Current Challenges in Deciphering Sutton Nevi—Literature Review and Personal Experience
Abstract
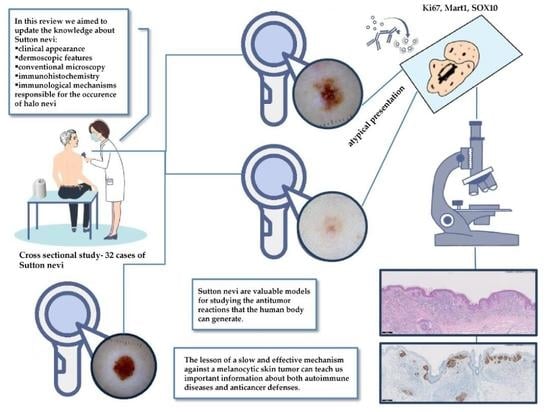
:1. Introduction
2. Why Measure the Diameter?—The Average Size of Halo Nevi, the Relationship between the Halo and the Nevus
3. Dermoscopic Pattern—Same Look as in Common Nevi?
4. The Microscopic Features—What Matters in Atypical Tumors?
5. Association with Diseases—What to (Not) Investigate?
6. Key Antimelanocyte Immune Reaction Lesson
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nazzaro, G.; Rovaris, M. The men or women behind nevi: Richard Sutton. JAMA Dermatol. 2014, 150, 302. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, D. The man behind the eponyms: Richard L Sutton: Periadenitis mucosa necroticarecurrens (Sutton’s ulcer) and leukoderma acquisitumcentrifugum-Sutton’s (halo) nevus. Am. J. Dermatopathol. 2003, 25, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.H. Clinical note on leukoderma acquisitumcontrifigum. Arch. Derm. Syphilol. 1923, 8, 178. [Google Scholar]
- Kopf, A.W.; Morrill, S.D.; Silberberg, I. Broad spectrum of leukoderma acquisitumcentrifugum. Arch. Dermatol. 1965, 92, 14–33. [Google Scholar] [CrossRef]
- Rivers, J.K.; MacLennan, R.; Kelly, J.W.; Lewis, A.E.; Tate, B.J.; Harrison, S.; McCarthy, W.H. The eastern Australian childhood nevus study: Prevalence of atypical nevi, congenital nevus-like nevi, and other pigmented lesions. J. Am. Acad. Derm. 1995, 32, 957–963. [Google Scholar] [CrossRef]
- Larsson, P.; Liden, S. Prevalence of skin diseases among adolescents 12–16 years of age. Acta Derm. Venereol. 1980, 60, 415–423. [Google Scholar] [PubMed]
- Mollet, I.; Ongenae, K.; Naeyaert, J.M. Origin, Clinical Presentation, and Diagnosis of Hypomelanotic Skin Disroders. Derm. Clin. 2007, 25, 363–371. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Passeron, T. Vitiligo and other disorders of hypopigmentation. In Textbook of Dermatology, 3rd ed.; Bolognia, J.L., Jorizzo, J.L., Schaffer, J.V., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 1023–1048. [Google Scholar]
- Aouthmany, M.; Weinstein, M.; Zirwas, M.J.; Brodell, R.T. The natural history of halo nevi: A retrospective case series. J. Am. Acad. Derm. 2012, 67, 582–586. [Google Scholar] [CrossRef]
- Frank, S.B.; Cohen, H.J. The Halo Nevus. Arch. Dermatol. 1964, 89, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Soyer, H.P.; Argenziano, G.; Hofmann-Wellenhof, R.; Johr, R.H. Color Atlas of Melanocytic Lesions of the Skin; Springer: Berlin/Heidelberg, Germany, 2007; pp. 124–128. [Google Scholar]
- Inamadar, A.C.; Palit, A.; Athanikar, S.B.; Sampagavi, V.V.; Deshmukh, N.S. Unusual course of a halo nevus. Pediatr. Derm. 2003, 20, 542–543. [Google Scholar] [CrossRef]
- Speeckaert, R.; Van Geel, N.; Vermaelen, K.V.; Lambert, J.; Van Gele, M.; Speeckaert, M.M.; Brochez, L. Immune reactions in benign and malignant melanocytic lesions: Lessons for immunotherapy. Pigment Cell Melanoma Res. 2011, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Rongioletti, F.; Cecchi, F.; Rebora, A. Halo phenomenon in melanocytic nevi (Sutton’s nevi). Does the diameter matter? J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1231–1232. [Google Scholar] [CrossRef]
- Kaminska-Winciorek, G.; Szymszal, J. Dermoscopy of halo nevus in own observation. Postepy Derm. Alergol. 2014, 31, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolm, I.; Di Stefani, A.; Hofmann-Wellenhof, R.; Fink-Puches, R.; Wolf, I.H.; Richtig, E.; Smolle, J.; Kerl, H.; Soyer, H.P.; Zalaudek, I. Dermoscopy patterns of halo nevi. Arch. Derm. 2006, 142, 1627–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larre Borges, A.; Zalaudek, I.; Longo, C.; Dufrechou, L.; Argenziano, G.; Lallas, A.; Piana, S.; Moscarella, E. Melanocytic nevi with special features: Clinical-dermoscopic and reflectance confocal microscopic findings. J. Eur. Acad. Derm. Venereol. 2014, 28, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, N.; Speeckaert, R.; Lambert, J.; Mollet, I.; De Keyser, S.; De Schepper, S.; Brochez, L. Halo naevi with associated vitiligo-like depigmentations: Pathogenetic hypothesis. J. Eur. Acad. Derm. Venereol. 2011, 26, 755–761. [Google Scholar] [CrossRef]
- Nedelcu, R.I.; Zurac, S.A.; Brinzea, A.; Cioplea, M.D.; Turcu, G.; Popescu, R.; Popescu, C.M.; Ion, D.A. Morphological features of melanocytic tumors with depigmented halo: Review of the literature and personal results. Rom J. Morphol. Embryol. 2015, 56, 659–663. [Google Scholar]
- Naveh, H.P.; Rao, U.N.M.; Butterfield, L.H. Melanoma-associated leukoderma—Immunology in black and white? Pigment Cell Melanoma Res. 2013, 26, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.; Bhat, M.R.; Dandeli, S.; Ali, N.M. Throwing Light onto the Core of a Halo Nevus: A New Finding. Indian J. Derm. 2016, 61, 238. [Google Scholar] [CrossRef] [PubMed]
- Brugues, A.; Ribero, S.; Da Silva, V.M.; Aguilera, P.; Garcia, A.P.; Alos, L.; Malvehy, J.; Puig, S.; Carrera, C. Sutton Naevi as Melanoma Simulators: Can Confocal Microscopy Help in the Diagnosis? Acta Derm. Venereol. 2020, 100, adv00134. [Google Scholar] [CrossRef]
- Ferrara, G.; Argenziano, G.; Soyer, H.P.; Corona, R.; Sera, F.; Brunetti, B.; Cerroni, L.; Chimenti, S.; El Shabrawi-Caelen, L.; Ferrari, A.; et al. Dermoscopic and histopathologic diagnosis of equivocal melanocytic skin lesions: An interdisciplinary study on 107 cases. Cancer 2002, 95, 1094–1100. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B.; Ivan, D.; Schwartz, M.R.; Tschen, J.A. The immunopathology of regression in benign lichenoid keratosis, keratoacanthoma and halo nevus. Clin. Med. Res. 2004, 2, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Ruby, K.N.; Li, Z.; Yan, S. Aberrant expression of HMB45 and negative PRAME expression in halo nevi. J. Cutan. Pathol. 2021, 48, 519–525. [Google Scholar] [CrossRef]
- Akasu, R.; From, L.; Kahn, H.J. Characterization of the mononuclear infiltrate involved in regression of halo nevi. J. Cutan. Pathol. 1994, 21, 302–311. [Google Scholar] [CrossRef] [PubMed]
- De Schriver, S.; Theate, I.; Vanhooteghem, O. Halo Nevi Are Not Trivial: About 2 Young Patients of Regressed Primary Melnaoma That Simulates Halo Nevi. Case Rep. Derm. 2021, 2021, 6672528. [Google Scholar]
- Speeckaert, R.; Van Geel, N.; Luiten, R.M.; Van Gele, M.; Speeckaert, M.; Lambert, J.; Vermaelen, K.; Tjin, E.P.M.; Borchez, L. Melanocyte-specific Immune Response in a Patient with Multiple Regressing Nevi and a History of Melanoma. Anticancer Res. 2011, 31, 3697–3703. [Google Scholar] [PubMed]
- Musette, P.; Bachelez, H.; Flageul, B.; Delarbre, C.; Kourilsky, P.; Dubertret, L.; Gachelin, G. Immune-Mediated Destruction of Melanocytes in Halo Nevi Is Associated with the Local Expansion of a Limited Number of T Cell Clones. J. Immunol. 1999, 162, 1789–1794. [Google Scholar] [PubMed]
- Botella-Estrada, R.; Kutzner, H. Study of the immunophenotype of the inflammatory cells in melanomas with regression and halo nevi. Am. J. Derm. 2015, 37, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jin, S.A.; Choi, Y.D.; Shin, M.H.; Lee, S.E.; Yun, S.J. Foxp3(+) regulatory T cells are increased in the early stages of halo nevi: Clinicopathological features of 30 halo nevi. Dermatology 2012, 225, 172–178. [Google Scholar] [CrossRef]
- Cioplea, M.; Nichita, L.; Georgescu, D.; Sticlaru, L.; Cioroianu, A.; Nedelcu, R.; Turcu, G.; Rauta, A.; Mogodici, C.; Zurac, S.; et al. FOXP3 in Melanoma with Regression: Between Tumoral Expression and Regulatory T Cell Upregulation. J. Immunol. Res. 2020, 2020. [Google Scholar] [CrossRef]
- Moretti, S.; Spallanzani, A.; Pinzi, C.; Prignano, F.; Fabbri, P. Fibrosis in regressing melanoma versus nonfibrosis in halo nevus upon melanocyte disappearance: Could it be related to a different cytokine microenvironment? J. Cutan. Pathol. 2007, 34, 301–308. [Google Scholar] [CrossRef]
- Martín, J.M.; Rubio, M.; Bella, R.; Jordá, E.; Monteagudo, C. Regresióncompleta de nevosmelanocíticos: Correlaciónclínica, dermatoscópica e histológica de una serie de 13 casos. Actas Dermo-Sifiliográficas 2012, 103, 401–410. [Google Scholar] [CrossRef]
- Uguen, A.; Talagas, M.; Costa, S.; Duigou, S.; Bouvier, S.; De Braekeleer, M.; Marcorelles, P. A p16-Ki-67-HMB45 immunohistochemistry scoring system as an ancillary diagnostic tool in the diagnosis of melanoma. Diagn. Pathol. 2015, 10, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME expression in melanocytic tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.C.; Chen, M.K.; Liao, Q.M.; Mao, R.X.; Han, J.D. Factors Associated with Development of Vitiligo in Patients withHalo Nevus. Chin. Med. J. 2017, 130, 2703–2708. [Google Scholar] [CrossRef]
- Lorentzen, H.F. Eruptive Halo Naevi: A Possible Indicator of Malignant Disease in a Case Series of Post-adolescent Patients. Acta Derm. Venereol. 2020, 100, adv00228. [Google Scholar] [CrossRef]
- Patrizi, A.; Bentivogli, M.; Raone, B.; Dondi, A.; Tabanelli, M.; Neri, I. Association of halo nevus/i and vitiligo in childhood: A retrospective observational study. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e148–e152. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Mu, E.W.; Orlow, S.J. Comparison of Childhood Vitiligo Presenting with or without Associated Halo Nevi. Pediatr Derm. 2016, 33, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, L.Y.; Fu, W.W.; Kang, K.F. Childhood vitiligo in China: Clinical profiles and immunological findings in620 cases. Am. J. Clin. Derm. 2011, 12, 277–281. [Google Scholar] [CrossRef]
- Birnbaum, M.R.; Ma, M.W.; Casey, M.A.; Amin, B.D.; Jacobson, M.; Cheng, H.; McLellan, B.N. Development of Halo Nevi in a Lung Cancer Patient: A Novel Immune-Related Cutaneous Event from Atezolizumab. J. Drugs Derm. 2017, 16, 1047–1049. [Google Scholar]
- Plaquevent, M.; Greliak, A.; Pinard, C.; Duval-Modeste, A.B.; Joly, P. Simultaneous long-lasting regression of multiple nevi and melanoma metastases after ipilimumab therapy. Melanoma Res. 2019, 29, 311–312. [Google Scholar] [CrossRef] [PubMed]







| Global Structure | |
|---|---|
| Symmetric | 16/32 (50%) |
| Asymmetric | 6/32 (18.75%) |
| Regressed | 10/32 (31.25%) |
| Size- mean, median, min, max (mm) | 5,22, 5, 3, 10 |
| Distribution of Pigmentation | |
| Uniform | 16/32 (50%) |
| Centralhyperpigmentedannular type | 2/32 (6.25%) |
| Eccentric hyperpigmented type | 1/32 (3.1%) |
| Multifocalhyper/hypopigmentedtype | 3/32 (9.4 %) |
| No pigmentation | 10/32 (31.25%) |
| Structural Patterns | |
| Globular | 12/32 (37.5%) |
| Regular | 7/32 (21.9 %) |
| Irregular | 4/32 (12.5%) |
| Cobblestone | 1/32 (3.1%) |
| Network | 3/32 (9.4 %) |
| Typical | 1/32 (3.1%) |
| Inverse | 1/32 (3.1%) |
| Patchy Peripheral | 1/32 (3.1%) |
| Homogeneous | 20/32 (62.5%) |
| One Color | 18/32 (56.25%) |
| Two Colors | 2/32 (6.25%) |
| Regression | 25/32 (59.4 %) |
| Partial | 15/32 (46.9 %) |
| Total | 10/32 (31.25%) |
| Local Features | |
| Vascular Pattern—Coma-Like Vessels | 5/32 (15.6%) |
| Poliosis | 14/32 (43.75%) |
| Regression Structures | |
| Blue White Veil | 1/32 (3.1%) |
| Peppering | 2/32 (6.25%) |
| Surrounding Halo | |
| Symmetric | 21/32 (65.6%) |
| Asymmetric | 7/32 (21.9 %) |
| Regional | 2/32 (6.25%) |
| Without | 2/32 (6.25%) |
| Size- mean, median, min, max (mm) | 12.06, 12, 5, 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedelcu, R.; Dobre, A.; Brinzea, A.; Hulea, I.; Andrei, R.; Zurac, S.; Balaban, M.; Antohe, M.; Manea, L.; Calinescu, A.; et al. Current Challenges in Deciphering Sutton Nevi—Literature Review and Personal Experience. J. Pers. Med. 2021, 11, 904. https://doi.org/10.3390/jpm11090904
Nedelcu R, Dobre A, Brinzea A, Hulea I, Andrei R, Zurac S, Balaban M, Antohe M, Manea L, Calinescu A, et al. Current Challenges in Deciphering Sutton Nevi—Literature Review and Personal Experience. Journal of Personalized Medicine. 2021; 11(9):904. https://doi.org/10.3390/jpm11090904
Chicago/Turabian StyleNedelcu, Roxana, Alexandra Dobre, Alice Brinzea, Ionela Hulea, Razvan Andrei, Sabina Zurac, Mihaela Balaban, Mihaela Antohe, Lorena Manea, Andreea Calinescu, and et al. 2021. "Current Challenges in Deciphering Sutton Nevi—Literature Review and Personal Experience" Journal of Personalized Medicine 11, no. 9: 904. https://doi.org/10.3390/jpm11090904