A Deep Learning Algorithm for Detecting Acute Pericarditis by Electrocardiogram
Abstract
:1. Introduction
2. Method
2.1. Study Population
2.2. Data Source
2.3. The Implementation of the Deep Learning Model
2.4. Human–Machine Competition
2.5. Statistical Analysis
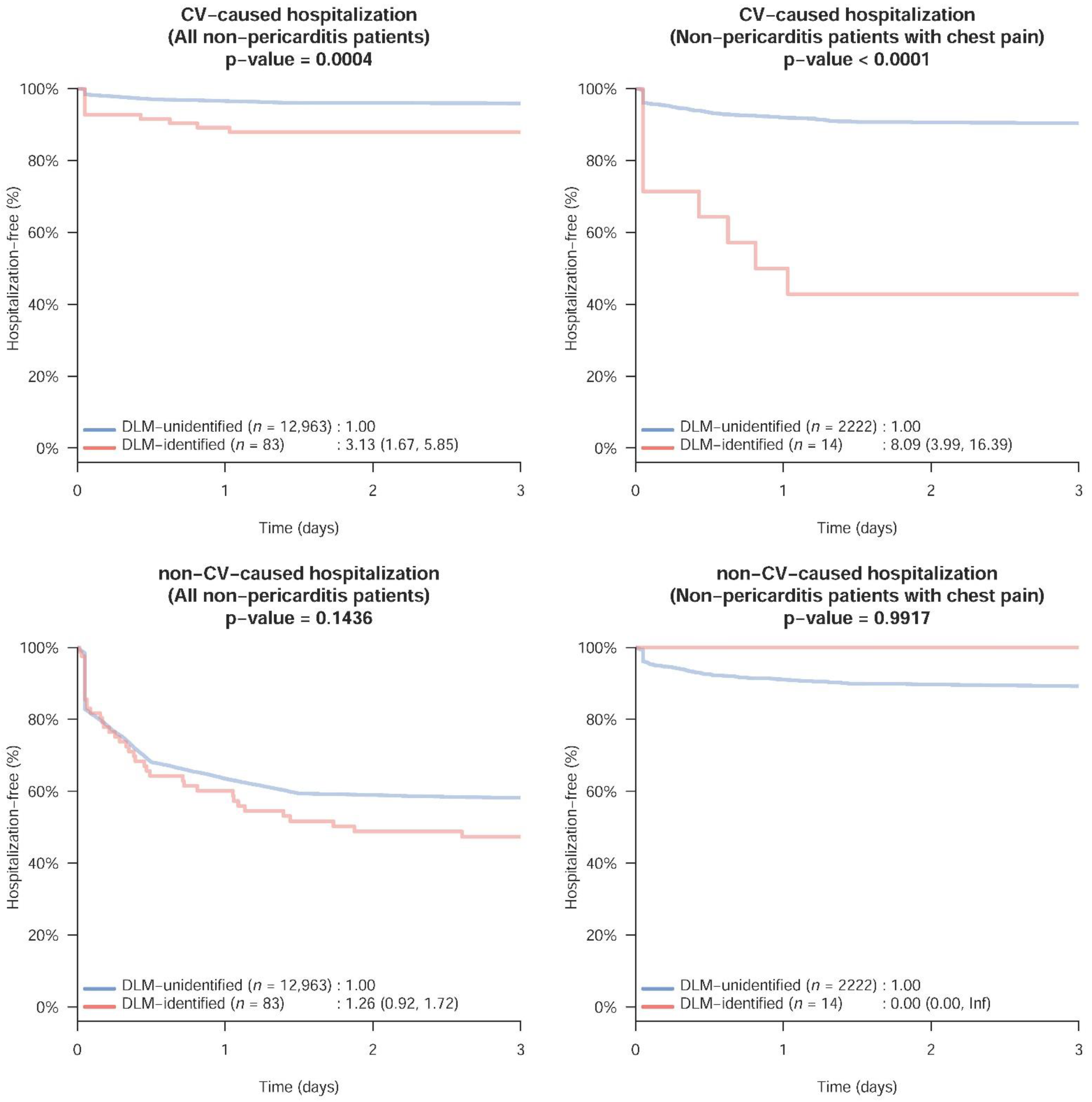
3. Results
4. Discussion
 Central Illustration. The flow diagram of the development, validation, and future application of the pericarditis deep learning model (DLM). The pericarditis-DLM was developed and trained by 39,919 ECGs. The DLM had the best performance compared to the built-in algorithm of ECG machine, another machine-learning model, and human specialists. The DLM achieved a good diagnostic ability, with an AUC of 0.95, a sensitivity of 78.9% and a specificity of 97.7%. The pericarditis-DLM and the previously developed STEMI-DLM were combined to a new strategy, which exhibited an excellent power to differentiate between acute pericarditis and STEMI, with a sensitivity of 73.7% and a positive predictive value of 50%. The applications of our DLM in emergency department, telemedicine and wearable devices may possibly help to improve the healthcare and outcomes of cardiovascular diseases in the future.
Central Illustration. The flow diagram of the development, validation, and future application of the pericarditis deep learning model (DLM). The pericarditis-DLM was developed and trained by 39,919 ECGs. The DLM had the best performance compared to the built-in algorithm of ECG machine, another machine-learning model, and human specialists. The DLM achieved a good diagnostic ability, with an AUC of 0.95, a sensitivity of 78.9% and a specificity of 97.7%. The pericarditis-DLM and the previously developed STEMI-DLM were combined to a new strategy, which exhibited an excellent power to differentiate between acute pericarditis and STEMI, with a sensitivity of 73.7% and a positive predictive value of 50%. The applications of our DLM in emergency department, telemedicine and wearable devices may possibly help to improve the healthcare and outcomes of cardiovascular diseases in the future.Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiabrando, J.G.; Bonaventura, A.; Vecchié, A.; Wohlford, G.F.; Mauro, A.G.; Jordan, J.H.; Grizzard, J.D.; Montecucco, F.; Berrocal, D.H.; Brucato, A.; et al. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015, 314, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Fardman, A.; Charron, P.; Imazio, M.; Adler, Y. European Guidelines on Pericardial Diseases: A Focused Review of Novel Aspects. Curr. Cardiol. Rep. 2016, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Maestroni, S.; Cumetti, D.; Belli, R.; Trinchero, R.; Adler, Y. Risk of Constrictive Pericarditis after Acute Pericarditis. Circulation 2011, 124, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [PubMed]
- Imazio, M.; Brucato, A.; Spodick, D.H.; Adler, Y. Prognosis of myopericarditis as determined from previously published reports. J. Cardiovasc. Med. 2014, 15, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Mlllaire, A.; de Groote, P.; Decoulx, E.; Leroy, O.; Ducloux, G. Outcome after thrombolytic therapy of nine cases of myopericarditis misdiagnosed as myocardial infarction. Eur. Heart J. 1995, 16, 333–338. [Google Scholar] [CrossRef]
- Fanari, Z.; Abraham, N.; Kolm, P.; Doorey, J.; Herman, A.; Hoban, A.; Reddy, V.; Hammami, S.; Leonovich, J.; Rahman, E.; et al. Aggressive Measures to Decrease “Door to Balloon” Time and Incidence of Unnecessary Cardiac Catheterization: Potential Risks and Role of Quality Improvement. Mayo Clin. Proc. 2015, 90, 1614–1622. [Google Scholar] [CrossRef] [Green Version]
- Salisbury, A.C.; Olalla-Gómez, C.; Rihal, C.S.; Bell, M.R.; Ting, H.H.; Casaclang-Verzosa, G.; Oh, J.K. Frequency and Predictors of Urgent Coronary Angiography in Patients with Acute Pericarditis. Mayo Clin. Proc. 2009, 84, 11–15. [Google Scholar] [CrossRef]
- McNamara, N.; Ibrahim, A.; Satti, Z.; Ibrahim, M.; Kiernan, T.J. Acute pericarditis: A review of current diagnostic and management guidelines. Future Cardiol. 2019, 15, 119–126. [Google Scholar] [CrossRef]
- Shoaib, M.; Huish, W.; Woollard, E.L.; Aguila, J.; Coxall, D.; Alexander, M.; Hicks, D.; McQuillan, B. Impact of Pre-Hospital Activation of STEMI on False Positive Activation Rate and Door to Balloon Time. Heart Lung Circ. 2022, 31, 447–455. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.E.; Al Ali, A.S.; Brady, W.J.; Ghaemmaghami, C.A.; Menon, V.; Welsford, M.; Shuster, M. Part 9: Acute Coronary Syndromes: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132 (Suppl. 2), S483–S500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosson, N.; Sanko, S.; Stickney, R.E.; Niemann, J.; French, W.J.; Jollis, J.G.; Kontos, M.C.; Taylor, T.; Macfarlane, P.W.; Tadeo, R.; et al. Causes of Prehospital Misinterpretations of ST Elevation Myocardial Infarction. Prehospital. Emerg. Care 2017, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Schläpfer, J.; Wellens, H.J. Computer-Interpreted Electrocardiograms: Benefits and Limitations. J. Am. Coll. Cardiol. 2017, 70, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Lin, C.-S.; Tsao, T.-P.; Lee, C.-C.; Cheng, C.-C.; Chen, J.-T.; Tsai, C.-S.; Lin, W.-S. A Deep-Learning Algorithm-Enhanced System Integrating Electrocardiograms and Chest X-rays for Diagnosing Aortic Dissection. Can. J. Cardiol. 2022, 38, 160–168. [Google Scholar] [CrossRef]
- Hsiang, C.-W.; Lin, C.; Liu, W.-C.; Chang, W.-C.; Hsu, H.-H.; Huang, G.-S.; Lou, Y.-S.; Lee, C.-C.; Wang, C.-H.; Fang, W.-H. Detection of Left Ventricular Systolic Dysfunction Using an Artificial Intelligence–Enabled Chest X-Ray. Can. J. Cardiol. 2022, 38, 763–773. [Google Scholar] [CrossRef]
- Lou, Y.-S.; Lin, C.-S.; Fang, W.-H.; Lee, C.-C.; Ho, C.-L.; Wang, C.-H.; Lin, C. Artificial Intelligence-Enabled Electrocardiogram Estimates Left Atrium Enlargement as a Predictor of Future Cardiovascular Disease. J. Pers. Med. 2022, 12, 315. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lin, C.-S.; Fang, W.-H.; Lou, Y.-S.; Cheng, C.-C.; Lee, C.-C.; Lin, C. Artificial Intelligence-Enabled Electrocardiography Predicts Left Ventricular Dysfunction and Future Cardiovascular Outcomes: A Retrospective Analysis. J. Pers. Med. 2022, 12, 455. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, C.; Yin, H.; Li, X.; Zuo, P.; Ding, J.; Lin, F.; Wang, J.; Zhou, B.; Li, Y.; et al. Automatic multilabel electrocardiogram diagnosis of heart rhythm or conduction abnormalities with deep learning: A cohort study. Lancet Digit. Health 2020, 2, e348–e357. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Wu, L.; Huang, G.; Yu, X.; Ye, M.; Liu, L.; Ling, Y.; Liu, X.; Liu, D.; Zhou, B.; Liu, Y.; et al. Deep Learning Networks Accurately Detect ST-Segment Elevation Myocardial Infarction and Culprit Vessel. Front. Cardiovasc. Med. 2022, 9, 797207. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Lin, C.-S.; Tsai, C.-S.; Tsao, T.-P.; Cheng, C.-C.; Liou, J.-T.; Lin, W.-S.; Cheng, S.-M.; Lou, Y.-S.; Lee, C.-C.; et al. A deep learning algorithm for detecting acute myocardial infarction. EuroIntervention 2021, 17, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Lee, Y.T.; Fang, W.H.; Lou, Y.S.; Kuo, F.C.; Lee, C.C.; Lin, C. Deep Learning Algorithm for Management of Diabetes Mellitus via Electrocardiogram-Based Glycated Hemoglobin (ECG-HbA1c): A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 725. [Google Scholar] [CrossRef]
- Lin, C.S.; Fang, W.-H.; Hsu, C.-J.; Chen, S.-J.; Huang, K.-H.; Lin, W.-S.; Tsai, C.-S.; Kuo, C.-C.; Chau, T.; Yang, S.J.; et al. A Deep-Learning Algorithm (ECG12Net) for Detecting Hypokalemia and Hyperkalemia by Electrocardiography: Algorithm Development. JMIR Med. Inform. 2020, 8, e15931. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-W.; Lin, C.-S.; Tsao, T.-P.; Lee, C.-C.; Chen, J.-T.; Tsai, C.-S.; Lin, W.-S.; Lin, C. Detecting Digoxin Toxicity by Artificial Intelligence-Assisted Electrocardiography. Int. J. Environ. Res. Public Health 2021, 18, 3839. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Peterson, L.R.; Soto, P.F.; Herrero, P.; Mohammed, B.S.; Avidan, M.; Schechtman, K.B.; Dence, C.; Gropler, R.J. Impact of Gender on the Myocardial Metabolic Response to Obesity. JACC Cardiovasc. Imaging 2008, 1, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-C.; Lin, C.-S.; Tsai, C.-S.; Tsao, T.-P.; Cheng, C.-C.; Liou, J.-T.; Lin, W.-S.; Lee, C.-C.; Chen, J.-T.; Lin, C. A deep learning-based system capable of detecting pneumothorax via electrocardiogram. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef]
- Liu, W.-C.; Lin, C.; Lin, C.-S.; Tsai, M.-C.; Chen, S.-J.; Tsai, S.-H.; Lin, W.-S.; Lee, C.-C.; Tsao, T.-P.; Cheng, C.-C. An Artificial Intelligence-Based Alarm Strategy Facilitates Management of Acute Myocardial Infarction. J. Pers. Med. 2021, 11, 1149. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Behr, E.R.; Friedman, P.A. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021, 42, 4717–4730. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F. Diagnosis and treatment of pericarditis. Heart 2015, 101, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spodick, D.H. Electrocardiogram in acute pericarditis: Distributions of morphologic and axial changes by stages. Am. J. Cardiol. 1974, 33, 470–474. [Google Scholar] [CrossRef]
- Ariyarajah, V.; Spodick, D.H. Acute pericarditis: Diagnostic cues and common electrocardiographic manifestations. Cardiol. Rev. 2007, 15, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.R.; Gersh, B.J.; Stuckey, T.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Dudek, D.; Grines, C.L.; Cox, D.; Parise, H.; et al. When Is Door-to-Balloon Time Critical? Analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) Trials. J. Am. Coll. Cardiol. 2010, 56, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, H.; Nakagawa, Y.; Morimoto, T.; Furukawa, Y.; Nakano, A.; Shirai, S.; Taniguchi, R.; Yamaji, K.; Nagao, K.; Suyama, T.; et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: Observational study. BMJ 2012, 344, e3257. [Google Scholar] [CrossRef] [Green Version]
- Solhpour, A.; Chang, K.-W.; Arain, S.A.; Balan, P.; Loghin, C.; McCarthy, J.J.; Anderson, H.V.; Smalling, R.W. Ischemic time is a better predictor than door-to-balloon time for mortality and infarct size in ST-elevation myocardial infarction. Catheter. Cardiovasc. Interv. 2016, 87, 1194–1200. [Google Scholar] [CrossRef]
- Raghunath, S.; Cerna, A.E.U.; Jing, L.; Vanmaanen, D.P.; Stough, J.; Hartzel, D.N.; Leader, J.B.; Kirchner, H.L.; Stumpe, M.C.; Hafez, A.; et al. Prediction of mortality from 12-lead electrocardiogram voltage data using a deep neural network. Nat. Med. 2020, 26, 886–891. [Google Scholar] [CrossRef]
- Raghunath, S.; Pfeifer, J.M.; Ulloa-Cerna, A.E.; Nemani, A.; Carbonati, T.; Jing, L.; Vanmaanen, D.P.; Hartzel, D.N.; Ruhl, J.A.; Lagerman, B.F.; et al. Deep Neural Networks Can Predict New-Onset Atrial Fibrillation From the 12-Lead ECG and Help Identify Those at Risk of Atrial Fibrillation–Related Stroke. Circulation 2021, 143, 1287–1298. [Google Scholar] [CrossRef]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef]
- Han, C.; Kang, K.-W.; Kim, T.Y.; Uhm, J.-S.; Park, J.-W.; Jung, I.H.; Kim, M.; Bae, S.; Lim, H.-S.; Yoon, D. Artificial Intelligence-Enabled ECG Algorithm for the Prediction of Coronary Artery Calcification. Front. Cardiovasc. Med. 2022, 9, 849223. [Google Scholar] [CrossRef]
- Lin, C.; Chau, T.; Shang, H.-S.; Fang, W.-H.; Lee, D.-J.; Lee, C.-C.; Tsai, S.-H.; Wang, C.-H.; Lin, S.-H. Point-of-care artificial intelligence-enabled ECG for dyskalemia: A retrospective cohort analysis for accuracy and outcome prediction. NPJ Digit. Med. 2022, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.D.; Hu, K.M.; Westreich, A.A.; Tewelde, S.; Farzad, A.; Mattu, A. Evaluation of Spodick’s Sign and Other Electrocardiographic Findings as Indicators of STEMI and Pericarditis. J. Emerg. Med. 2020, 58, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Lazaros, G.; Anastassopoulou, C.; Hatziantoniou, S.; Kalos, T.; Soulaidopoulos, S.; Lazarou, E.; Vlachopoulos, C.; Vassilopoulos, D.; Tsakris, A.; Tsioufis, C. A case series of acute pericarditis following COVID-19 vaccination in the context of recent reports from Europe and the United States. Vaccine 2021, 39, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Pepe, S.; Gregory, A.T.; Denniss, A.R. Myocarditis, Pericarditis and Cardiomyopathy After COVID-19 Vaccination. Heart Lung Circ. 2021, 30, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Bokolo, A.J. Exploring the adoption of telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic. Ir. J. Med Sci. 2021, 190, 1–10. [Google Scholar] [CrossRef]
- Mastoris, I.; Sauer, A.J. Opening the “Black Box” of Artificial Intelligence for Detecting Heart Failure. ASAIO J. 2021, 67, 322–323. [Google Scholar] [CrossRef]



| Development Set | Tuning Set | Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pericarditis (n = 99) | Non-Pericarditis (n = 39,820) | p Value | Pericarditis (n = 10) | Non-Pericarditis (n = 13,767) | p Value | Pericarditis (n = 19) | Non-Pericarditis (n = 13,046) | p Value | |
| Clinical features | |||||||||
| Chest pain | 99 (100.0%) | 7637 (19.2%) | <0.001 | 10 (100.0%) | 2316 (16.8%) | <0.001 | 19 (100.0%) | 2236 (17.1%) | <0.001 |
| STEMI | 6 (6.1%) | 665 (1.7%) | 0.007 | 0 (0.0%) | 42 (0.3%) | 1.000 | 0 (0.0%) | 49 (0.4%) | 1.000 |
| Demographic data | |||||||||
| Gender (male) | 81 (81.8%) | 20,983 (52.7%) | <0.001 | 7 (70.0%) | 7002 (50.9%) | 0.344 | 15 (78.9%) | 7058 (54.1%) | 0.030 |
| Age (years) | 43.9 ± 18.7 | 62.1 ± 19.6 | <0.001 | 35.9 ± 1.1 | 66.0 ± 18.8 | <0.001 | 51.7 ± 22.9 | 66.2 ± 18.4 | 0.007 |
| BMI (kg/m2) | 24.4 ± 4.3 | 24.3 ± 5.8 | 0.977 | 22.9 ± 3.1 | 24.3 ± 6.5 | 0.491 | 26.5 ± 3.2 | 24.4 ± 6.0 | 0.056 |
| Disease history | |||||||||
| AMI | 11 (11.1%) | 2136 (5.4%) | 0.011 | 0 (0.0%) | 725 (5.3%) | 1.000 | 0 (0.0%) | 833 (6.4%) | 0.630 |
| Stroke | 7 (7.1%) | 6697 (16.8%) | 0.010 | 0 (0.0%) | 3205 (23.3%) | 0.130 | 1 (5.3%) | 3622 (27.8%) | 0.029 |
| CAD | 28 (28.3%) | 9828 (24.7%) | 0.407 | 3 (30.0%) | 4241 (30.8%) | 1.000 | 6 (31.6%) | 4871 (37.3%) | 0.604 |
| HF | 0 (0.0%) | 3568 (9.0%) | 0.002 | 0 (0.0%) | 2076 (15.1%) | 0.377 | 1 (5.3%) | 2489 (19.1%) | 0.152 |
| AF | 0 (0.0%) | 2722 (6.8%) | 0.007 | 0 (0.0%) | 1401 (10.2%) | 0.613 | 1 (5.3%) | 1299 (10.0%) | 1.000 |
| DM | 6 (6.1%) | 9387 (23.6%) | <0.001 | 0 (0.0%) | 4442 (32.3%) | 0.037 | 1 (5.3%) | 4900 (37.6%) | 0.004 |
| HTN | 19 (19.2%) | 15,111 (37.9%) | <0.001 | 0 (0.0%) | 7008 (50.9%) | 0.001 | 6 (31.6%) | 7284 (55.8%) | 0.033 |
| CKD | 1 (1.0%) | 4512 (11.3%) | 0.001 | 0 (0.0%) | 2795 (20.3%) | 0.229 | 1 (5.3%) | 3047 (23.4%) | 0.098 |
| HLP | 16 (16.2%) | 11,463 (28.8%) | 0.006 | 4 (40.0%) | 4984 (36.2%) | 0.755 | 0 (0.0%) | 5144 (39.4%) | <0.001 |
| COPD | 15 (15.2%) | 6533 (16.4%) | 0.736 | 3 (30.0%) | 3314 (24.1%) | 0.712 | 1 (5.3%) | 3581 (27.4%) | 0.030 |
| Laboratory test | |||||||||
| eGFR (ml/min) | 97.4 ± 32.1 | 77.2 ± 39.5 | <0.001 | 94.9 ± 9.0 | 70.4 ± 40.6 | 0.010 | 123.6 ± 74.7 | 69.6 ± 42.5 | <0.001 |
| Cr (mg/dL) | 1.0 ± 0.8 | 1.5 ± 1.9 | 0.016 | 0.9 ± 0.2 | 1.8 ± 2.3 | 0.251 | 0.8 ± 0.2 | 1.9 ± 2.4 | 0.006 |
| BUN (mg/dL) | 16.3 ± 8.8 | 24.9 ± 22.3 | 0.003 | 11.3 ± 3.5 | 28.1 ± 24.6 | <0.001 | 13.9 ± 4.9 | 28.6 ± 25.8 | 0.001 |
| Na+ (mmol/L) | 135.8 ± 3.3 | 136.7 ± 5.1 | 0.101 | 135.2 ± 1.5 | 136.4 ± 5.1 | 0.049 | 135.8 ± 2.5 | 136.3 ± 5.5 | 0.144 |
| K+ (mmol/L) | 3.8 ± 0.5 | 3.9 ± 0.6 | 0.166 | 4.2 ± 0.7 | 4.0 ± 0.7 | 0.461 | 4.0 ± 0.5 | 4.0 ± 0.7 | 0.696 |
| Cl− (mmol/L) | 101.4 ± 4.4 | 102.6 ± 5.9 | 0.202 | 100.8 ± 2.3 | 102.2 ± 5.8 | 0.128 | 107.6 ± 3.1 | 102.0 ± 6.2 | 0.012 |
| tCa++ (mg/dL) | 8.6 ± 0.6 | 8.5 ± 0.7 | 0.769 | 8.4 ± 0.3 | 8.6 ± 0.8 | 0.724 | 8.2 ± 0.4 | 8.6 ± 0.7 | 0.014 |
| Mg++ (mg/dL) | 1.9 ± 0.3 | 2.1 ± 0.4 | 0.029 | 2.2 ± 0.2 | 2.1 ± 0.4 | 0.103 | 2.0 ± 0.0 | 2.1 ± 0.4 | 0.528 |
| TnI (pg/mL) | 1912.7 ± 3393.7 | 607.7 ± 5459.5 | 0.049 | 125.6 ± 109.7 | 240.4 ± 2545.5 | 0.027 | 1073.6 ± 3789.5 | 245.5 ± 2863.0 | 0.622 |
| CK (U/L) | 217.7 ± 226.0 | 222.1 ± 811.5 | 0.965 | 69.6 ± 15.4 | 186.7 ± 766.2 | 0.160 | 181.2 ± 210.7 | 168.1 ± 712.5 | 0.104 |
| BNP (pg/mL) | 361.1 ± 415.1 | 528.4 ± 938.9 | 0.314 | 33.2 ± 28.5 | 569.7 ± 987.0 | 0.015 | 144.3 ± 39.3 | 673.1 ± 1120.2 | 0.642 |
| GLU (gm/dL) | 115.2 ± 18.5 | 150.3 ± 88.0 | 0.024 | 109.2 ± 24.0 | 149.2 ± 80.9 | 0.109 | 120.0 ± 20.7 | 151.8 ± 89.4 | 0.258 |
| Hb (g/dL) | 13.9 ± 2.1 | 12.7 ± 2.4 | <0.001 | 14.8 ± 2.2 | 12.3 ± 2.5 | 0.002 | 14.1 ± 1.9 | 12.1 ± 2.5 | 0.001 |
| WBC (103/uL) | 11.8 ± 4.3 | 9.5 ± 6.2 | 0.002 | 10.3 ± 6.0 | 9.3 ± 4.7 | 0.660 | 12.9 ± 5.5 | 9.2 ± 7.0 | 0.001 |
| PLT (103/uL) | 216.8 ± 72.7 | 238.0 ± 90.4 | 0.047 | 281.2 ± 64.6 | 233.8 ± 92.0 | 0.037 | 200.0 ± 49.2 | 230.3 ± 95.2 | 0.144 |
| AST (U/L) | 43.1 ± 46.0 | 52.6 ± 174.4 | 0.632 | 29.9 ± 18.7 | 40.5 ± 117.2 | 0.652 | 25.3 ± 20.6 | 44.3 ± 137.8 | 0.083 |
| ALT (U/L) | 60.6 ± 123.1 | 36.1 ± 126.7 | 0.180 | 40.3 ± 37.7 | 31.1 ± 81.4 | 0.804 | 23.6 ± 8.5 | 34.3 ± 122.6 | 0.141 |
| TG (gm/dL) | 108.2 ± 35.8 | 126.0 ± 142.1 | 0.532 | 235.0 ± 0.0 | 122.2 ± 146.6 | 0.030 | 69.6 ± 14.6 | 120.8 ± 132.4 | 0.041 |
| TC (gm/dL) | 135.9 ± 32.3 | 153.0 ± 48.4 | 0.003 | 174.1 ± 22.1 | 149.6 ± 48.8 | 0.016 | 137.0 ± 16.3 | 148.0 ± 45.6 | 0.416 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-L.; Lin, C.-S.; Cheng, C.-C.; Lin, C. A Deep Learning Algorithm for Detecting Acute Pericarditis by Electrocardiogram. J. Pers. Med. 2022, 12, 1150. https://doi.org/10.3390/jpm12071150
Liu Y-L, Lin C-S, Cheng C-C, Lin C. A Deep Learning Algorithm for Detecting Acute Pericarditis by Electrocardiogram. Journal of Personalized Medicine. 2022; 12(7):1150. https://doi.org/10.3390/jpm12071150
Chicago/Turabian StyleLiu, Yu-Lan, Chin-Sheng Lin, Cheng-Chung Cheng, and Chin Lin. 2022. "A Deep Learning Algorithm for Detecting Acute Pericarditis by Electrocardiogram" Journal of Personalized Medicine 12, no. 7: 1150. https://doi.org/10.3390/jpm12071150






