Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of OIR
2.3. Cell Culture and Treatment
2.4. Immunostaining
2.5. RNA Sequencing Analysis
2.5.1. Single-Cell RNA Sequencing
2.5.2. Bulk RNA Sequencing
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blotting
2.8. Cell Proliferation Assay
2.9. Cell Migration Assay
2.10. OIR Lesion Assessment
2.11. ERG Analysis
2.12. Statistics
3. Results
3.1. Spp1 Is Characteristically Upregulated in Microglia in OIR
3.2. Expression of Spp1 Is Regulated by HYPOXIA-Inducible Factor 1 (HIF-1) Signaling Pathway and NF-Kappa B (NF-κB) Signaling Pathway
3.3. Spp1 Mediates Microglia-EC Interaction and Promotes EC Proliferation and Migration In Vitro
3.4. Spp1 Activates Kit/Akt/mTOR Signaling Cascade in EC
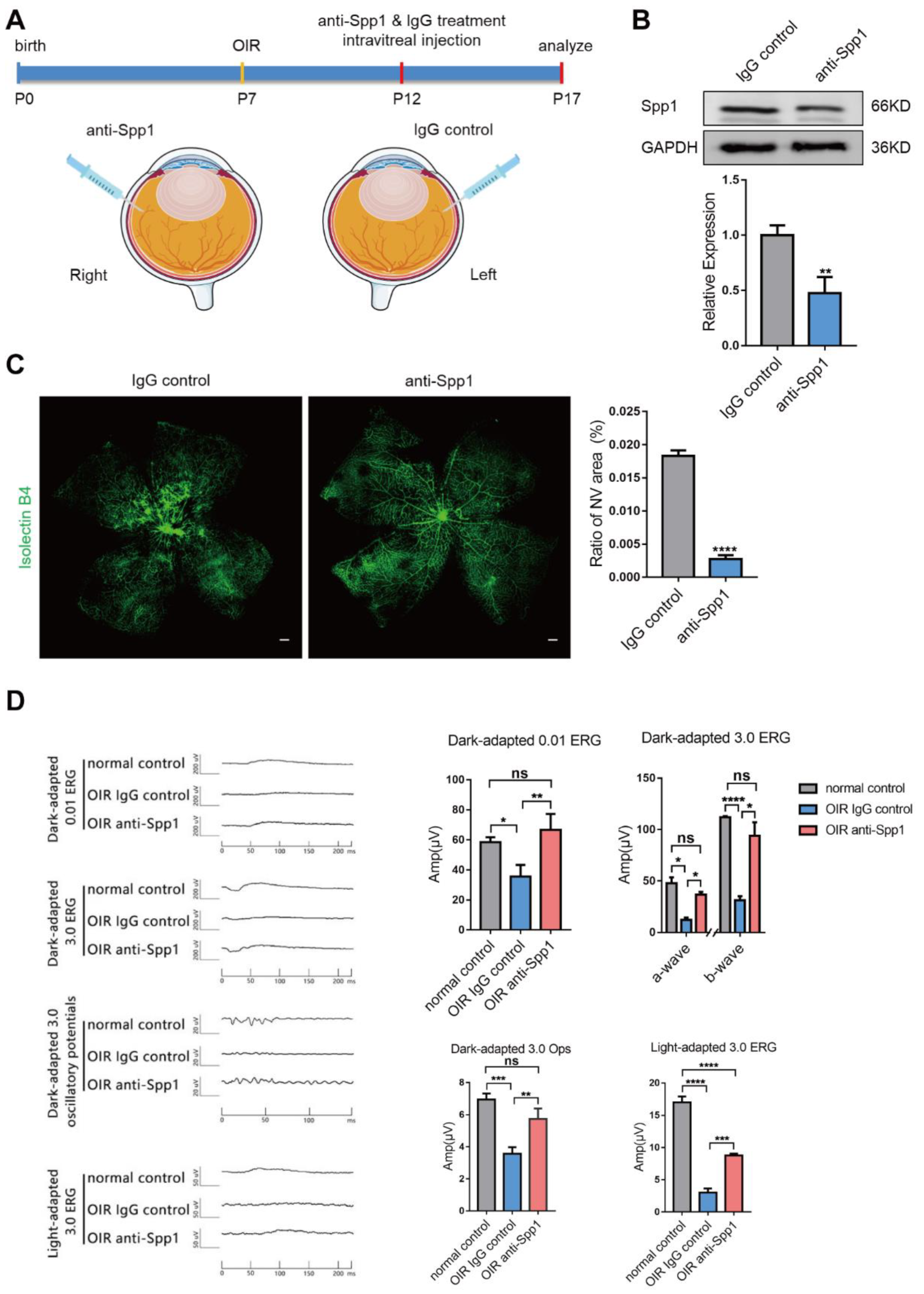
3.5. Interference of Spp1 Inhibits RNV and Alleviate Visual Injury in OIR Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usui, Y.; Westenskow, P.D.; Murinello, S.; Dorrell, M.I.; Scheppke, L.; Bucher, F.; Sakimoto, S.; Paris, L.P.; Aguilar, E.; Friedlander, M. Angiogenesis and Eye Disease. Annu. Rev. Vis. Sci. 2015, 1, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef] [PubMed]
- Rathnasamy, G.; Foulds, W.S.; Ling, E.A.; Kaur, C. Retinal microglia—A key player in healthy and diseased retina. Prog. Neurobiol. 2019, 173, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodero, M.; Pezard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Mao, X.; Chen, M.; Wu, X.; Zhu, T.; Liu, Y.; Zhang, Z.; Fan, W.; Xie, P.; Yuan, S.; et al. Single-Cell Transcriptomics Reveals Novel Role of Microglia in Fibrovascular Membrane of Proliferative Diabetic Retinopathy. Diabetes 2022, 71, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Usui-Ouchi, A.; Usui, Y.; Kurihara, T.; Aguilar, E.; Dorrell, M.I.; Ideguchi, Y.; Sakimoto, S.; Bravo, S.; Friedlander, M. Retinal microglia are critical for subretinal neovascular formation. JCI Insight 2020, 5, e137317. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Suda, T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends. Cardiovasc. Med. 2009, 19, 38–43. [Google Scholar] [CrossRef]
- Xu, W.Q.; Wang, Y.S. The role of Toll-like receptors in retinal ischemic diseases. Int. J. Ophthalmol. 2016, 9, 1343–1351. [Google Scholar] [CrossRef]
- Yin, J.; Xu, W.Q.; Ye, M.X.; Zhang, Y.; Wang, H.Y.; Zhang, J.; Li, Y.; Wang, Y.S. Up-regulated basigin-2 in microglia induced by hypoxia promotes retinal angiogenesis. J. Cell Mol. Med. 2017, 21, 3467–3480. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Liu, Y.; Yang, Z.; Ni, B.; Zhu, X.; Huang, Z.; Xu, H.; Feng, Q.; Lin, X.; He, C.; et al. IL-17 signaling induces iNOS+ microglia activation in retinal vascular diseases. Glia 2021, 69, 2644–2657. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Huang, Z.; Yang, Z.; Zhou, T.; Liu, S.; Hao, Z.; Wang, J.; Feng, Q.; Liu, Y.; et al. A specific RIP3(+) subpopulation of microglia promotes retinopathy through a hypoxia-triggered necroptotic mechanism. Proc. Natl. Acad. Sci. USA 2021, 118, e2023290118. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Fernandes, R.; Santiago, A.R.; Ambrósio, A.F. Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration. Cells 2020, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Scatena, M.; Almeida, M.; Chaisson, M.L.; Fausto, N.; Nicosia, R.F.; Giachelli, C.M. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J. Cell Biol. 1998, 141, 1083–1093. [Google Scholar] [CrossRef]
- Liaw, L.; Almeida, M.; Hart, C.E.; Schwartz, S.M.; Giachelli, C.M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ. Res. 1994, 74, 214–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giachelli, C.M.; Lombardi, D.; Johnson, R.J.; Murry, C.E.; Almeida, M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J. Pathol. 1998, 152, 353–358. [Google Scholar] [PubMed]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Puputti, M.; Tynninen, O.; Pernilä, P.; Salmi, M.; Jalkanen, S.; Paetau, A.; Sihto, H.; Joensuu, H. Expression of KIT receptor tyrosine kinase in endothelial cells of juvenile brain tumors. Brain Pathol. 2010, 20, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Wu, Y.L.; Chen, B.J.; Zhang, W.; Tanaka, Y.; Sugiyama, H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int. J. Biol. Sci. 2013, 9, 435–443. [Google Scholar] [CrossRef]
- Sun, L.; Hui, A.M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R.; et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef]
- Matsui, J.; Wakabayashi, T.; Asada, M.; Yoshimatsu, K.; Okada, M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J. Biol. Chem. 2004, 279, 18600–18607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.L.; Seo, S.; Kim, J.T.; Kim, J.; Kim, W.; Yeo, Y.; Sung, J.H.; Park, S.G.; Suh, W. SCF (Stem Cell Factor) and cKIT Modulate Pathological Ocular Neovascularization. Arter. Thromb. Vasc. Biol. 2019, 39, 2120–2131. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Chang, T.F.; Li, M.H.; Sun, L.J.; Yan, X.C.; Yang, Z.Y.; Liu, Y.; Xu, W.Q.; Lv, Y.; Su, J.B.; et al. SNAI1, an endothelial-mesenchymal transition transcription factor, promotes the early phase of ocular neovascularization. Angiogenesis 2018, 21, 635–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Jing, Y.; Niu, Y.; Chang, T.; Sun, J.; Guo, C.; Wang, Y.; Dou, G. Distinguished Functions of Microglia in the Two Stages of Oxygen-Induced Retinopathy: A Novel Target in the Treatment of Ischemic Retinopathy. Life 2022, 12, 1676. [Google Scholar] [CrossRef]
- Xu, W.; Yin, J.; Sun, L.; Hu, Z.; Dou, G.; Zhang, Z.; Wang, H.; Guo, C.; Wang, Y. Impact of minocycline on vascularization and visual function in an immature mouse model of ischemic retinopathy. Sci. Rep. 2017, 7, 7535. [Google Scholar] [CrossRef] [Green Version]
- Dou, G.R.; Li, N.; Chang, T.F.; Zhang, P.; Gao, X.; Yan, X.C.; Liang, L.; Han, H.; Wang, Y.S. Myeloid-Specific Blockade of Notch Signaling Attenuates Choroidal Neovascularization through Compromised Macrophage Infiltration and Polarization in Mice. Sci. Rep. 2016, 6, 28617. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.X.; Dou, G.R.; Yang, Z.Y.; Liang, L.; Duan, J.L.; Ruan, B.; Li, M.H.; Chang, T.F.; Xu, X.Y.; Chen, J.J.; et al. Notch activation promotes endothelial quiescence by repressing MYC expression via miR-218. Mol. Ther. Nucleic. Acids 2021, 25, 554–566. [Google Scholar] [CrossRef]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Fischer, F.; Martin, G.; Agostini, H.T. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J. Neuroinflammation 2011, 8, 120. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Z.; Lv, Y.; Dou, G.; Zhang, Z.; Wang, H.; Wang, Y. Microglial density determines the appearance of pathological neovascular tufts in oxygen-induced retinopathy. Cell Tissue Res. 2018, 374, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, X.; Zhong, Y. The Effect of Osteopontin on Microglia. Biomed. Res. Int. 2017, 2017, 1879437. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Kim, H.L.; Choi, J.S.; Choi, J.Y.; Cha, J.H.; Lee, M.Y. Osteopontin: Correlation with phagocytosis by brain macrophages in a rat model of stroke. Glia 2011, 59, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Duvall, C.L.; Weiss, D.; Robinson, S.T.; Alameddine, F.M.; Guldberg, R.E.; Taylor, W.R. The role of osteopontin in recovery from hind limb ischemia. Arter. Thromb. Vasc. Biol. 2008, 28, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyle, A.N.; Joseph, G.; Fan, A.E.; Weiss, D.; Landázuri, N.; Taylor, W.R. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arter. Thromb. Vasc. Biol. 2012, 32, 1383–1391. [Google Scholar] [CrossRef] [Green Version]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef]
- Koshikawa, M.; Aizawa, K.; Kasai, H.; Izawa, A.; Tomita, T.; Kumazaki, S.; Tsutsui, H.; Koyama, J.; Shimodaira, S.; Takahashi, M.; et al. Elevated osteopontin levels in patients with peripheral arterial disease. Angiology 2009, 60, 42–45. [Google Scholar] [CrossRef]
- Schlecht, A.; Zhang, P.; Wolf, J.; Thien, A.; Rosmus, D.D.; Boneva, S.; Schlunck, G.; Lange, C.; Wieghofer, P. Secreted Phosphoprotein 1 Expression in Retinal Mononuclear Phagocytes Links Murine to Human Choroidal Neovascularization. Front. Cell Dev. Biol. 2020, 8, 618598. [Google Scholar] [CrossRef]
- Fujita, N.; Fujita, S.; Ogata, N.; Matsuoka, M.; Okada, Y.; Kon, S.; Uede, T.; Saika, S. Endogenous osteopontin involvement in laser-induced choroidal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9310–9315. [Google Scholar] [CrossRef]
- Beguier, F.; Housset, M.; Roubeix, C.; Augustin, S.; Zagar, Y.; Nous, C.; Mathis, T.; Eandi, C.; Benchaboune, M.; Drame-Maigné, A.; et al. The 10q26 Risk Haplotype of Age-Related Macular Degeneration Aggravates Subretinal Inflammation by Impairing Monocyte Elimination. Immunity 2020, 53, 429–441.e428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Johnson, J.N.; Singh, K.; Singh, M. Impairment of myocardial angiogenic response in the absence of osteopontin. Microcirculation 2007, 14, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.S.; Malek, G.; Espinosa-Heidmann, D.G.; Wu, K.; Saloupis, P.; Spiga, M.G.; Cousins, S.W. Osteopontin Drives Fibrosis in a Mouse Model of Neovascular Age Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 942. [Google Scholar]
- Wang, Y.; Yan, W.; Lu, X.; Qian, C.; Zhang, J.; Li, P.; Shi, L.; Zhao, P.; Fu, Z.; Pu, P.; et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur. J. Cell Biol. 2011, 90, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Cao, Y.; Wang, D.; Lv, Y.; Liu, Y.; Gu, X.; Wang, Y.; Wang, X.; Yu, B. Single-cell sequencing reveals microglia induced angiogenesis by specific subsets of endothelial cells following spinal cord injury. Faseb. J. 2022, 36, e22393. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Behera, R.; Ahmed, M.; Sharma, P.; Kumar, V.; Kundu, G.C. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr. Mol. Med. 2006, 6, 819–830. [Google Scholar] [CrossRef]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arter. Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Li, L.; Yao, J.Q.; Chen, X.; Liu, Q.H. Osteopontin expression in vitreous and proliferative retinal membranes of patients with proliferative vitreous retinopathy. Int. J. Ophthalmol. 2011, 4, 406–409. [Google Scholar] [CrossRef]
- Rosmus, D.D.; Lange, C.; Ludwig, F.; Ajami, B.; Wieghofer, P. The Role of Osteopontin in Microglia Biology: Current Concepts and Future Perspectives. Biomedicines 2022, 10, 840. [Google Scholar] [CrossRef]
- Roberts, R.; Govender, D. Gene of the month: KIT. J. Clin. Pathol. 2015, 68, 671–674. [Google Scholar] [CrossRef]
- Lennartsson, J.; Jelacic, T.; Linnekin, D.; Shivakrupa, R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells 2005, 23, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Reboll, M.R.; Klede, S.; Taft, M.H.; Cai, C.L.; Field, L.J.; Lavine, K.J.; Koenig, A.L.; Fleischauer, J.; Meyer, J.; Schambach, A.; et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science 2022, 376, 1343–1347. [Google Scholar] [CrossRef]
- Duan, J.L.; Zhou, Z.Y.; Ruan, B.; Fang, Z.Q.; Ding, J.; Liu, J.J.; Song, P.; Xu, H.; Xu, C.; Yue, Z.S.; et al. Notch-Regulated c-Kit-Positive Liver Sinusoidal Endothelial Cells Contribute to Liver Zonation and Regeneration. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, J.; Rönnstrand, L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.X.; Ravindranath, N.; Dym, M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J. Biol. Chem. 2000, 275, 25572–25576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, B.; Marinov, M.; Arcaro, A. Targeting receptor tyrosine kinase signalling in small cell lung cancer (SCLC): What have we learned so far? Cancer Treat. Rev. 2007, 33, 391–406. [Google Scholar] [CrossRef]
- Spitzer, D.; Guérit, S.; Puetz, T.; Khel, M.I.; Armbrust, M.; Dunst, M.; Macas, J.; Zinke, J.; Devraj, G.; Jia, X.; et al. Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke. Acta Neuropathol. 2022, 144, 305–337. [Google Scholar] [CrossRef]





| β-actin forward | GGCTGTATTCCCCTCCATCG |
| β-actin reverse | CCAGTTGGTAACAATGCCATGT |
| Kit forward | GAGTTCCATAGACTCCAGCGTC |
| Kit reverse | AATGAGCAGCGGCGTGAACAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Wang, X.; Yan, H.; Wen, L.; Zhou, Z.; Ye, Y.; Jing, Y.; Niu, Y.; Wang, L.; Zhang, Z.; et al. Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling. J. Pers. Med. 2023, 13, 146. https://doi.org/10.3390/jpm13010146
Bai Q, Wang X, Yan H, Wen L, Zhou Z, Ye Y, Jing Y, Niu Y, Wang L, Zhang Z, et al. Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling. Journal of Personalized Medicine. 2023; 13(1):146. https://doi.org/10.3390/jpm13010146
Chicago/Turabian StyleBai, Qian, Xin Wang, Hongxiang Yan, Lishi Wen, Ziyi Zhou, Yating Ye, Yutong Jing, Yali Niu, Liang Wang, Zifeng Zhang, and et al. 2023. "Microglia-Derived Spp1 Promotes Pathological Retinal Neovascularization via Activating Endothelial Kit/Akt/mTOR Signaling" Journal of Personalized Medicine 13, no. 1: 146. https://doi.org/10.3390/jpm13010146





