Personalized Nutrition in Patients with Type 2 Diabetes and Chronic Kidney Disease: The Two-Edged Sword of Dietary Protein Intake
Abstract
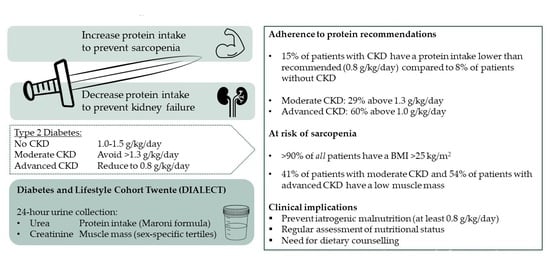
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Renal Function
2.4. Dietary Assessment
2.5. Assessment of Nutritional Status
2.6. Statistical Analysis
3. Results
3.1. Adherence to the Protein Recommendations
3.2. Assessment of Nutritional Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31 (Suppl. 1), S61–S78. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of medical care in diabetes–2021. Diabetes Care 2018, 44 (Suppl. 1), S151–S167. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ. Tech. Rep. Ser. 2007, 935, 1–265. [Google Scholar] [CrossRef]
- American Diabetes Association. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of medical care in diabetes–2021. Diabetes Care 2021, 44 (Suppl. 1), S53–S72. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 73–90. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Oosterwijk, M.M.; Groothof, D.; Navis, G.; Bakker, S.J.; Laverman, G.D. High-Normal Protein Intake Is Not Associated With Faster Renal Function Deterioration in Patients With Type 2 Diabetes: A Prospective Analysis in the DIALECT Cohort. Diabetes Care 2021, 45, 35–41. [Google Scholar] [CrossRef]
- Dunkler, D.; Kohl, M.; Teo, K.K.; Heinze, G.; Dehghan, M.; Clase, C.M.; Gao, P.; Yusuf, S.; Mann, J.F.E.; Oberbauer, R. Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol. Dial. Transplant. 2015, 30 (Suppl. 4), iv76–iv85. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gant, C.M.; Binnenmars, S.H.; van den Berg, E.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LifEstyle Cohort Twente (DIALECT). Nutrients 2017, 9, 709. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Mussap, M.; Vestra, M.D.; Fioretto, P.; Saller, A.; Varagnolo, M.; Nosadini, R.; Plebani, M. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002, 61, 1453–1461. [Google Scholar] [CrossRef]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Wendel-Vos, G.W.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Hagedoorn, I.J.M.; Braber, N.D.; Oosterwijk, M.M.; Gant, C.M.; Navis, G.; Vollenbroek-Hutten, M.M.R.; Van Beijnum, B.-J.F.; Bakker, S.J.L.; Laverman, G.D. Low physical activity in patients with complicated type 2 diabetes mellitus is associated with low muscle mass and low protein intake. J. Clin. Med. 2020, 9, 3104. [Google Scholar] [CrossRef]
- Levey, A.S.; Greene, T.; Beck, G.J.; Caggiula, A.W.; Kusek, J.W.; Hunsicker, L.G.; Klahr, S. Dietary protein restriction and the progression of chronic renal disease: What have all of the results of the MDRD study shown? J. Am. Soc. Nephrol. 1999, 10, 2426–2439. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.; Smits, M.; Van Bommel, E.J.; Heerspink, H.J.L.; Van Raalte, D.H.; Joles, J.A. Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Greene, T.; Sarnak, M.J.; Wang, X.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Kopple, J.D. Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2006, 48, 879–888. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.; Doehner, W.; Fearon, K.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Slee, A.D. Exploring metabolic dysfunction in chronic kidney disease. Nutr. Metab. 2012, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y.; Peng, C.H.; Hung, S.C.; Tarng, D.C. Body composition is associated with clinical outcomes in patients with non–dialysis-dependent chronic kidney disease. Kidney Int. 2018, 93, 733–740. [Google Scholar] [CrossRef]
- Jalving, A.C.; Oosterwijk, M.M.; Hagedoorn, I.J.M.; Navis, G.; Bakker, S.J.L.; Laverman, G.D. Clinical and Dietary Determinants of Muscle Mass in Patients with Type 2 Diabetes: Data from the Diabetes and Lifestyle Cohort Twente. J. Clin. Med. 2021, 10, 5227. [Google Scholar] [CrossRef] [PubMed]
- Gant, C.M.; Mensink, I.; Binnenmars, S.H.; van der Palen, J.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Body weight course in the DIAbetes and LifEstyle Cohort Twente (DIALECT-1)—A 20-year observational study. PLoS ONE 2019, 14, e0218400. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.J.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-D.; Tsauo, J.-Y.; Wu, Y.-T.; Cheng, C.-P.; Chen, H.-C.; Huang, Y.-C.; Chen, H.-C.; Liou, T.-H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef] [Green Version]
- Oosterwijk, M.M.; den Braber, N.; Bakker, S.J.L.; Laverman, G.D. Urinary Creatinine Excretion is an Indicator of Whole Body Physical Performance and Function. J. Cachexia Sarcopenia Muscle, 2022; in press. [Google Scholar]

| Total Population | Mild to No CKD (eGFR > 60) | Moderate CKD (eGFR 30−60) | Advanced CKD (eGFR < 30) | p-Value | |
|---|---|---|---|---|---|
| n | 361 | 246 (68) | 100 (28) | 15 (4) | |
| Age (years) | 63 ± 9 | 61 ± 9 | 68 ± 7 | 70 ± 7 | <0.001 |
| Sex (male) | 209 (58) | 149 (61) | 50 (50) | 10 (67) | 0.15 |
| BMI (kg/m2) | 32.7 ± 5.7 | 32.6 ± 5.7 | 33.2 ± 5.7 | 30.6 ± 4.8 | 0.22 |
| Normal weight (BMI < 25) | 18 (5) | 15 (6) | 2 (2) | 1 (7) | 0.44 |
| Overweight (BMI 25–30) | 105 (29) | 68 (28) | 31 (31) | 6 (40) | |
| Obese (BMI > 30) | 238 (66) | 163 (66) | 67 (67) | 8 (53) | |
| Muscle mass (CER/m2) | 4.64 ± 1.40 | 4.84 ± 1.42 | 4.28 ± 1.28 | 3.93 ± 1.00 | <0.001 |
| Energy intake (kcal/day) | 2004 ± 677 | 2075 ± 704 | 1818 ± 584 | 2072 ± 589 | 0.005 |
| Dietary protein intake (g/kg/day) | 1.22 ± 0.33 | 1.27 ± 0.33 | 1.12 ± 0.29 | 1.05 ± 0.23 | <0.001 |
| <0.8 g/kg/day | 37 (10) | 20 (8) | 15 (15) | 2 (13) | 0.017 |
| 0.8–1.0 g/kg/day | 47 (13) | 25 (10) | 18 (18) | 4 (27) | |
| 1.0–1.3 g/kg/day | 140 (39) | 95 (39) | 38 (38) | 7 (47) | |
| >1.3 g/kg/day | 137 (38) | 106 (43) | 29 (29) | 2 (13) | |
| Adherence to protein recommendations | |||||
| Above recommendations | 62 (17) | 45 (18) | 15 (15) | 2 (13) | 0.006 |
| According to recommendations | 212 (59) | 152 (62) | 56 (56) | 4 (27) | |
| Below recommendations | 87 (24) | 49 (20) | 29 (29) | 9 (60) | |
| Adherence to the Dutch Healthy Exercise Norm | |||||
| No adherence | 150 (42) | 93 (38) | 51(51) | 6 (40) | 0.08 |
| Adherence | 211 (58) | 153 (62) | 49 (49) | 9 (60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosterwijk, M.M.; Navis, G.; Bakker, S.J.L.; Laverman, G.D. Personalized Nutrition in Patients with Type 2 Diabetes and Chronic Kidney Disease: The Two-Edged Sword of Dietary Protein Intake. J. Pers. Med. 2022, 12, 300. https://doi.org/10.3390/jpm12020300
Oosterwijk MM, Navis G, Bakker SJL, Laverman GD. Personalized Nutrition in Patients with Type 2 Diabetes and Chronic Kidney Disease: The Two-Edged Sword of Dietary Protein Intake. Journal of Personalized Medicine. 2022; 12(2):300. https://doi.org/10.3390/jpm12020300
Chicago/Turabian StyleOosterwijk, Milou M., Gerjan Navis, Stephan J. L. Bakker, and Gozewijn D. Laverman. 2022. "Personalized Nutrition in Patients with Type 2 Diabetes and Chronic Kidney Disease: The Two-Edged Sword of Dietary Protein Intake" Journal of Personalized Medicine 12, no. 2: 300. https://doi.org/10.3390/jpm12020300