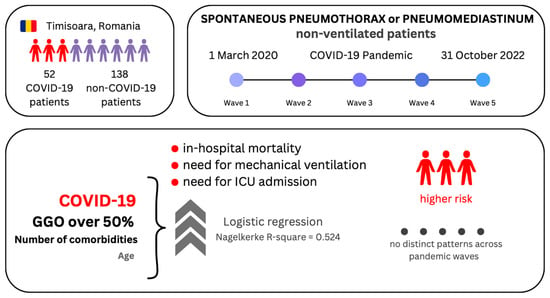
Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Data Analysis
2.4. Ethics
3. Results
3.1. Characteristics of Patients with SP–SPM
3.2. Logistic Regression Models of the Binary Outcomes
4. Discussion
4.1. Mortality and Severity of Disease across the Five Pandemic Waves
4.2. Medical History and Comorbities of Patients with SP–SPM
4.3. Imaging and Laboratory Findings in Patients with SP–SPM
4.4. Treatment Options in Patients with SP–SPM
4.5. Limitations of This Debriefing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberetchs, E.J.; Jacob, J.; Alquézar-Arbé, A.; et al. Frequency, Risk Factors, Clinical Characteristics, and Outcomes of Spontaneous Pneumothorax in Patients with Coronavirus Disease 2019. Chest 2021, 159, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Melhorn, J.; Davies, H.E.; Rostron, A.J.; Adeni, A.; et al. COVID-19 and Pneumothorax: A Multicentre Retrospective Case Series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef] [PubMed]
- Marza, A.M.; Petrica, A.; Lungeanu, D.; Sutoi, D.; Mocanu, A.; Petrache, I.; Mederle, O.A. Risk Factors, Characteristics, and Outcome in Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with SARS-CoV-2 Infection. Int. J. Gen. Med. 2022, 15, 489–500. [Google Scholar] [CrossRef]
- Taha, M.; Elahi, M.; Wahby, K.; Samavati, L. Incidence and Risk Factors of COVID-19 Associated Pneumothorax. PLoS ONE 2022, 17, e0271964. [Google Scholar] [CrossRef] [PubMed]
- Shahsavarinia, K.; Rahvar, G.; Soleimanpour, H.; Saadati, M.; Vahedi, L.; Mahmoodpoor, A. Spontaneous Pneumomediastinum, Pneumothorax and Subcutaneous Emphysema in Critically Ill COVID-19 Patients: A Systematic Review. Pak. J. Med. Sci. 2022, 38, 730. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Sedhai, Y.R.; Budhathoki, P.; Adhikari, A.; Pokharel, N.; Dhakal, R.; Kafle, S.; Yadullahi Mir, W.A.; Acharya, R.; Kashiouris, M.G.; et al. Pulmonary Barotrauma in COVID-19: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2022, 73, 103221. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Hu, K.; Chopra, A. The Incidence, Clinical Characteristics, and Outcomes of Pneumothorax in Hospitalized COVID-19 Patients: A Systematic Review. Heart Lung 2021, 50, 599–608. [Google Scholar] [CrossRef]
- Woo, W.; Kipkorir, V.; Marza, A.M.; Hamouri, S.; Albawaih, O.; Dhali, A.; Kim, W.; Udwadia, Z.F.; Nashwan, A.J.; Shaikh, N.; et al. Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score. J. Clin. Med. 2022, 11, 7132. [Google Scholar] [CrossRef]
- Palumbo, D.; Campochiaro, C.; Belletti, A.; Marinosci, A.; Dagna, L.; Zangrillo, A.; De Cobelli, F. Pneumothorax/Pneumomediastinum in Non-Intubated COVID-19 Patients: Differences between First and Second Italian Pandemic Wave. Eur. J. Intern. Med. 2021, 88, 144–146. [Google Scholar] [CrossRef]
- Tacconi, F.; Rogliani, P.; Leonardis, F.; Sarmati, L.; Fabbi, E.; De Carolis, G.; La Rocca, E.; Vanni, G.; Ambrogi, V. Incidence of Pneumomediastinum in COVID-19: A Single-Center Comparison between 1st and 2nd Wave. Respir. Investig. 2021, 59, 661–665. [Google Scholar] [CrossRef]
- Situația COVID-19 În Timișoara. Available online: https://covid19.primariatm.ro/ (accessed on 10 March 2023).
- Infecția Cu Noul Coronavirus (SARS-CoV-2). Available online: http://www.cnscbt.ro/ (accessed on 10 March 2023).
- Fericean, R.M.; Citu, C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Tudorache, E.; Oancea, C. Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves. J. Clin. Med. 2022, 11, 2916. [Google Scholar] [CrossRef]
- Weekly Epidemiological Update on COVID-19—14 September 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---14-september-2021 (accessed on 10 March 2023).
- Rzymski, P.; Kasianchuk, N.; Sikora, D.; Poniedziałek, B. COVID-19 Vaccinations and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in Europe during SARS-CoV-2 Omicron Wave in the First Quarter of 2022. J. Med. Virol. 2023, 95, e28131. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Genovese, D.; Fallucca, A.; Ferro, P.; Sparacia, B.; D’Azzo, L.; Fertitta, A.; Maida, C.M.; Vitale, F. Clinical Severity in Different Waves of SARS-CoV-2 Infection in Sicily: A Model of Smith’s “Law of Declining Virulence” from Real-World Data. Viruses 2022, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Zawbaa, H.M.; Osama, H.; El-Gendy, A.; Saeed, H.; Harb, H.S.; Madney, Y.M.; Abdelrahman, M.; Mohsen, M.; Ali, A.M.A.; Nicola, M.; et al. Effect of Mutation and Vaccination on Spread, Severity, and Mortality of COVID-19 Disease. J. Med. Virol. 2022, 94, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Akyil, M.; Bayram, S.; Erdizci, P.; Tokgoz Akyil, F.; Ulusoy, A.; Evman, S.; Alpay, L.; Baysungur, V. The Prognostic Effect of Concomitant COVID-19 with Spontaneous Pneumothorax. Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 352–357. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Rapparini, C.; Gomes, B.M.; Pinto, L.A.C. Pneumothorax as a Late Complication of COVID-19. Rev. Inst. Med. Trop. Sao Paulo 2020, 62. [Google Scholar] [CrossRef] [PubMed]
- Marzocchi, G.; Vassallo, A.; Monteduro, F. Spontaneous Pneumothorax as a Delayed Complication after Recovery from COVID-19. BMJ Case Rep. 2021, 14, e243578. [Google Scholar] [CrossRef]
- Nunna, K.; Braun, A.B. Development of a Large Spontaneous Pneumothorax after Recovery from Mild COVID-19 Infection. BMJ Case Rep. 2021, 14, e238863. [Google Scholar] [CrossRef] [PubMed]
- Abushahin, A.; Degliuomini, J.; Aronow, W.S.; Newman, T. A Case of Spontaneous Pneumothorax 21 Days After Diagnosis of Coronavirus Disease 2019 (COVID-19) Pneumonia. Am. J. Case Rep. 2020, 21, e925787-1. [Google Scholar] [CrossRef]
- Tudora, A.; Lungeanu, D.; Pop-Moldovan, A.; Puschita, M.; Lala, R.I. Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania. Healthcare 2023, 11, 1183. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, W.; Liu, L.; Dong, M.; Ji, J.; Hu, D.; Zhang, N. Does Asthma Increase the Mortality of Patients with COVID-19?: A Systematic Review and Meta-Analysis. Int. Arch. Allergy Immunol. 2021, 182, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sunjaya, A.P.; Allida, S.M.; Di Tanna, G.L.; Jenkins, C. Asthma and Risk of Infection, Hospitalization, ICU Admission and Mortality from COVID-19: Systematic Review and Meta-Analysis. J. Asthma 2022, 59, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Lazureanu, V.; Cut, T.; Laza, R.; Musta, V.; Nicolescu, N.; Marinescu, A.; Nelson-Twakor, A.; Dumache, R.; Mederle, O. Angiocatheter Decompression on a COVID-19 Patient with Severe Pneumonia, Pneumothorax, and Subcutaneous Emphysema. Clin. Lab. 2022, 68, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-J.; Hui, C.K.M.; Hull, J.H.; Birring, S.S.; McGarvey, L.; Mazzone, S.B.; Chung, K.F. Confronting COVID-19-Associated Cough and the Post-COVID Syndrome: Role of Viral Neurotropism, Neuroinflammation, and Neuroimmune Responses. Lancet Respir. Med. 2021, 9, 533–544. [Google Scholar] [CrossRef]
- Al Armashi, A.R.; Somoza-Cano, F.J.; Patell, K.; Homeida, M.; Desai, O.; Al Zubaidi, A.; Altaqi, B.; Ravakhah, K. Spontaneous Pneumomediastinum: A Collaborative Sequelae between COVID-19 and Self-inflicted Lung Injury—A Case Report and Literature Review. Radiol. Case Rep. 2021, 16, 3655–3658. [Google Scholar] [CrossRef]
- Wadhawan, G.; Thakkar, K.; Singh, R. Spontaneous Pneumothorax in Post COVID-19 Patients—A Case Series. J. Surg. Res. 2022, 5, 46–50. [Google Scholar] [CrossRef]
- Kasturi, S.; Muthirevula, A.; Chinthareddy, R.R.; Lingaraju, V.C. Delayed Recurrent Spontaneous Pneumothorax Post-Recovery from COVID-19 Infection. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 551–553. [Google Scholar] [CrossRef]
- Janssen, M.L.; van Manen, M.J.G.; Cretier, S.E.; Braunstahl, G.-J. Pneumothorax in Patients with Prior or Current COVID-19 Pneumonia. Respir. Med. Case Rep. 2020, 31, 101187. [Google Scholar] [CrossRef]
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; de Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for Pleural Disease. Thorax 2023, 78 (Suppl. S3), S1–S42. [Google Scholar] [CrossRef]
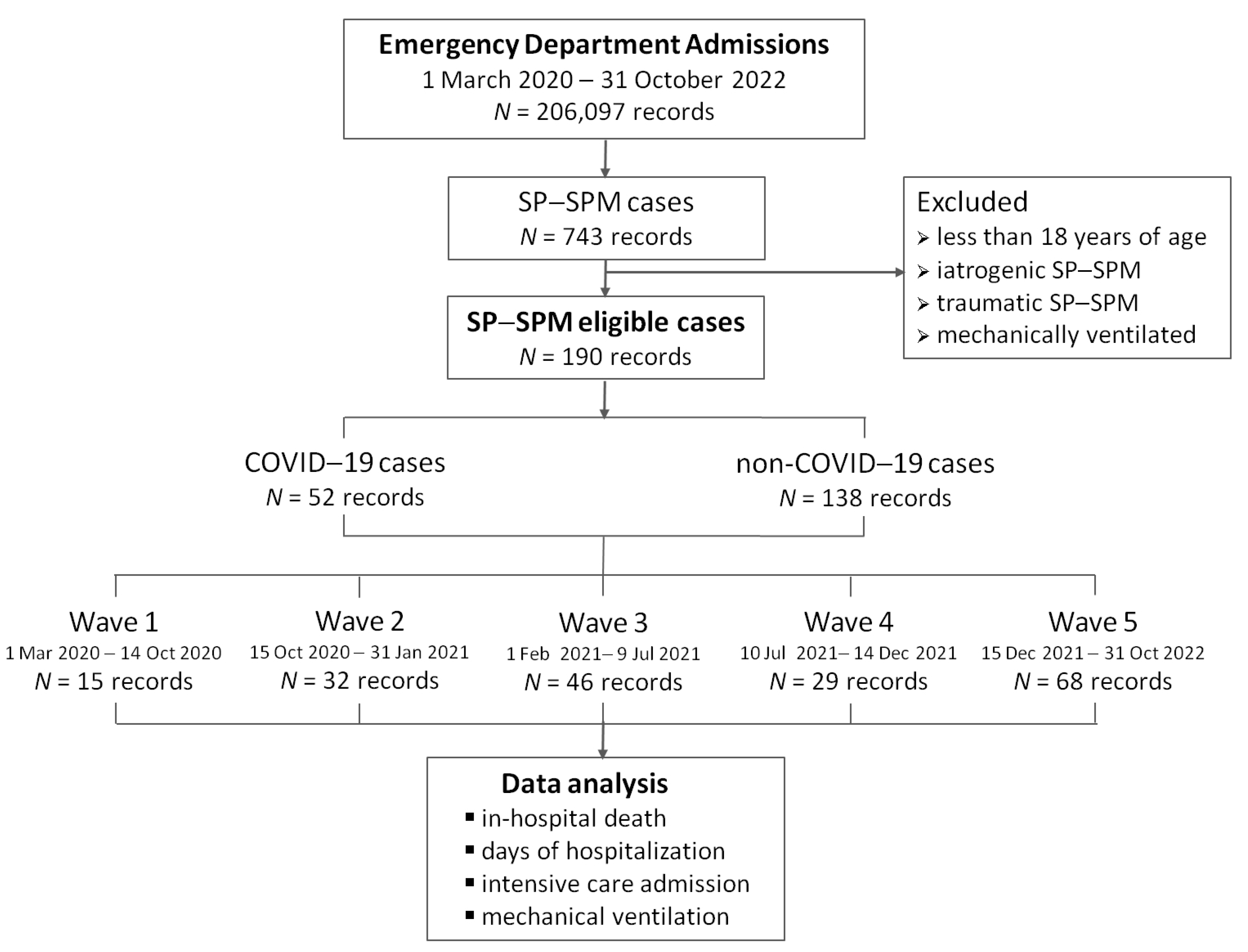

| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | ||
|---|---|---|---|---|---|---|
| Wave period | 1 Mar 2020–14 Oct 2020 | 15 Oct 2020–31 Jan 2021 | 1 Feb 2021–09 Jul 2021 | 10 Jul 2021–14 Dec 2021 | 15 Dec 2021–31 Oct 2022 | |
| Wave spike(s) | – | 22 Oct 2020–4 Jan 2021 | 16 Feb 2021–7 Apr 2021 | 15 Sep 2021–14 Nov 2021 | 5 Jan 2022–13 Feb 2022 | 19 Jul 2022–13 Sep 2022 |
| Spike duration (in days) | – | 74 | 50 | 60 | 39 | 56 |
| Frequent VOC | Wuhan-Hu-1 NC_045512.2 | Clade S:D614G | Alpha B.1.1.7 | Delta B.1.617.2 | Omicron B.1.1.529 | |
| Daily average case count during the spike | – | 143.16 | 164.64 | 236.71 | 608.61 | 137.32 |
| SP–SPM cases | 15 | 32 | 46 | 29 | 68 | |
| SP–SPM and COVID-19 cases | – | 18 (55.9%) | 15 (31.1%) | 6 (20.7%) | 13 (19.1%) | |
| Variable | All Patients (N = 190) | Wave 1 (N = 15) | Wave 2 (N = 32) | Wave 3 (N = 46) | Wave 4 (N = 29) | Wave 5 (N = 68) | p-Value (a),(b) | ||
|---|---|---|---|---|---|---|---|---|---|
| Age (a) | + COVID-19 | 63.37 ± 13.9 | - | 60.4 ± 12.56 | 71 ± 13.5 | 51.83 ± 9.58 | 63.92 ± 13.96 | 0.019 * | |
| − COVID-19 | 48.83 ± 18.51 | 46.67 ± 16.41 | 53.64 ± 12.51 | 44.45 ± 19.27 | 46.13 ± 17.19 | 51.78 ± 20.12 | 0.314 | ||
| Gender (male) (b) | + COVID-19 | 40 (21%) | - | 14 (35%) | 9 (22.5%) | 5 (12.5%) | 12 (30%) | <0.001 ** | |
| − COVID-19 | 98 (51.57%) | 13 (13.3%) | 7 (7.1%) | 23 (23.5%) | 14 (14.3%) | 41 (41.8%) | |||
| Active smoker (b) | + COVID-19 | 10 (5.26%) | - | 3 (30%) | 3 (30%%) | 1 (10%) | 3 (30%) | <0.001 ** | |
| − COVID-19 | 59 (31.05%) | 12 (20.3%) | 8 (13.6%) | 14 (23.7%) | 5 (8.5%) | 20 (33.9%) | |||
| Comorbidities (b) | |||||||||
| none | + COVID-19 | 19 (21.3%) | - | 5 (26.3%) | 4 (21.1%) | 5 (26.3%) | 5 (26.3%) | 0.043 * | |
| − COVID-19 | 70 (78.7%) | 7 (10%) | 4 (5.7%) | 19 (27.1%) | 13 (18.6%) | 27 (38.6%) | |||
| one | + COVID-19 | 10 (22.2%) | - | 5 (50%) | 1 (10%) | - | 4 (40%) | ||
| − COVID-19 | 35 (77.8%) | 3 (8.6%) | 3 (8.6%) | 6 (17.1%) | 8 (22.9%) | 15 (42.9%) | |||
| two | + COVID-19 | 12 (36.4%) | - | 7 (58.3%) | 3 (25%) | - | 2 (16.7%) | ||
| − COVID-19 | 21 (63.6%) | 2 (9.5%) | 4 (19%) | 3 (14.3%) | 2 (9.5%) | 10 (47.6%) | |||
| three or more | + COVID-19 | 11 (47.8%) | - | 1 (9.1%) | 7 (63.6%) | 1 (9.1%) | 2 (18.2%) | ||
| − COVID-19 | 12 (52.2%) | 3 (25%) | 3 (25%) | 3 (25%) | - | 3 (25%) | |||
| Variable (a) | All Patients (N = 190) | Wave 1 (N = 15) | Wave 2 (N = 32) | Wave 3 (N = 46) | Wave 4 (N = 29) | Wave 5 (N = 68) | p-Value (a) | |
|---|---|---|---|---|---|---|---|---|
| Lung infiltrates | + COVID-19 | 20 (71.4%) | - | 8 (40%) | 8 (40%) | 1 (5%) | 3 (15%) | <0.001 ** (a) |
| − COVID-19 | 8 (28.6%) | 1 (12.5%) | 2 (25%) | 3 (37.5%) | 1 (12.5%) | 1 (12.5%) | ||
| Ground-glass opacities | + COVID-19 | 32 (84.2%) | - | 16 (50%) | 10 (31.3%) | 2 (6.3%) | 4 (12.5%) | <0.001 ** (a) |
| − COVID-19 | 6 (15.8%) | - | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 3 (50%) | ||
| Pneumomediastinum | + COVID-19 | 23 (60.5%) | - | 9 (39.1%) | 8 (34.8%) | 1 (4.3%) | 5 (21.7%) | <0.001 ** (a) |
| − COVID-19 | 15 (39.5%) | - | 1 (6.7%) | 4 (26.7%) | 3 (20%) | 7 (46.7%) | ||
| Subcutaneous emphysema | + COVID-19 | 16 (53.3%) | - | 7 (43.8%) | 6 (37.5%) | 1 (6.3%) | 2 (12.5%) | <0.001 ** (a) |
| − COVID-19 | 14 (46.7%) | 1 (7.1%) | 2 (14.3%) | - | 4 (28.6%) | 7 (50%) | ||
| Observation | + COVID-19 | 22 (64.7%) | - | 7 (30.4%) | 6 (26.1%) | 2 (8.7%) | 8 (34.8%) | <0.001 ** (b) |
| − COVID-19 | 14 (37.8%) | - | - | 3 (21.4%) | 2 (14.3%) | 9 (64.3%) | ||
| Chest tube | + COVID-19 | 29 (19%) | - | 11 (37.9%) | 9 (31%) | 4 (13.8%) | 5 (17.2%) | |
| − COVID-19 | 124 (81%) | 15 (12.1%) | 14 (11.3%) | 28 (22.6%) | 21 (16.9%) | 46 (37.1%) | ||
| Variable | All Patients N = 52 (#) | Wave 2 N = 18 | Wave 3 N = 15 | Wave 4 N = 6 (#) | Wave 5 N = 13 | p-Value (a),(b) | |
|---|---|---|---|---|---|---|---|
| GGO (a) | 0.035 * | ||||||
| no GGO | 17 (33.3%) | 2 (11.8%) | 5 (29.4%) | 2 (11.8%) | 8 (47.1%) | ||
| GGO < 20% | 4 (7.8%) | 3 (75%) | - | 1 (25%) | - | ||
| GGO 20–50% | 10 (19.6%) | 4 (40%) | 4 (40%) | - | 2 (20%%) | ||
| GGO > 50% | 20 (39.2%) | 9 (45%) | 6 (30%) | 2 (10%) | 3 (15%) | ||
| SpO2 on room air (b) | 80.55 ± 13.81 | 75.72 ± 16.37 | 82.27 ± 10.96 | 84.67 ± 14.81 | 83.58 ± 11.75 | 0.363 | |
| Variable | All Patients (N = 190) | Wave 1 (N = 15) | Wave 2 (N = 32) | Wave 3 (N = 46) | Wave 4 (N = 29) | Wave 5 (N = 68) | p-Value (a),(b) | |
|---|---|---|---|---|---|---|---|---|
| Deceased (a) | + COVID-19 | 24 (77.4%) | - | 7 (29.2%) | 9 (37.5%) | 3 (12.5%) | 5 (20.8%) | <0.001 ** |
| − COVID-19 | 7 (22.6%) | - | 2 (28.6%) | 4 (57.1%) | - | 1 (14.3%) | ||
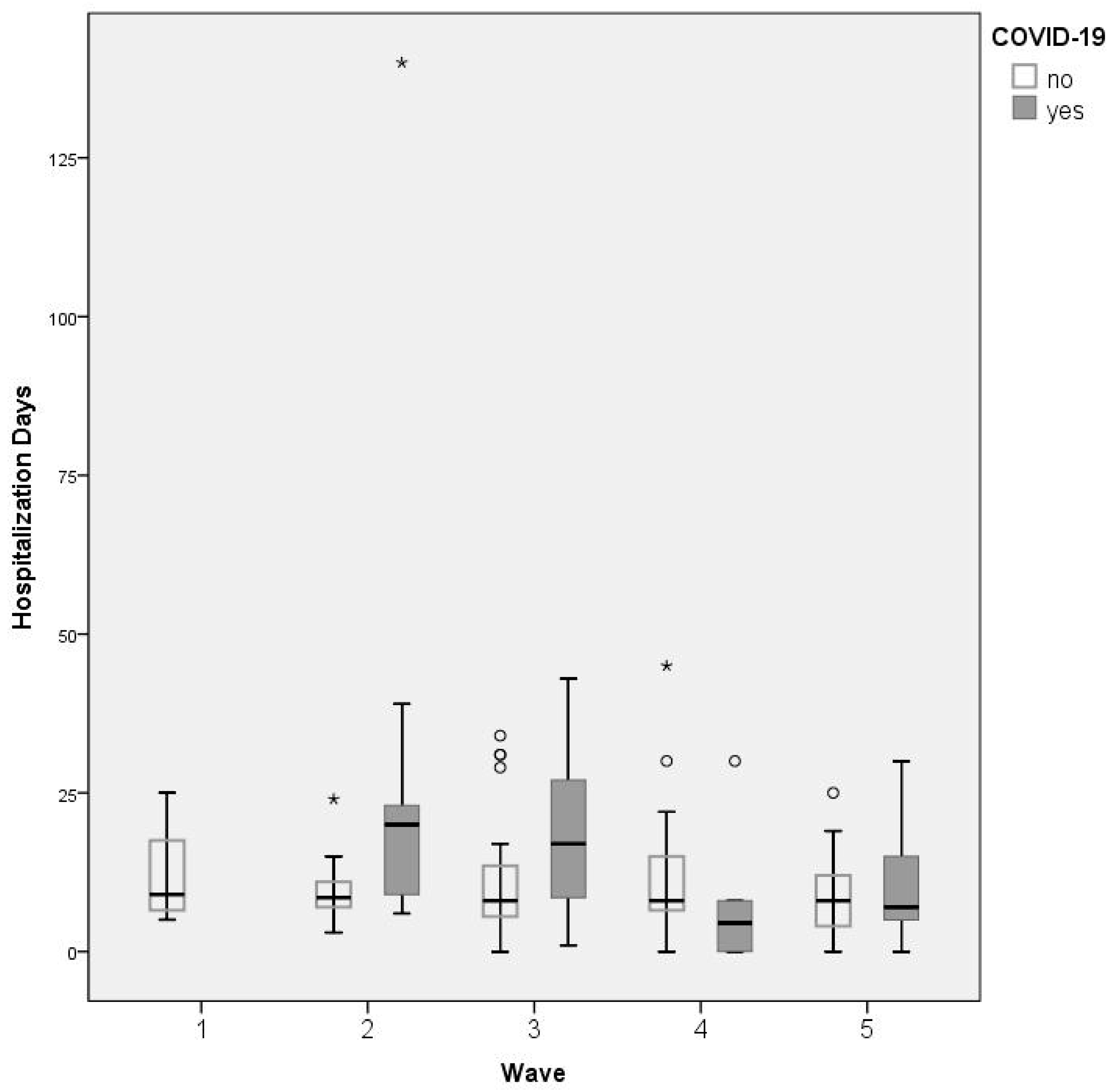
| Hospitalization days (b) | + COVID-19 | 17.63 ± 20.82 | - | 24.72 ± 30.38 | 18.27 ± 12.58 | 7.83 ± 11.39 | 11.08 ± 10.04 | 0.035 * |
| − COVID-19 | 9.88 ± 7.58 | 12.07 ± 7 | 9.5 ± 5.19 | 10.81 ± 9.09 | 12 ± 10.02 | 7.94 ± 5.62 | 0.285 | |
| Required ICU (a) | + COVID-19 | 23 (71.9%) | - | 9 (39.1%) | 6 (26.1%) | 2 (8.7%) | 6 (26.1%) | <0.001 ** |
| − COVID-19 | 9 (28.1%) | - | 2 (22.2%) | 2 (22.2%) | 2 (22.2%) | 3 (33.3%) | ||
| Required MV (a) | + COVID-19 | 19 (65.5%) | - | 6 (31.6%) | 5 (26.3%) | 2 (10.5%) | 6 (31.6%) | <0.001 ** |
| − COVID-19 | 10 (34.5%) | - | 2 (20%) | 2 (20%) | 3 (30%) | 3 (30%) | ||
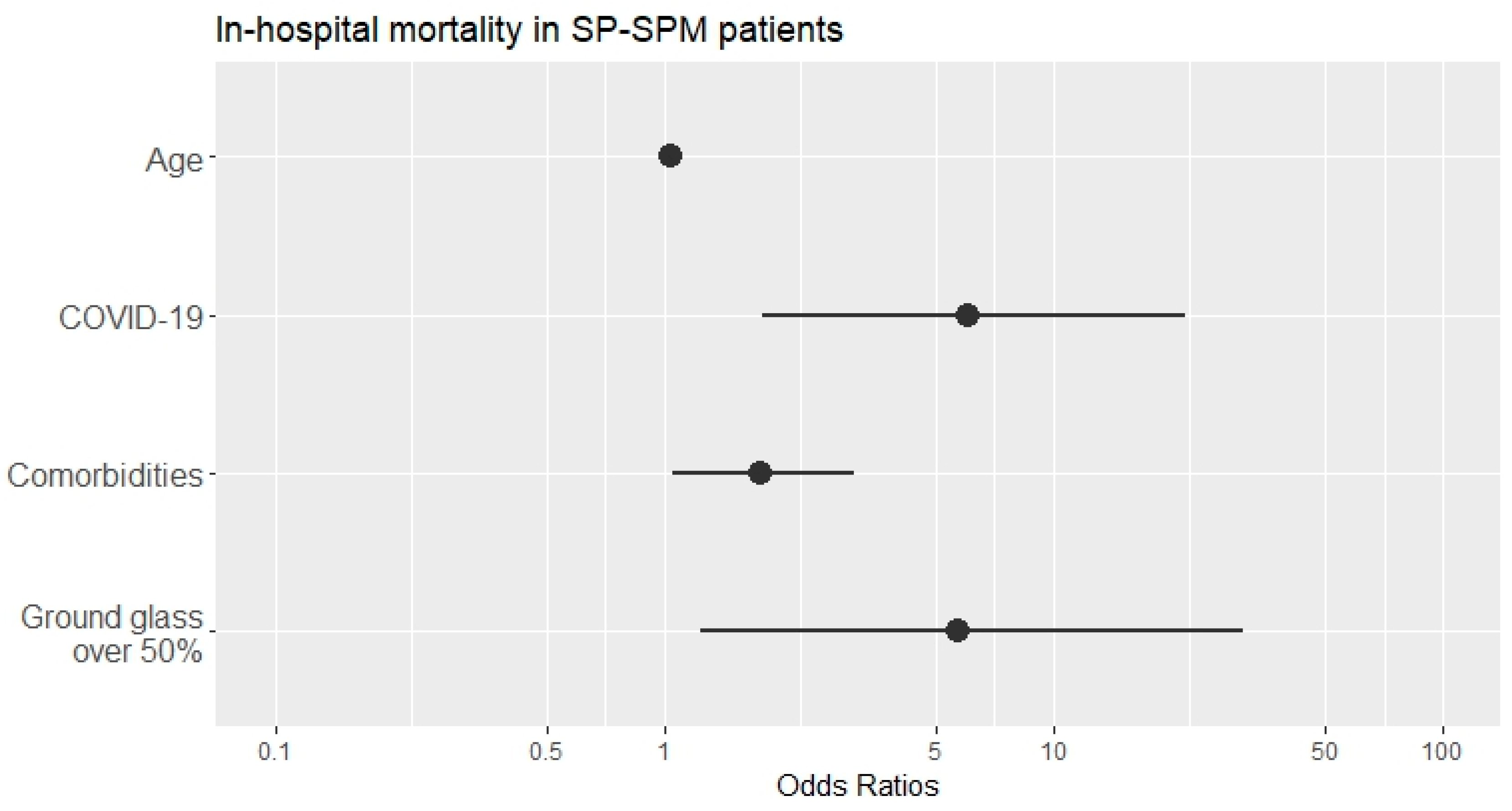
| Model: Deceased ~ Age + COVID-19 + N comorbidities + Ground glass opacities over 50% Controlling for: GenderM + ActiveSmoker + pandemicWave (categorical) + extension of SP–SPM (categorical) | ||||
| Predictor | B ± Std. err | p-Value | Exp (B) (95% CI) | |
| Age | 0.042 ± 0.02 | 0.039 * | 1.043 (1.002–1.085) | |
| COVID-19 | 1.797 ± 0.629 | 0.004 ** | 6.032 (1.757–20.712) | |
| N comorbidities | 0.572 ± 0.269 | 0.033 * | 1.772 (1.046–3.001) | |
| Ground glass opacities over 50% | 1.739 ± 0.808 | 0.031 * | 5.694 (1.169–27.746) | |
| Nagelkerke R-square = 0.524 | ||||
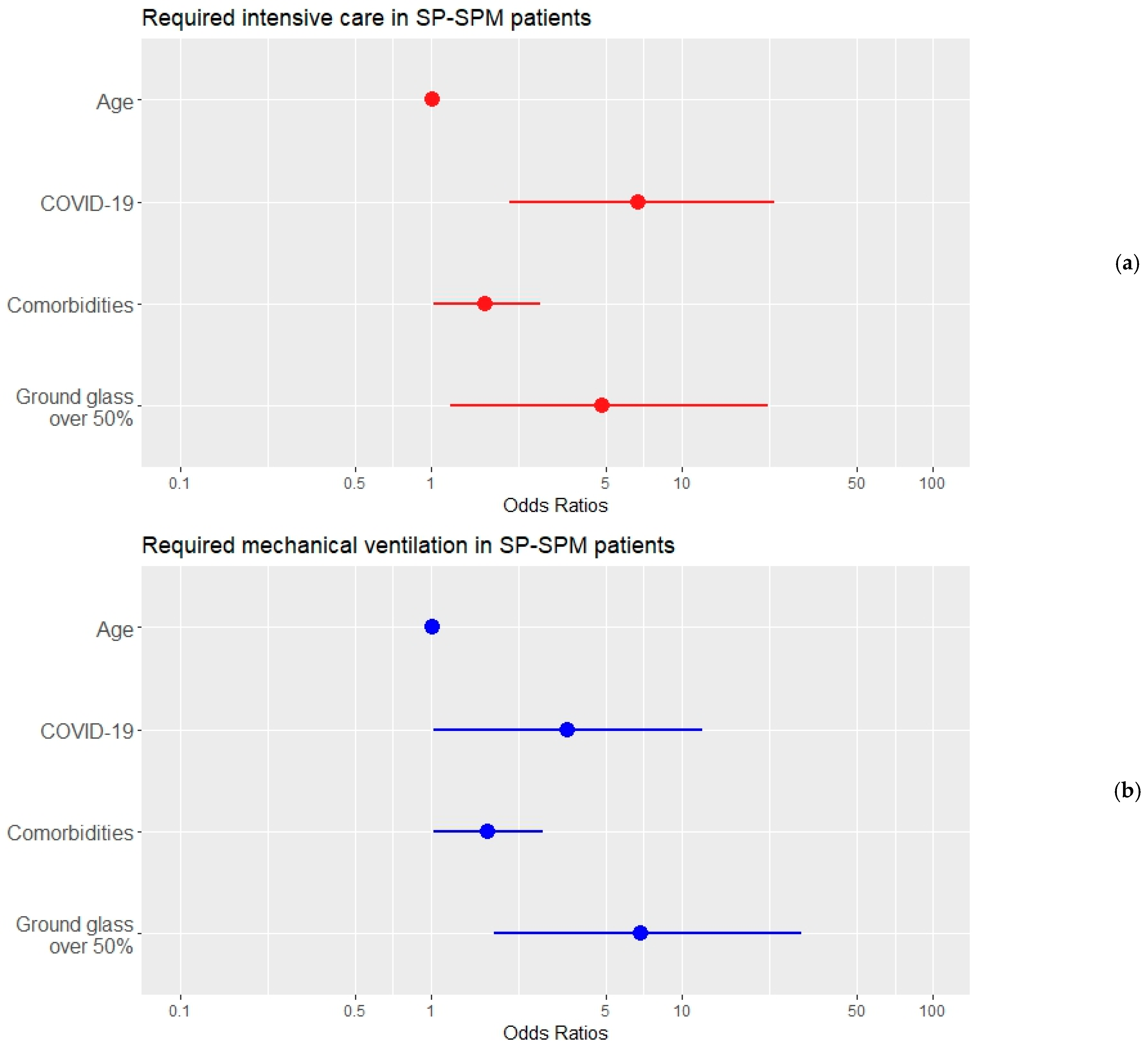
| Model: ICU ~ Age + COVID-19 + N comorbidities + Ground glass opacities over 50% Controlling for: GenderM + ActiveSmoker + pandemicWave (categorical) + extension of SP–SPM (categorical) | ||||
| Predictor | B ± Std. err | p-Value | Exp (B) (95% CI) | |
| Age | 0.011 ± 0.016 | 0.511 | 1.011 (0.979–1.043) | |
| COVID-19 | 1.901 ± 0.613 | 0.002 ** | 6.693 (2.013–22.261) | |
| N comorbidities | 0.504 ± 0.246 | 0.041 * | 1.656 (1.022–2.683) | |
| Ground glass opacities over 50% | 1.570 ± 0.736 | 0.033 * | 4.807 (1.137–20.326) | |
| Nagelkerke R-square = 0.419 | ||||
| Model: MV ~ Age + COVID-19 + N comorbidities + Ground glass opacities over 50% Controlling for: GenderM + ActiveSmoker + pandemicWave (categorical) + extension of SP–SPM (categorical) | ||||
| Predictor | B ± Std. err | p-Value | Exp (B) (95% CI) | |
| Age | 0.012 ± 0.016 | 0.454 | 1.012 (0.981–1.044) | |
| COVID-19 | 1.249 ± 0.622 | 0.045 * | 3.488 (1.031–11.801) | |
| N comorbidities | 0.519 ± 0.254 | 0.041 * | 1.681 (1.022–2.766) | |
| Ground glass opacities over 50% | 1.928 ± 0.712 | 0.007 * | 6.876 (1.704–27.735) | |
| Nagelkerke R-square = 0.373 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marza, A.M.; Cindrea, A.C.; Petrica, A.; Stanciugelu, A.V.; Barsac, C.; Mocanu, A.; Critu, R.; Botea, M.O.; Trebuian, C.I.; Lungeanu, D. Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves. J. Pers. Med. 2023, 13, 1497. https://doi.org/10.3390/jpm13101497
Marza AM, Cindrea AC, Petrica A, Stanciugelu AV, Barsac C, Mocanu A, Critu R, Botea MO, Trebuian CI, Lungeanu D. Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves. Journal of Personalized Medicine. 2023; 13(10):1497. https://doi.org/10.3390/jpm13101497
Chicago/Turabian StyleMarza, Adina Maria, Alexandru Cristian Cindrea, Alina Petrica, Alexandra Valentina Stanciugelu, Claudiu Barsac, Alexandra Mocanu, Roxana Critu, Mihai Octavian Botea, Cosmin Iosif Trebuian, and Diana Lungeanu. 2023. "Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves" Journal of Personalized Medicine 13, no. 10: 1497. https://doi.org/10.3390/jpm13101497