Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis
Abstract
:1. Introduction
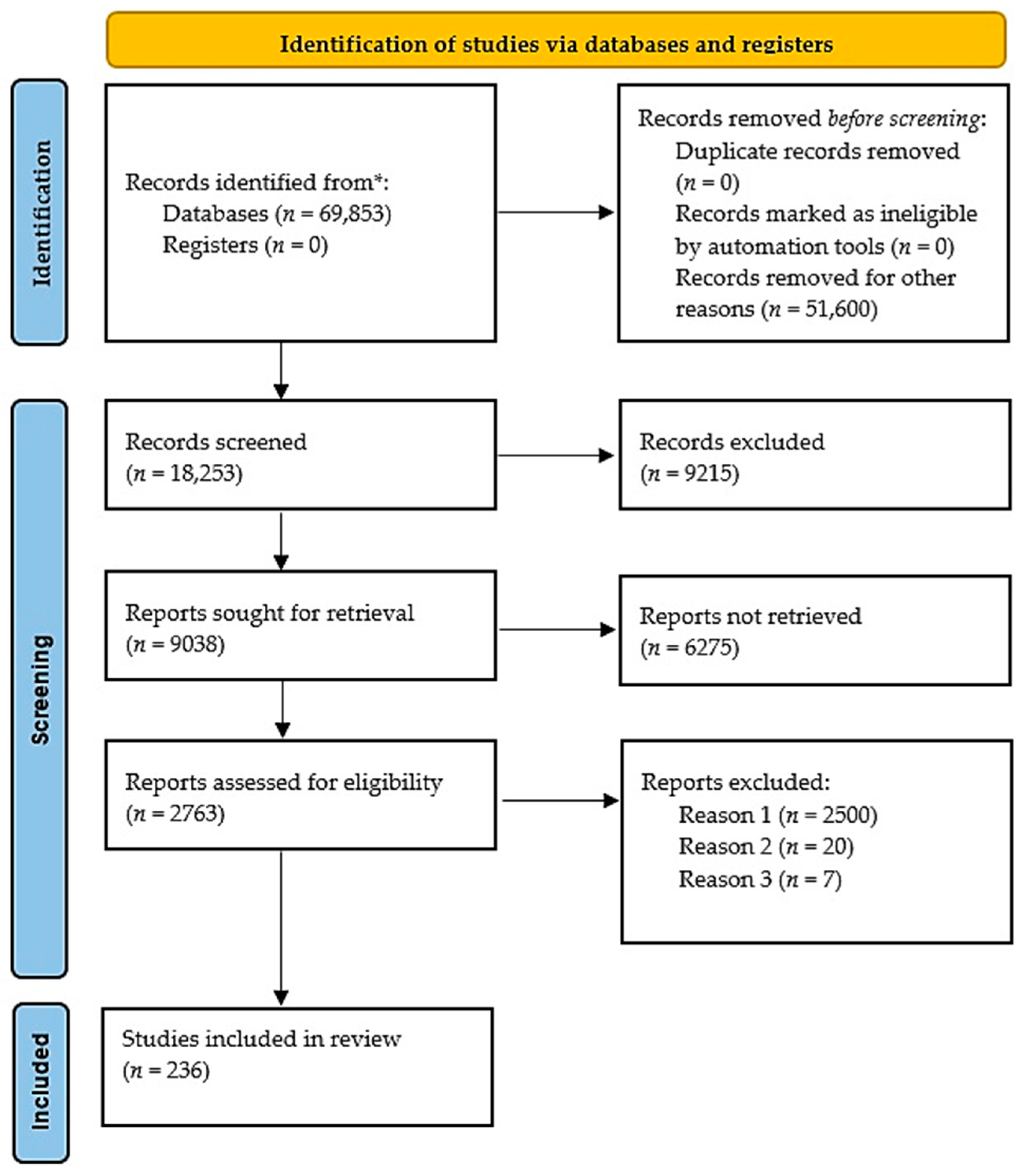
2. Materials and Methods
Study Strategy
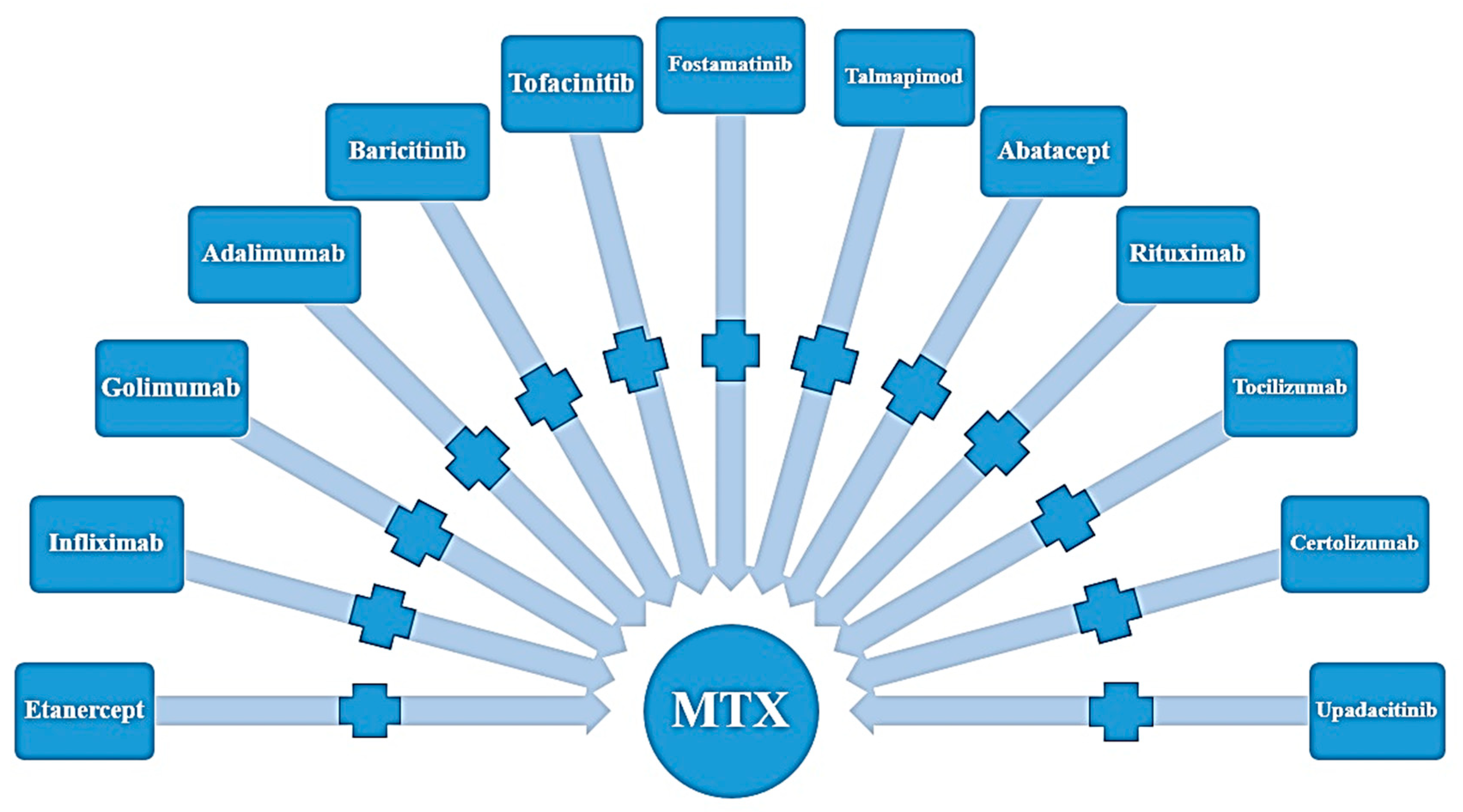
3. Results
3.1. Results from the Literature
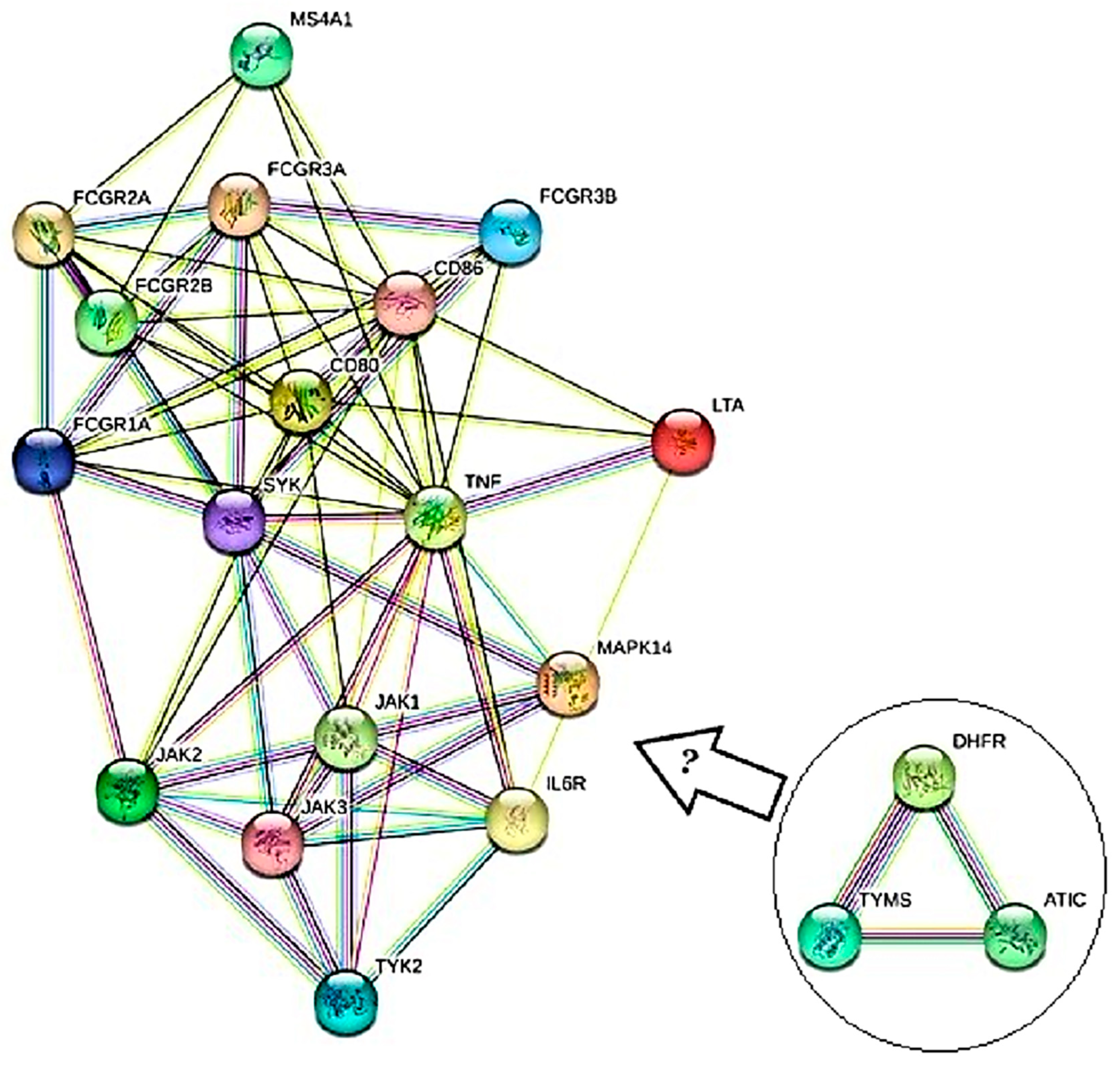
3.2. Results from Bioinformatics Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D.; Tanasescu, R. Extra-articular manifestations in rheumatoid arthritis. Maedica 2010, 5, 286. [Google Scholar]
- Xu, Y.; Wu, Q. Prevalence Trend and Disparities in Rheumatoid Arthritis among US Adults, 2005–2018. J. Clin. Med. 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Breedveld, F.C.; Dougados, M.; Kalden, J.R.; Schiff, M.H.; Smolen, J.S. Early referral recommendation for newly diagnosed rheumatoid arthritis: Evidence based development of a clinical guide. Ann. Rheum. Dis. 2002, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T.; Kautiainen, H.; Pincus, T.; Verstappen, S.M.; Aggarwal, A.; Alten, R.; Andersone, D.; Badsha, H.; Baecklund, E.; Belmonte, M.; et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: Data from 32 countries in the QUEST-RA Study. Arthritis Res. Ther. 2010, 12, R42. [Google Scholar] [CrossRef]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current Therapeutic Options in the Treatment of Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Kim, I.J.; Cho, M.-S.; Lee, J. A Case of Rheumatoid Vasculitis Involving Hepatic Artery in Early Rheumatoid Arthritis. J. Korean Med. Sci. 2017, 32, 1207–1210. [Google Scholar] [CrossRef]
- Ostrowska, M.; Maśliński, W.; Prochorec-Sobieszek, M.; Nieciecki, M.; Sudoł-Szopińska, I. Cartilage and bone damage in rheumatoid arthritis. Rheumatology 2018, 56, 111–120. [Google Scholar] [CrossRef]
- Scott, D.L.; Houssien, D.A. Clinical and Laboratory Assessments in Rheumatoid Arthritis and Osteoarthritis. Rheumatology 1996, 35, 6–9. [Google Scholar] [CrossRef]
- Grassi, W.; De Angelis, R.; Lamanna, G.; Cervini, C. The clinical features of rheumatoid arthritis. Eur. J. Radiol. 1998, 27, S18–S24. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- Marok, R.; Winyard, P.G.; Coumbe, A.; Kus, M.L.; Gaffney, K.; Blades, S.; Mapp, P.I.; Morris, C.J.; Blake, D.R.; Kaltschmidt, C.; et al. Activation of the transcription factor nuclear factor-κB in human inflamed synovial tissue. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1996, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.L.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.-L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Chang, Y.; Wei, W. The role of BAFF in the progression of rheumatoid arthritis. Cytokine 2015, 76, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Staheli, L.T.; Hall, J.G.; Jaffe, K.M.; Paholke, D.O. (Eds.) Arthrogryposis: A Text Atlas; Cambridge University Press: Cambridge, UK, 1998; 184p, ISBN 0521571065/9780521571067. [Google Scholar]
- Ong, C.; Lirk, P.; Tan, C.; Seymour, R. An Evidence-Based Update on Nonsteroidal Anti-Inflammatory Drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Devi, A.; Kumari, S.; Modgil, S.; Thakur, S.; Yadav, R. RHUMATOID ARTHRITIS: A REVIEW. YMER 2022, 21, 8. [Google Scholar]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Lee, R.G.; Tolman, K.G. Liver histology in rheumatoid arthritis patients receiving long-term methotrexate therapy. A Prospective Study with Baseline and Sequential Biopsy Samples. Arthritis Rheum. 1989, 32, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Kaye, G.I.; Kaye, N.W.; Ishak, K.G.; Axiotis, C.A. Light and electron microscopic analysis of sequential liver biopsy samples from rheumatoid arthritis patients receiving long-term methotrexate therapy. Followup over long treatment intervals and correlation with clinical and laboratory variables. Arthritis Rheum. 1995, 38, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Herrmann, M.L.; Frangou, C.G.; Wahl, G.M.; Morris, R.E.; Strande, V.; Kirschbaum, B.J. Mechanism of Action for Leflunomide in Rheumatoid Arthritis. Clin. Immunol. 1999, 93, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Osiri, M.; Shea, B.; Welch, V.; E Suarez-Almazor, M.; Strand, V.; Tugwell, P.; A Wells, G. Leflunomide for the treatment of rheumatoid arthritis. Cochrane Database Syst. Rev. 2002, 2010, CD002047. [Google Scholar] [CrossRef] [PubMed]
- Osiri, M.; Shea, B.; Robinson, V.; Suarez-Almazor, M.; Strand, V.; Tugwell, P.; Wells, G. Leflunomide for the treatment of rheumatoid arthritis: A systematic review and metaanalysis. J. Rheumatol. 2003, 30, 1182–1190. [Google Scholar] [PubMed]
- Sinha, S.; Sardana, K.; Sachdeva, S. Hydroxychloroquine in dermatology and beyond: Recent update. Indian Dermatol. Online J. 2020, 11, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Smedegård, G.; Björk, J. Sulphasalazine: Mechanism of action in rheumatoid arthritis. Rheumatology 1995, 34 (Suppl. 2), 7–15. [Google Scholar] [CrossRef]
- Agarwal, S.K. Biologic Agents in Rheumatoid Arthritis: An Update for Managed Care Professionals. J. Manag. Care Pharm. 2011, 17, S14–S18. [Google Scholar] [CrossRef]
- Hazlewood, G.S.; Barnabe, C.; Tomlinson, G.; Marshall, D.; Devoe, D.; Bombardier, C. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: Abridged Cochrane systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2016, 353, i1777. [Google Scholar] [CrossRef]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Fleischmann, R.; Hall, S.; Wilkinson, B.; Bradley, J.D.; Gruben, D.; Koncz, T.; Krishnaswami, S.; Wallenstein, G.V.; Zang, C.; et al. Tofacitinib versus Methotrexate in Rheumatoid Arthritis. N. Engl. J. Med. 2014, 370, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. Int. J. Rheum. Dis. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Lis, K.; Kuzawińska, O.; Bałkowiec-Iskra, E. State of the art paper Tumor necrosis factor inhibitors–state of knowledge. Arch. Med. Sci. 2014, 6, 1175–1185. [Google Scholar] [CrossRef]
- Taylor, P.C. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr. Opin. Pharmacol. 2010, 10, 308–315. [Google Scholar] [CrossRef]
- Shin, I.-S.J.; Baer, A.N.; Kwon, H.J.; Papadopoulos, E.J.; Siegel, J.N. Guillain-Barré and Miller Fisher syndromes occurring with tumor necrosis factor α antagonist therapy. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2006, 54, 1429–1434. [Google Scholar] [CrossRef]
- Braddock, M.; Quinn, A. Targeting IL-1 in inflammatory disease: New opportunities for therapeutic intervention. Nat. Rev. Drug Discov. 2004, 3, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Innate Immunity and the Failing Heart. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Singh, J.A. Use of Biologics in Rheumatoid Arthritis: Current and Emerging Paradigms of Care. Clin. Ther. 2011, 33, 679–707. [Google Scholar] [CrossRef]
- Shaw, T.; Quan, J.; Totoritis, M.C. B cell therapy for rheumatoid arthritis: The rituximab (anti-CD20) experience. Ann. Rheum. Dis. 2003, 62 (Suppl. 2), ii55–ii59. [Google Scholar] [CrossRef]
- Mok, C.C. Rituximab for the treatment of rheumatoid arthritis: An update. Drug Des. Dev. Ther. 2014, 8, 87–100. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Calatrava, M.J.M.; Castañeda, S. Abatacept mechanism of action: Concordance with its clinical profile. Reumatol. Clin. 2012, 8, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sebba, A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health Pharm. 2008, 65, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Baricitinib: First Global Approval. Drugs 2017, 77, 697–704. [Google Scholar] [CrossRef]
- Dougados, M.; van der Heijde, D.; Chen, Y.-C.; Greenwald, M.; Drescher, E.; Liu, J.; Beattie, S.; Witt, S.; de la Torre, I.; Gaich, C.; et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: Results from the RA-BUILD study. Ann. Rheum. Dis. 2016, 76, 88–95. [Google Scholar] [CrossRef]
- Taylor, P.C.; Keystone, E.C.; Van Der Heijde, D.; Weinblatt, M.E.; Del Carmen Morales, L.; Gonzaga, J.R.; Yakushin, S.; Ishii, T.; Emoto, K.; Beattie, S.; et al. Baricitinib versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2017, 376, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Kremer, J.M.; Bosch, F.V.D.; Kivitz, A.; Bessette, L.; Li, Y.; Zhou, Y.; A Othman, A.; Pangan, A.L.; Camp, H.S. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 2503–2512. [Google Scholar] [CrossRef]
- Vyas, D.; O’dell, K.M.; Bandy, J.L.; Boyce, E.G. Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. Ann. Pharmacother. 2013, 47, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Suzuki, M.; Nakamura, H.; Toyoizumi, S.; Zwillich, S.H.; Tofacitinib Study Investigators. Phase II study of tofacitinib (CP-690,550) combined with MTX in patients with rheumatoid arthritis and an inadequate response to MTX. Arthritis Care Res. 2011, 63, 1150–1158. [Google Scholar] [CrossRef]
- Singh, G.; Fries, J.F.; A Williams, C.; Zatarain, E.; Spitz, P.; A Bloch, D. Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis. J. Rheumatol. 1991, 18, 188–194. [Google Scholar]
- Bykerk, V.P.; Akhavan, P.; Hazlewood, G.S.; Schieir, O.; Dooley, A.; Haraoui, B.; Khraishi, M.; Leclercq, S.A.; Légaré, J.; Mosher, D.P.; et al. Canadian Rheumatology Association Recommendations for Pharmacological Management of Rheumatoid Arthritis with Traditional and Biologic Disease-modifying Antirheumatic Drugs. J. Rheumatol. 2012, 39, 1559–1582. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.J.; Kedves, M.; Hamar, A.; van der Goes, M.C.; Kent, A.; Bakkers, M.; Pchelnikova, P.; Blaas, E.; et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 2022, 81, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Chatzidionysiou, K.; Hetland, M.L.; Frisell, T.; Di Giuseppe, D.; Hellgren, K.; Glintborg, B.; Nordström, D.; Peltomaa, R.; Aaltonen, K.; Trokovic, N.; et al. Effectiveness of a Second Biologic After Failure of a Non–tumor Necrosis Factor Inhibitor As First Biologic in Rheumatoid Arthritis. J. Rheumatol. 2021, 48, 1512–1518. [Google Scholar] [CrossRef]
- Goll, G.L.; Kvien, T.K. What Next after Biologic Therapy Fails in Rheumatoid Arthritis? N. Engl. J. Med. 2020, 383, 1588–1589. [Google Scholar] [CrossRef]
- Saito, K.; Tanaka, Y. New biologic and non biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis. Nihon Rinsho Men’eki Gakkai Kaishi=Jpn. J. Clin. Immunol. 2009, 32, 149–159. [Google Scholar] [CrossRef]
- Provan, S.A.; Berg, I.J.; Hammer, H.B.; Mathiessen, A.; Kvien, T.K.; Semb, A.G. The Impact of Newer Biological Disease Modifying Anti-Rheumatic Drugs on Cardiovascular Risk Factors: A 12-Month Longitudinal Study in Rheumatoid Arthritis Patients Treated with Rituximab, Abatacept and Tociliziumab. PLoS ONE 2015, 10, e0130709. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Raimondo, M.G.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Spotlight on mavrilimumab for the treatment of rheumatoid arthritis: Evidence to date. Drug Des. Dev. Ther. 2017, 11, 211–223. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.A.; Todd, D.J. Kinase inhibitors: The next generation of therapies in the treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 2014, 17, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Mocsai, A.; Humphrey, M.B.; Van Ziffle, J.A.; Hu, Y.; Burghardt, A.; Spusta, S.C.; Majumdar, S.; Lanier, L.L.; Lowell, C.A.; Nakamura, M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteo-clasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 6158–6163. [Google Scholar] [CrossRef]
- Damjanov, N.; Kauffman, R.S.; Spencer-Green, G.T. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: Results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009, 60, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Burmester, G.; Nash, P.; Zerbini, C.A.F.; Soma, K.; Kwok, K.; Hendrikx, T.; Bananis, E.; Fleischmann, R. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2016, 75, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.L.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Xavier, R.M. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N. Engl. J. Med. 2020, 383, 1511–1521. [Google Scholar] [CrossRef]
- Combe, B.; Kivitz, A.; Tanaka, Y.; van der Heijde, D.; Simon, J.A.; Baraf, H.S.B.; Kumar, U.; Matzkies, F.; Bartok, B.; Ye, L.; et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: A phase III randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 848–858. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Lin, C.-H.; Huang, T.-H.; Fang, J.-Y. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials 2018, 8, 42. [Google Scholar] [CrossRef]
- Tanaka, K.; Yamaguchi, T.; Hara, M. Iguratimod for the treatment of rheumatoid arthritis in Japan. Expert Rev. Clin. Immunol. 2015, 11, 565–573. [Google Scholar] [CrossRef]
- Mimori, T.; Harigai, M.; Atsumi, T.; Fujii, T.; Kuwana, M.; Matsuno, H.; Momohara, S.; Takei, S.; Tamura, N.; Takasaki, Y.; et al. Safety and effectiveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: Interim analysis of a post-marketing surveillance study of 2679 patients in Japan. Mod. Rheumatol. 2017, 27, 755–765. [Google Scholar] [CrossRef]
- Cohen, S.; Tuckwell, K.; Katsumoto, T.R.; Zhao, R.; Galanter, J.; Lee, C.; Rae, J.; Toth, B.; Ramamoorthi, N.; Hackney, J.A.; et al. Fenebrutinib Versus Placebo or Adalimumab in Rheumatoid Arthritis: A Randomized, Double-Blind, Phase II Trial. Arthritis Rheumatol. 2020, 72, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Kochi, Y.; Suzuki, A.; Yamada, R.; Yamamoto, K. Ethnogenetic heterogeneity of rheumatoid arthritis—Implications for pathogenesis. Nat. Rev. Rheumatol. 2010, 6, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L.; Silva, C.; Stolt, P.; Alfredsson, L.; Klareskog, L. A gene–environment interaction between smoking and shared epitope genes in HLA–DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2004, 50, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Chujo, S.; Okamoto, S.; Sunahara, R.; Adachi, M.; Yamada, K.; Hayashi, H.; Takii, T.; Hayakawa, K.; Onozaki, K. Cigarette smoke condensate extracts augment collagen-induced arthritis in mice. Int. Immunopharmacol. 2010, 10, 1194–1199. [Google Scholar] [CrossRef]
- Okamoto, S.; Adachi, M.; Chujo, S.; Yamada, K.; Akita, K.; Itoh, S.; Takii, T.; Hayakawa, K.; Onozaki, K. Etiological role of cigarette smoking in rheumatoid arthritis: Nasal exposure to cigarette smoke condensate extracts augments the development of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 2011, 404, 1088–1092. [Google Scholar] [CrossRef]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2011, 51, 7–23. [Google Scholar] [CrossRef]
- Ospelt, C.; Brentano, F.; Rengel, Y.; Stanczyk, J.; Kolling, C.; Tak, P.P.; Gay, R.E.; Gay, S.; Kyburz, D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2008, 58, 3684–3692. [Google Scholar] [CrossRef]
- Seibl, R.; Birchler, T.; Loeliger, S.; Hossle, J.P.; Gay, R.E.; Saurenmann, T.; Michel, B.A.; Seger, R.A.; Gay, S.; Lauener, R.P. Expression and Regulation of Toll-Like Receptor 2 in Rheumatoid Arthritis Synovium. Am. J. Pathol. 2003, 162, 1221–1227. [Google Scholar] [CrossRef]
- Kim, K.-W.; Cho, M.-L.; Lee, S.-H.; Oh, H.-J.; Kang, C.-M.; Ju, J.H.; Min, S.-Y.; Cho, Y.-G.; Park, S.-H.; Kim, H.-Y. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol. Lett. 2007, 110, 54–64. [Google Scholar] [CrossRef]
- Brentano, F.; Schorr, O.; Gay, R.E.; Gay, S.; Kyburz, D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via toll-like receptor 3. Arthritis Rheum. 2005, 52, 2656–2665. [Google Scholar] [CrossRef]
- Roelofs, M.F.; Joosten, L.A.; Abdollahi-Roodsaz, S.; Van Lieshout, A.W.; Sprong, T.; Van Den Hoogen, F.H.; Van Den Berg, W.B.; Radstake, T.R. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2005, 52, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.H.; Zhang, J.; Yu, X.; Qin, L.; Kang, L.; Zhang, P. New Therapeutic Approaches for the Treatment of Rheumatoid Arthritis may Rise from the Cholinergic Anti-Inflammatory Pathway and Antinociceptive Pathway. Sci. World J. 2010, 10, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Plum, S.M.; Park, E.J.; Strawn, S.J.; Moore, E.G.; Sidor, C.F.; E Fogler, W. Disease modifying and antiangiogenic activity of 2-Methoxyestradiol in a murine model of rheumatoid arthritis. BMC Musculoskelet. Disord. 2009, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Larionova, R.V.; Brooks, W.H.; Bettacchioli, E.; Renaudineau, Y. Toll-like Receptors, Infections, and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2020, 58, 172–181. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, W.; Jiang, C.; He, X.; Hou, W.; Zheng, F.; Holmdahl, R.; Lu, S. Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res. Ther. 2010, 12, R103. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, M.; Takeda, K.; Rezaei, N. Prophylactic and therapeutic implications of toll-like receptor ligands. Med. Res. Rev. 2010, 32, 294–325. [Google Scholar] [CrossRef]
- Makkouk, A.; Abdelnoor, A.M. The potential use of toll-like receptor (TLR) agonists and antagonists as prophylactic and/or therapeutic agents. Immunopharmacol. Immunotoxicol. 2009, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Clanchy, F.I.; Sacre, S.M. Modulation of toll-like receptor function has therapeutic potential in autoimmune disease. Expert Opin. Biol. Ther. 2010, 10, 1703–1716. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Unterberger, S.; A Davies, K.; Rambhatla, S.B.; Sacre, S. Contribution of Toll-Like Receptors and the NLRP3 Inflammasome in Rheumatoid Arthritis Pathophysiology. ImmunoTargets Ther. 2021, 10, 285–298. [Google Scholar] [CrossRef]
- Choulaki, C.; Papadaki, G.; Repa, A.; Kampouraki, E.; Kambas, K.; Ritis, K.; Bertsias, G.; Boumpas, D.T.; Sidiropoulos, P. Enhanced activity of NLRP3 inflammasome in peripheral blood cells of patients with active rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 257. [Google Scholar] [CrossRef]
- Guo, C.; Fu, R.; Wang, S.; Huang, Y.; Li, X.; Zhou, M.; Zhao, J.; Yang, N. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin. Exp. Immunol. 2018, 194, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Kolly, L.; Busso, N.; Palmer, G.; Talabot-Ayer, D.; Chobaz, V.; So, A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology 2010, 129, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Q.; Zhu, J.; Guo, H.; Zhai, Q.; Li, B.; Jin, Y.; He, X.; Jin, F. Comparison of therapeutic effects of different mesenchymal stem cells on rheumatoid arthritis in mice. PeerJ 2019, 7, e7023. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Shin, I.S.; Song, J.W.; Lee, M.; Yun, T.W.; Yang, J.; Choi, K.-S.; Kim, S.-J. Effects of Transplantation of CTLA4Ig-Overexpressing Adipose Tissue-Derived Mesenchymal Stem Cells in Mice with Sustained Severe Rheumatoid Arthritis. Cell Transplant. 2016, 25, 243–259. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Monteiro, A.P.T.; Tsoporis, J.N.; Mei, S.H.J.; Stewart, D.J.; dos Santos, C.C. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev. Rep. 2020, 16, 812–827. [Google Scholar] [CrossRef]
- Park, N.; Rim, Y.A.; Jung, H.; Kim, J.; Yi, H.; Kim, Y.; Jang, Y.; Jung, S.M.; Lee, J.; Kwok, S.-K.; et al. Etanercept-Synthesising Mesenchymal Stem Cells Efficiently Ameliorate Collagen-Induced Arthritis. Sci. Rep. 2017, 7, 39593. [Google Scholar] [CrossRef]
- Liu, L.N.; Wang, G.; Hendricks, K.; Lee, K.; Bohnlein, E.; Junker, U.; Mosca, J.D. Comparison of Drug and Cell-Based Delivery: Engineered Adult Mesenchymal Stem Cells Expressing Soluble Tumor Necrosis Factor Receptor II Prevent Arthritis in Mouse and Rat Animal Models. STEM CELLS Transl. Med. 2013, 2, 362–375. [Google Scholar] [CrossRef]
- Lopez-Santalla, M.; Fernandez-Perez, R.; Garin, M.I. Mesenchymal Stem/Stromal Cells for Rheumatoid Arthritis Treatment: An Update on Clinical Applications. Cells 2020, 9, 1852. [Google Scholar] [CrossRef]
- Glocker, M.O.; Guthke, R.; Kekow, J.; Thiesen, H.J. Rheumatoid arthritis, a complex multifactorial disease: On the way toward in-dividualized medicine. Med. Res. Rev. 2006, 26, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Furst, D.E.; Bharat, A.; Curtis, J.R.; Kavanaugh, A.F.; Kremer, J.M.; Moreland, L.W.; O’Dell, J.; Winthrop, K.L.; Beukelman, T.; et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012, 64, 625–639. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, J.R.; Mikuls, T.R.; Taylor, T.H.; Ahluwalia, V.; Brophy, M.; Warren, S.R.; Lew, R.A.; Cannella, A.C.; Kunkel, G.; Phibbs, C.S.; et al. Therapies for Active Rheumatoid Arthritis after Methotrexate Failure. N. Engl. J. Med. 2013, 369, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, Z.; Ning, D.; Fu, Y. Rosmanol and Carnosol Synergistically Alleviate Rheumatoid Arthritis through Inhibiting TLR4/NF-κB/MAPK Pathway. Molecules 2021, 27, 78. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Liu, Y.; Wu, H.; He, Y.; Li, C.; Wang, Q.; Su, X.; Yan, S.; Su, W.; et al. A Novel Drug Combination of Mangiferin and Cinnamic Acid Alleviates Rheumatoid Arthritis by Inhibiting TLR4/NFκB/NLRP3 Activation-Induced Pyroptosis. Front. Immunol. 2022, 13, 912933. [Google Scholar] [CrossRef]
- Werner, L.E.; Wagner, U. Calcium-sensing receptor-mediated NLRP3 inflammasome activation in rheumatoid arthritis and autoinflammation. Front. Physiol. 2023, 13, 1078569. [Google Scholar] [CrossRef]
- Li, R.-N.; Ou, T.-T.; Lin, C.-H.; Lin, Y.-Z.; Fang, T.-J.; Chen, Y.-J.; Tseng, C.-C.; Sung, W.-Y.; Wu, C.-C.; Yen, J.-H. NLRP3 Gene Polymorphisms in Rheumatoid Arthritis and Primary Sjogren’s Syndrome Patients. Diagnostics 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Zhang, C.; Huang, M.; Yu, H.; Wang, Y.; Wang, Y. Mitochondrion-NLRP3 inflammasome activation in macrophages: A novel mechanism of the anti-inflammatory effect of Notopterygium in rheumatoid arthritis treatment. Biomed. Pharmacother. 2023, 167, 115560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Dong, S.; Yin, H.; Yang, Y.; Xiong, G. Regulatory effect of zinc finger protein A20 on rheumatoid arthritis through NLRP3/Caspase-1 signaling axis mediating pyroptosis of HFLS- RA cells. Cell. Mol. Biol. 2023, 69, 179–184. [Google Scholar] [CrossRef]
- Sun, H.-G.; Jiang, Q.; Fan, W.-J.; Shen, X.-Y.; Wang, Z.-W.; Wang, X. TAGAP activates Th17 cell differentiation by promoting RhoA and NLRP3 to accelerate rheumatoid arthritis development. Clin. Exp. Immunol. 2023, uxad084. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, T.; Shen, J.; Shi, X.; Luo, F.; Ren, Y. Sulforaphene targets NLRP3 inflammasome to suppress M1 polarization of macrophages and inflammatory response in rheumatoid arthritis. J. Biochem. Mol. Toxicol. 2023, 37, e23362. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Z.; Zhang, Q.; Yu, J.; Han, D.; Liu, J.; Li, P.; Li, F. Osthole: A potential AMPK agonist that inhibits NLRP3 inflammasome activation by regulating mitochondrial homeostasis for combating rheumatoid arthritis. Phytomedicine 2023, 110, 154640. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gao, Y.; Gao, Y.; Ding, Y.; Ding, Y.; Jiang, Y.; Jiang, Y.; Chen, H.; Chen, H.; et al. Targeting KAT2A inhibits inflammatory macrophage activation and rheumatoid arthritis through epigenetic and metabolic reprogramming. Medcomm 2023, 4, e306. [Google Scholar] [CrossRef]
- Elbasha, Y.I.; Mesbah, N.M.; Abdel-Hamed, A.R.; Abo-Elmatty, D.M.; Bakry, S.; Mansour, A.M.; Elbeialy, A.A. Effect of autologous bone marrow derived mesenchymal stem cells in treatment of rheumatoid arthritis. Transpl. Immunol. 2023, 80, 101890. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Khorashad, M.; Ghoryani, M.; Shabgah, A.G.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. The Effects of Mesenchymal Stem Cells on the Gene Expression of TGF-beta and IFN-gamma in Patients with Rheumatoid Arthritis. Iran. J. Allergy Asthma Immunol. 2023, 22, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Lim, I.-R.; Park, J.H.; Song, J.; Choi, B.; Kim, S. Exosomes derived from mesenchymal stem cells primed with disease-condition-serum improved therapeutic efficacy in a mouse rheumatoid arthritis model via enhanced TGF-β1 production. Stem Cell Res. Ther. 2023, 14, 283. [Google Scholar] [CrossRef]
- Zeng, Y.-X.; Chou, K.-Y.; Hwang, J.-J.; Wang, H.-S. The effects of IL-1β stimulated human umbilical cord mesenchymal stem cells on polarization and apoptosis of macrophages in rheumatoid arthritis. Sci. Rep. 2023, 13, 10612. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Amirsaadat, S.; Jalil, A.T.; Kadhim, M.M.; Abasi, M.; Pilehvar, Y. Curcumin-Loaded Mesenchymal Stem Cell–Derived Exosomes Efficiently Attenuate Proliferation and Inflammatory Response in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Appl. Biochem. Biotechnol. 2022, 195, 51–67. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, X.; Abdollahi, E.; Tavasolian, F. Genetically Engineered Exosomes as a Potential Regulator of Th1 Cells Response in Rheumatoid Arthritis. Biopreservation Biobanking 2023, 21, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Rui, K.; Tang, X.; Shen, Z.; Jiang, C.; Zhu, Q.; Liu, S.; Che, N.; Tian, J.; Ling, J.; Yang, Y. Exosome inspired photo-triggered gelation hydrogel composite on modulating immune pathogenesis for treating rheumatoid arthritis. J. Nanobiotechnology 2023, 21, 111. [Google Scholar] [CrossRef]

| Emerging Therapy | Author | Findings | Year | Ref. |
|---|---|---|---|---|
| TLR4 | Li et al. | Rosmanol and Carnosol synergistically alleviate RA by inhibiting TLR4/NF-κB/MAPK pathway | 2021 | [105] |
| TLR4 | Li et al. | A novel drug combination of mangiferin and cinnamic acid alleviates RA by inhibiting TLR4/NFκB/NLRP3 activation-induced pyroptosis | 2022 | [106] |
| NLRP3 inflammasome | Werner and Wagner | Increased extracellular Ca2+, calciprotein particles, and pro-inflammatory cytokines drive a vicious cycle of inflammation and bone destruction which in turn offers new potential therapeutic approaches. | 2023 | [107] |
| NLRP3 inflammasome | Li et al. | NLRP3 gene polymorphisms may play a role in the pathogenesis of RA and primary SS. The T allele of rs4612666 CT increased the susceptibility to RA disease. | 2023 | [108] |
| NLRP3 inflammasome | Liu et al. | The anti-inflammatory and antirheumatic effect of notopterygium may involve regulating NLRP3 inflammasome activation through mitochondria and NLRP3 is probably the key target molecule of notopterygium in the treatment of RA. | 2023 | [109] |
| NLRP3 inflammasome | Zhao et al. | Regulatory effect of zinc finger protein A20 on rheumatoid arthritis through NLRP3/Caspase-1 signaling axis mediating pyroptosis of Human Fibroblast-Like Synoviocytes (HFL)-RA cells | 2023 | [110] |
| NLRP3 inflammasome | Sun et al. | T-cell activation Rho GTPase activating protein (TAGAP) activates Th17 cell differentiation by promoting RhoA and NLRP3 to accelerate rheumatoid arthritis development | 2023 | [111] |
| NLRP3 inflammasome | Ye et al. | Sulforaphene targets NLRP3 inflammasome to suppress M1 polarization of macrophages and inflammatory response in rheumatoid arthritis | 2023 | [112] |
| NLRP3 inflammasome | Jiang et al. | Osthole (OST), a characteristic coumarin compound which is demonstrated as a potential AMPK agonist that inhibits NLRP3 inflammasome activation by regulating mitochondrial homeostasis for combating rheumatoid arthritis | 2023 | [113] |
| NLRP3 inflammasome | Zhang et al. | acetyltransferase KAT2A licenses metabolic and epigenetic reprogramming for NLRP3 inflammasome activation in inflammatory macrophages, thereby targeting KAT2A represents a potential therapeutic approach for patients suffering from RA. | 2023 | [114] |
| Mesenchymal stem cells | Elbasha et al. | Effect of autologous bone marrow-derived mesenchymal stem cells in treatment of rheumatoid arthritis | 2023 | [115] |
| Mesenchymal stem cells | Khorashad et al. | A significant change in the gene expression of TGFB1 and IFNG was consistent with the amelioration of clinical manifestations, suggesting a mechanism of action for MSCs in the treatment of RA. | 2023 | [116] |
| Mesenchymal stem cells | Choi et al. | Exosomes derived from mesenchymal stem cells primed with disease-condition-serum improved therapeutic efficacy in a mouse rheumatoid arthritis model via enhanced TGF-β1 production | 2023 | [117] |
| Mesenchymal stem cells | Zeng et al. | The effects of IL-1β stimulated human umbilical cord mesenchymal stem cells on polarization and apoptosis of macrophages in rheumatoid arthritis | 2023 | [118] |
| Mesenchymal stem cells | He et al. | Curcumin-loaded mesenchymal stem cell-derived exosomes efficiently attenuate proliferation and inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes | 2023 | [119] |
| Mesenchymal stem cells | Ren et al. | Genetically engineered MSC-derived exosomes as a potential regulator of Th1 cell response in rheumatoid arthritis | 2023 | [120] |
| Mesenchymal stem cells | Rui et al. | A promising PD-L1 expression was identified on the exosomes, which potently suppressed Tfh cell polarization via inhibiting the PI3K/AKT pathway. | 2023 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefi, M.; Lewandrowski, K.-U.; Lorio, M.; Fiorelli, R.K.A., on behalf of the Brazilian Society For Thoracic Surgery—Sociedade Brasileira de Cirurgia Torácica (SBCT); Landgraeber, S.; Sharafshah, A. Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis. J. Pers. Med. 2023, 13, 1550. https://doi.org/10.3390/jpm13111550
Assefi M, Lewandrowski K-U, Lorio M, Fiorelli RKA on behalf of the Brazilian Society For Thoracic Surgery—Sociedade Brasileira de Cirurgia Torácica (SBCT), Landgraeber S, Sharafshah A. Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis. Journal of Personalized Medicine. 2023; 13(11):1550. https://doi.org/10.3390/jpm13111550
Chicago/Turabian StyleAssefi, Marjan, Kai-Uwe Lewandrowski, Morgan Lorio, Rossano Kepler Alvim Fiorelli on behalf of the Brazilian Society For Thoracic Surgery—Sociedade Brasileira de Cirurgia Torácica (SBCT), Stefan Landgraeber, and Alireza Sharafshah. 2023. "Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis" Journal of Personalized Medicine 13, no. 11: 1550. https://doi.org/10.3390/jpm13111550






