatti, V.; Ravishankar, P.; Ray, S. Large Visual Stimuli Induce Two Distinct Gamma Oscillations in Primate Visual Cortex. J. Neurosci. 2018, 38, 2730–2744. [Google Scholar] [CrossRef] [Green Version]
- Todorov, M.I.; Kékesi, K.A.; Borhegyi, Z.; Galambos, R.; Juhász, G.; Hudetz, A.G. Retino-cortical stimulus frequency-dependent gamma coupling: Evidence and functional implications of oscillatory potentials. Physiol. Rep. 2016, 4, e12986. [Google Scholar] [CrossRef] [Green Version]
- Chorlian, D.B.; Porjesz, B.; Begleiter, H. Amplitude modulation of gamma band oscillations at alpha frequency produced by photic driving. Int. J. Psychophysiol. 2006, 61, 262–278. [Google Scholar] [CrossRef]
- Paszkiel, S. Data Acquisition Methods for Human Brain Activity. In Analysis and Classification of EEG Signals for Brain–Computer Interfaces; Studies in Computational Intelligence; Springer: Cham, Switzerland, 2020; Volume 852. [Google Scholar]
- Tsoneva, T.; Garcia-Molina, G.; Desain, P. Neural dynamics during repetitive visual stimulation. J. Neural Eng. 2015, 12, 66017. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Herrmann, C.; Engel, A.K.; Schneider, T.R. Different coupling modes mediate cortical cross-frequency interactions. NeuroImage 2016, 140, 76–82. [Google Scholar] [CrossRef]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring Phase-Amplitude Coupling Between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef]
- Otero, M.; Prado-Gutiérrez, P.; Weinstein, A.; Escobar, M.-J.; El-Deredy, W. Persistence of EEG Alpha Entrainment Depends on Stimulus Phase at Offset. Front. Hum. Neurosci. 2020, 14, 139. [Google Scholar] [CrossRef] [Green Version]
- Köster, M.; Finger, H.; Graetz, S.; Kater, M.; Gruber, T. Theta-gamma coupling binds visual perceptual features in an associative memory task. Sci. Rep. 2018, 8, 17688. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Martens, U.; Gruber, T. Memory entrainment by visually evoked theta-gamma coupling. NeuroImage 2019, 188, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Staudigl, T.; Fellner, M.C. Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Front. Hum. Neurosci. 2012, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanslmayr, S.; Staresina, B.P.; Bowman, H. Oscillations and Episodic Memory: Addressing the Synchronization/Desynchronization Conundrum. Trends Neurosci. 2016, 39, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, B.J.; Parish, G.; Roux, F.; Michelmann, S.; van der Plas, M.; Kolibius, L.D.; Chelvarajah, R.; Rollings, D.T.; Sawlani, V.; Hamer, H.; et al. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory. Proc. Natl. Acad. Sci. USA 2019, 116, 21834–21842. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Frequency Band | Baseline vs. 40 Hz-WL | Baseline vs. 40 Hz-VL | 40 Hz-WL vs. 40 Hz-VL | Baseline vs. Post-WL | Baseline vs. Post-VL | Post-WL vs. Post-VL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t38 | P | t38 | p | t38 | P | t38 | p | t38 | p | t38 | p | |
| Delta | 1.92 | 0.07 | 0.74 | 0.47 | 2.40 | 0.02 * | 0.23 | 0.81 | 0.67 | 0.51 | 0.47 | 0.64 |
| Theta | 2.15 | 0.04 * | 0.11 | 0.91 | 2.05 | 0.05 * | 0.21 | 0.83 | 0.07 | 0.94 | 0.27 | 0.78 |
| Alpha | 0.91 | 0.37 | 0.28 | 0.78 | 0.62 | 0.53 | 0.52 | 0.60 | 0.34 | 0.73 | 0.19 | 0.86 |
| Beta | 1.56 | 0.14 | 0.69 | 0.51 | 0.83 | 0.41 | 0.96 | 0.35 | 1.08 | 0.29 | 0.19 | 0.86 |
| Gamma | 1.34 | 0.20 | 0.99 | 0.34 | 0.45 | 0.66 | 0.93 | 0.36 | 1.87 | 0.06 | 0.94 | 0.35 |
| Frequency Band | Prefrontal Cortex 40 Hz-WL vs. 40 Hz-VL | Occipital Cortex 40 Hz-WL vs. 40 Hz-VL | ||
|---|---|---|---|---|
| t38 | p | t38 | p | |
| Delta | 1.89 | 0.07 | 1.87 | 0.04 * |
| Theta | 2.03 | 0.04 * | 1.34 | 0.18 |
| Alpha | 0.99 | 0.32 | 0.06 | 0.95 |
| Beta | 1.22 | 0.24 | 0.08 | 0.94 |
| Gamma | 1.18 | 0.24 | 0.30 | 0.77 |
| Figure | Prefrontal Cortex | Occipital Cortex | ||
|---|---|---|---|---|
| t38 | p | t38 | p | |
| Delta | 0.34 | 0.73 | 0.45 | 0.66 |
| Theta | 0.25 | 0.81 | 0.23 | 0.81 |
| Alpha | −0.03 | 0.98 | 0.15 | 0.87 |
| Beta | −0.04 | 0.96 | −0.22 | 0.82 |
| Gamma | −0.53 | 0.60 | −1.00 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, Y.; Takano, M.; Hayano, M.; Li, X.; Wada, M.; Nakajima, S.; Mimura, M.; Kondo, S.; Tsubota, K. Photobiological Neuromodulation of Resting-State EEG and Steady-State Visual-Evoked Potentials by 40 Hz Violet Light Optical Stimulation in Healthy Individuals. J. Pers. Med. 2021, 11, 557. https://doi.org/10.3390/jpm11060557
Noda Y, Takano M, Hayano M, Li X, Wada M, Nakajima S, Mimura M, Kondo S, Tsubota K. Photobiological Neuromodulation of Resting-State EEG and Steady-State Visual-Evoked Potentials by 40 Hz Violet Light Optical Stimulation in Healthy Individuals. Journal of Personalized Medicine. 2021; 11(6):557. https://doi.org/10.3390/jpm11060557
Chicago/Turabian StyleNoda, Yoshihiro, Mayuko Takano, Motoshi Hayano, Xuemei Li, Masataka Wada, Shinichiro Nakajima, Masaru Mimura, Shinichiro Kondo, and Kazuo Tsubota. 2021. "Photobiological Neuromodulation of Resting-State EEG and Steady-State Visual-Evoked Potentials by 40 Hz Violet Light Optical Stimulation in Healthy Individuals" Journal of Personalized Medicine 11, no. 6: 557. https://doi.org/10.3390/jpm11060557








