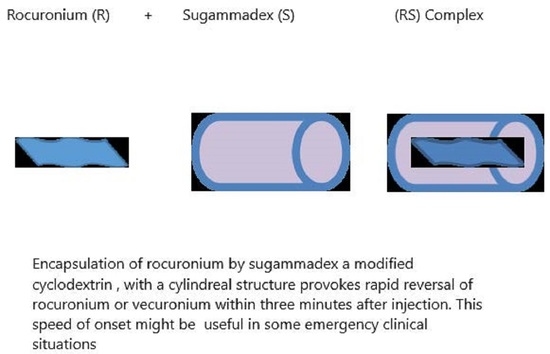
Sugammadex in Emergency Situations
Abstract
:1. Introduction
2. Operating Room Emergencies
2.1. Rapid Sequence Induction
2.2. CICV Situations
2.3. Anaphylaxis
2.4. Emergency Situations in Obese Patients
3. Non-Operating Room Emergencies
Rapid Arousal for Neurological Assessment
4. Pediatric Emergencies
5. Emergency Intubation after Sugammadex Administration
6. COVID-19 Pandemic
7. Monitoring Neuromuscular Blockade
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welliver, M. New drug sugammadex: A selective relaxant binding agent. AANA J 2006, 74, 357–363. [Google Scholar]
- Taha, S.; Siddik-Sayyid, S.; Alameddine, M.; Wakim, C.; Dahabra, C.; Moussa, A.; Khatib, M.; Baraka, A. Propofol is superior to thiopental for intubation without muscle relaxants. Can. J. Anaesth. 2005, 52, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Hristovska, A.M.; Duch, P.; Allingstrup, M.; Afshari, A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta—Analysis and trial sequential analysis. Anaesthesia 2018, 73, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, M.; Bretlau, C.; Gätke, M.; Sørensen, A.; Rasmussen, L. Rapid sequence induction and intubation with rocuronium–sugammadex compared with succinylcholine: A randomized trial. Br. J. Anaesth. 2012, 108, 682–689. [Google Scholar] [CrossRef] [Green Version]
- Puhringer, F.K.; Kristen, P.; Rex, C. Sugammadex reversal of rocuronium-induced neuromuscular block in Caesarean section patients: A series of seven cases. Br. J. Anaesth. 2010, 105, 657–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Klumpner, T.T.; Pancaro, C.; Rajala, B.; Kountanis, J.A. Sugammadex Administration in Pregnant Women: A Case Series of Maternal and Fetal Outcomes. A A Pract. 2021, 15, e01407. [Google Scholar] [CrossRef] [PubMed]
- Eichelsbacher, C.; Ilper, H.; Noppens, R.; Hinkelbein, J.; Loop, T. Rapid sequence induction and intubation in patients with risk of aspiration: Recommendations for action for practical management of anesthesia. Anaesthesist 2018, 67, 568–583. [Google Scholar] [CrossRef]
- Stourac, P.; Adamus, M.; Seidlova, D.; Pavlik, T.; Janku, P.; Krikava, I.; Mrozek, Z.; Prochazka, M.; Klucka, J.; Stoudek, R.; et al. Low-Dose or High-Dose Rocuronium Reversed with Neostigmine or Sugammadex for Cesarean Delivery Anesthesia: A Randomized Controlled Noninferiority Trial of Time to Tracheal Intubation and Extubation. Anesth. Analg. 2016, 122, 1536–1545. [Google Scholar] [CrossRef]
- Sastre, J.A.; Lopez, T.; Gomez-Rios, M.A.; Garzon, J.C.; Mariscal, M.L.; Martinez-Hurtado, E.; Freire-Otero, M.; Redondo, J.M.; Gomez, G.; Casalderrey-Rivas, M.; et al. Current practice of rapid sequence induction in adults: A national survey among anesthesiologists in Spain. Rev. Esp. Anestesiol. Reanim. 2020, 67, 381–390. [Google Scholar] [CrossRef]
- Bohringer, C.; Moua, H.; Liu, H. Is There Still a Role for Succinylcholine in Contemporary Clinical Practice? Transl. Perioper. Pain Med. 2019, 6, 129–135. [Google Scholar]
- Czarnetzki, C.; Albrecht, E.; Masouye, P.; Baeriswyl, M.; Poncet, A.; Robin, M.; Kern, C.; Tramer, M.R. Rapid Sequence Induction with a Standard Intubation Dose of Rocuronium After Magnesium Pretreatment Compared with Succinylcholine: A Randomized Clinical Trial. Anesth. Analg. 2021, 133, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.; Motamed, C.; Gaouar, H.; Chemam, S.; Amsler, E.; Frances, C. Severe reaction following sugammadex injection: Hypersensitivity? J. Investig. Allergol. Clin. Immunol. 2012, 22, 382. [Google Scholar] [PubMed]
- Fawcett, W.J. Suxamethonium or rocuronium for rapid sequence induction of anaesthesia? BJA Educ. 2019, 19, 380–382. [Google Scholar] [CrossRef]
- Curtis, R. Persistent’can’t intubate, can’t oxygenate’crisis despite reversal of rocuronium with sugammadex: The importance of timing. Anaesth. Intensive Care 2012, 40, 722. [Google Scholar]
- Bisschops, M.; Holleman, C.; Huitink, J. Can sugammadex save a patient in a simulated ‘cannot intubate, cannot ventilate’situation? Anaesthesia 2010, 65, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Brewer, L.; LaPierre, C.; Kopman, A.F.; Johnson, K.B. The Myth of Rescue Reversal in “Can’t Intubate, Can’t Ventilate” Scenarios. Anesth. Analg. 2016, 123, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Efune, P.N.; Alex, G.; Mehta, S.D. Emergency Sugammadex Reversal in an 850-G Premature Infant: A Case Report. J. Pediatr. Pharmacol. Ther. 2021, 26, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Luxen, J.; Trentzsch, H.; Urban, B. Rocuronium and sugammadex in emergency medicine: Requirements of a muscle relaxant for rapid sequence induction. Anaesthesist 2014, 63, 331–337. [Google Scholar] [CrossRef]
- Bailey, C.R. Sugammadex: When should we be giving it? Anaesthesia 2017, 72, 1170–1175. [Google Scholar] [CrossRef]
- Curtis, R.; Lomax, S.; Patel, B. Use of sugammadex in a ‘can’t intubate, can’t ventilate’ situation. Br. J. Anaesth. 2012, 108, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Lenz, A.; Hill, G.; White, P.F. Emergency use of sugammadex after failure of standard reversal drugs. Anesth. Analg. 2007, 104, 585–586. [Google Scholar] [CrossRef]
- Conte, B.; Zoric, L.; Bonada, G.; Debaene, B.; Ripart, J. Reversal of a rocuronium-induced grade IV anaphylaxis via early injection of a large dose of sugammadex. Can. J. Anaesth. 2014, 61, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Sato Boku, A.; Tachi, N.; Okumura, Y.; Kadoi, K.; Harada, J.; Okuda, M. Two Cases of Rocuronium-Induced Anaphylaxis/Anaphylactic Shock Successfully Treated With Sugammadex. Anesth. Prog. 2019, 66, 151–155. [Google Scholar] [CrossRef]
- Motamed, C.; Baguenard, P.; Bourgain, J.L. Possible mitigation of rocuronium-induced anaphylaxis after administration of sugammadex. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Murakawa, M. Rocuronium-induced anaphylaxis not improved by low dose sugammadex: A case report. Anaesth. Intensive Care 2016, 44, 522. [Google Scholar] [CrossRef] [Green Version]
- Takazawa, T.; Tomita, Y.; Yoshida, N.; Tomioka, A.; Horiuchi, T.; Nagata, C.; Orihara, M.; Yamada, M.H.; Saito, S. Three suspected cases of sugammadex-induced anaphylactic shock. BMC Anesthesiol. 2014, 14, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burbridge, M.A. Incidence of Anaphylaxis to Sugammadex in a Single-Center Cohort of 19,821 Patients. Anesth. Analg. 2021, 132, 93–97. [Google Scholar] [CrossRef]
- de Boer, H.D.; Hunter, J.M. Sugammadex or neostigmine: Should potential anaphylaxis be the overriding factor in the choice of a reversal drug? Comment on Br J Anaesth 2020; 124: 154-63. Br. J. Anaesth. 2020, 125, e220–e221. [Google Scholar] [CrossRef]
- Orihara, M.; Takazawa, T.; Horiuchi, T.; Sakamoto, S.; Nagumo, K.; Tomita, Y.; Tomioka, A.; Yoshida, N.; Yokohama, A.; Saito, S. Comparison of incidence of anaphylaxis between sugammadex and neostigmine: A retrospective multicentre observational study. Br. J. Anaesth. 2020, 124, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Ebo, D.G.; Baldo, B.A.; Van Gasse, A.L.; Mertens, C.; Elst, J.; Sermeus, L.; Bridts, C.H.; Hagendorens, M.M.; De Clerck, L.S.; Sabato, V. Anaphylaxis to sugammadex-rocuronium inclusion complex: An IgE-mediated reaction due to allergenic changes at the sugammadex primary rim. J. Allergy Clin. Immunol. Pract. 2020, 8, 1410–1415 e1413. [Google Scholar] [CrossRef]
- Kim, G.H.; Choi, W.S.; Kim, J.E.; Yun, M.J.; Koo, M.S.; Kwon, M.; Seo, H. Anaphylactic shock after sugammadex administration, induced by formation of a sugammadex-rocuronium complex—A case report. Korean J. Anesthesiol. 2019, 72, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Raigada, A.; Vega de la Osada, F.; Lopez-Sanz, C.; Mugica Garcia, M.V.; Alfranca, A.; Blanco, C. Severe Perioperative Anaphylaxis Due to Allergy to the Sugammadex-Rocuronium Complex. J. Investig. Allergol. Clin. Immunol. 2021, 32, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H. Recent clinical advance of rocuronium pharmacokinetics and a new neuromuscular reversal agent, sugammadex. Hokkaido Igaku Zasshi 2012, 87, 133–135. [Google Scholar] [PubMed]
- Riad, W.; Vaez, M.N.; Raveendran, R.; Tam, A.D.; Quereshy, F.A.; Chung, F.; Wong, D.T. Neck circumference as a predictor of difficult intubation and difficult mask ventilation in morbidly obese patients: A prospective observational study. Eur. J. Anaesthesiol. 2016, 33, 244–249. [Google Scholar] [CrossRef]
- Oda, Y. Appropriate dosing of sugammadex and rocuronium for reversal of neuromuscular blockade and reparalysis. J. Anesth. 2020, 34, 803–805. [Google Scholar] [CrossRef]
- Horrow, J.C.; Li, W.; Blobner, M.; Lombard, J.; Speek, M.; DeAngelis, M.; Herring, W.J. Actual versus ideal body weight dosing of sugammadex in morbidly obese patients offers faster reversal of rocuronium- or vecuronium-induced deep or moderate neuromuscular block: A randomized clinical trial. BMC Anesthesiol. 2021, 21, 62. [Google Scholar] [CrossRef]
- Hartley, E.L.; Alcock, R. Rocuronium Versus Suxamethonium: A Survey of First-line Muscle Relaxant Use in UK Prehospital Rapid Sequence Induction. Prehosp. Disaster Med. 2015, 30, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.; Ledingham, N.S.; Machi, P.; Czarnetzki, C.A. Rapid arousal from anaesthesia after reversal of deep rocuronium-induced neuromuscular block with sugammadex in a neuroradiological procedure. BMJ Case Rep. 2021, 14, e242820. [Google Scholar] [CrossRef]
- Patanwala, A.E. Paralytic Agents for Intubation in the Out-of-Hospital Setting. JAMA 2020, 323, 1506–1507. [Google Scholar] [CrossRef]
- Guihard, B.; Chollet-Xemard, C.; Lakhnati, P.; Vivien, B.; Broche, C.; Savary, D.; Ricard-Hibon, A.; Marianne Dit Cassou, P.J.; Adnet, F.; Wiel, E.; et al. Effect of Rocuronium vs Succinylcholine on Endotracheal Intubation Success Rate Among Patients Undergoing Out-of-Hospital Rapid Sequence Intubation: A Randomized Clinical Trial. JAMA 2019, 322, 2303–2312. [Google Scholar] [CrossRef]
- Bonhomme, V.; Hans, P. Management of the unstable cervical spine: Elective versus emergent cases. Curr. Opin. Anaesthesiol. 2009, 22, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Sugammadex: A Limited But Important Role in Emergency Medicine. Pediatr. Emerg. Care 2020, 36, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Hile, G.B.; Healy, K.J.; Almassalkhi, L.R. Rocuronium Reversal in the Emergency Department: Retrospective Evaluation of Hemodynamic Instability Following Administration of Sugammadex Versus Neostigmine With Glycopyrrolate. J. Pharm. Pract. 2021. [Google Scholar] [CrossRef]
- Jimenez, N.; Posner, K.L.; Cheney, F.W.; Caplan, R.A.; Lee, L.A.; Domino, K.B. An update on pediatric anesthesia liability: A closed claims analysis. Anesth. Analg. 2007, 104, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobias, J.D. Sugammadex: Applications in Pediatric Critical Care. J. Pediatr. Intensive Care 2020, 9, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, X.; Bash, L.D.; Bortnichak, E.; Horrow, J.; Koro, C. Neuromuscular Blocking Agents and Reversal Agents Among Hospitalized Children: A Cerner Database Study. Hosp. Pharm. 2021, 56, 424–429. [Google Scholar] [CrossRef]
- Cammu, G. Residual neuromuscular blockade and postoperative pulmonary complications: What does the recent evidence demonstrate? Curr. Anesthesiol. Rep. 2020, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Shores, R.; Fowler, K.; McDonough, J.; Suralis, A.; Scindele, D.; Müller-Wolff, T. Rocuronium vs Succinylcholine: Emergency Airway Management of the COVID-19 Patient. Anesth. eJournal 2021, 8, 1–5. [Google Scholar]
- Chaves-Cardona, H.; Hernandez-Torres, V.; Kiley, S.; Renew, J. Neuromuscular blockade management in patients with COVID-19. Korean J. Anesthesiol. 2021, 74, 285–292. [Google Scholar] [CrossRef]
- Colegrave, N.; Billard, V.; Motamed, C.; Bourgain, J.L. Comparison of the TOF-Scan acceleromyograph to TOF-Watch SX: Influence of calibration. Anaesth. Crit. Care Pain Med. 2016, 35, 223–227. [Google Scholar] [CrossRef]
- Plaud, B.; Baillard, C.; Bourgain, J.L.; Bouroche, G.; Desplanque, L.; Devys, J.M.; Fletcher, D.; Fuchs-Buder, T.; Lebuffe, G.; Meistelman, C.; et al. Guidelines on muscle relaxants and reversal in anaesthesia. Anaesth. Crit. Care Pain Med. 2020, 39, 125–142. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motamed, C. Sugammadex in Emergency Situations. J. Pers. Med. 2023, 13, 159. https://doi.org/10.3390/jpm13010159
Motamed C. Sugammadex in Emergency Situations. Journal of Personalized Medicine. 2023; 13(1):159. https://doi.org/10.3390/jpm13010159
Chicago/Turabian StyleMotamed, Cyrus. 2023. "Sugammadex in Emergency Situations" Journal of Personalized Medicine 13, no. 1: 159. https://doi.org/10.3390/jpm13010159