Enhancement of Immune Response of Bioconjugate Nanovaccine by Loading of CpG through Click Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Preparation of NP-OPS-CpG via Click Chemistry
2.3. Coomassie Blue Staining and Western Blotting
2.4. Stimulation of DC2.4 Mouse Dendritic Cell Line (DC2.4s) by Vaccines In Vitro
2.5. Lymph Node Imaging Assay
2.6. Mouse Immunization
2.7. T Cell Immune Response Induced by the Vaccines In Vivo
2.8. ELISA
2.9. Immune Effects of the NP-OPS-CpG in an S. flexneri 2a Infection Model
2.10. Statistical Analysis
3. Results
3.1. Conjugation of NP-OPS and CpG
3.2. Analysis of the NP-OPS-CpG Bioconjugate Nanovaccine
3.3. Evaluation of CpG Lymph Node Targeting
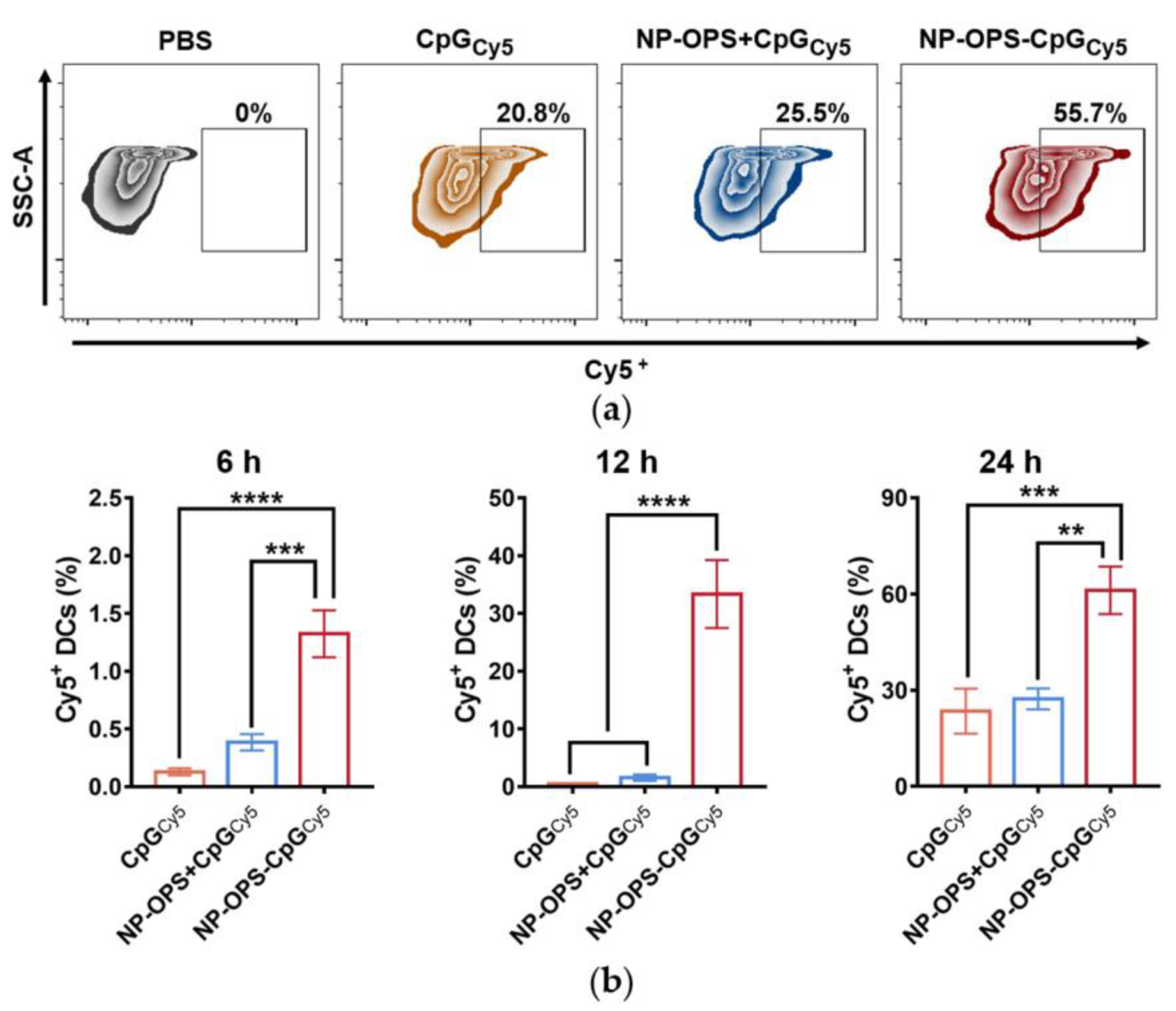
3.4. Analysis of CpG Phagocytosis by DC2.4s
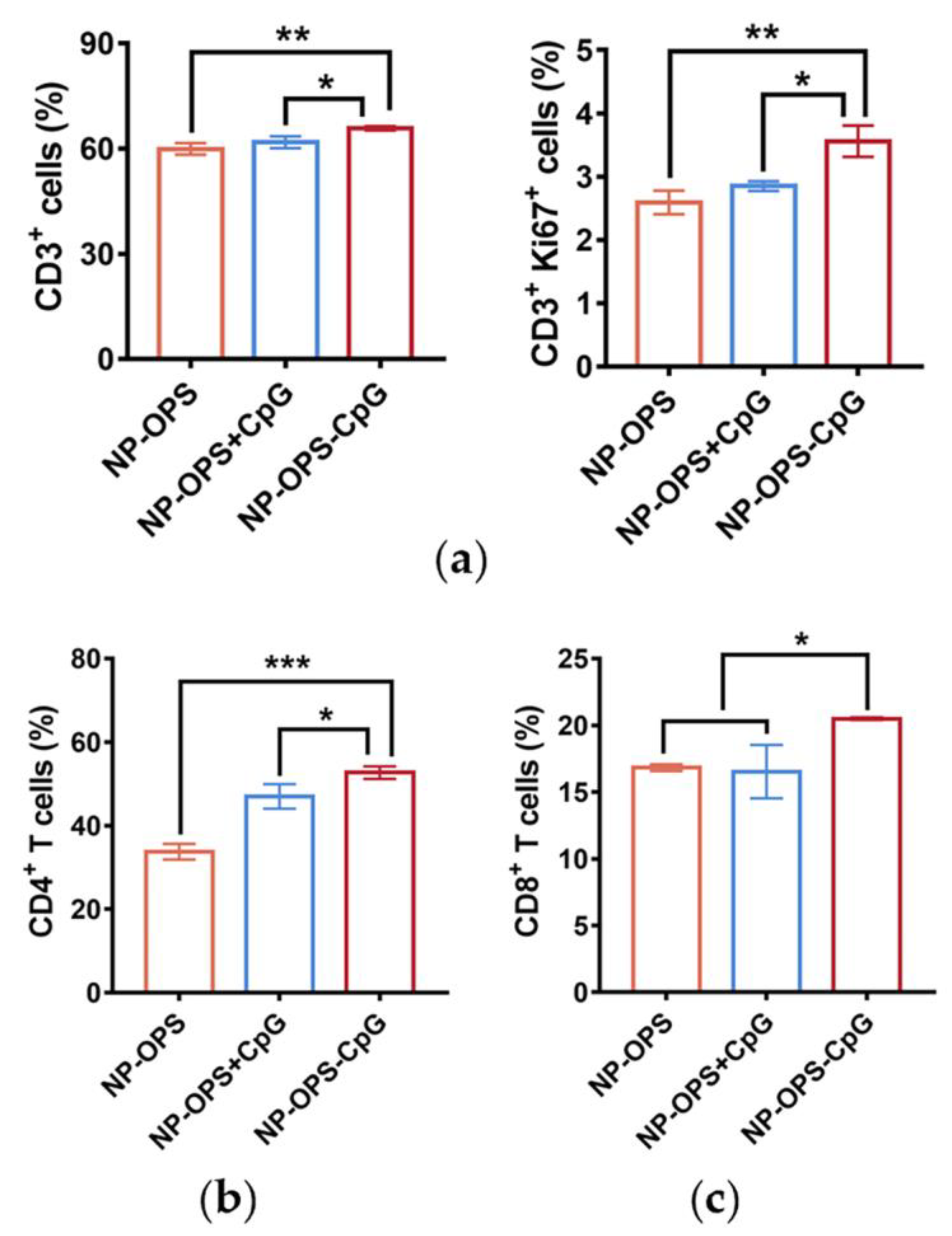
3.5. T Cell Proliferation and Differentiation Induced by the Nanovaccines
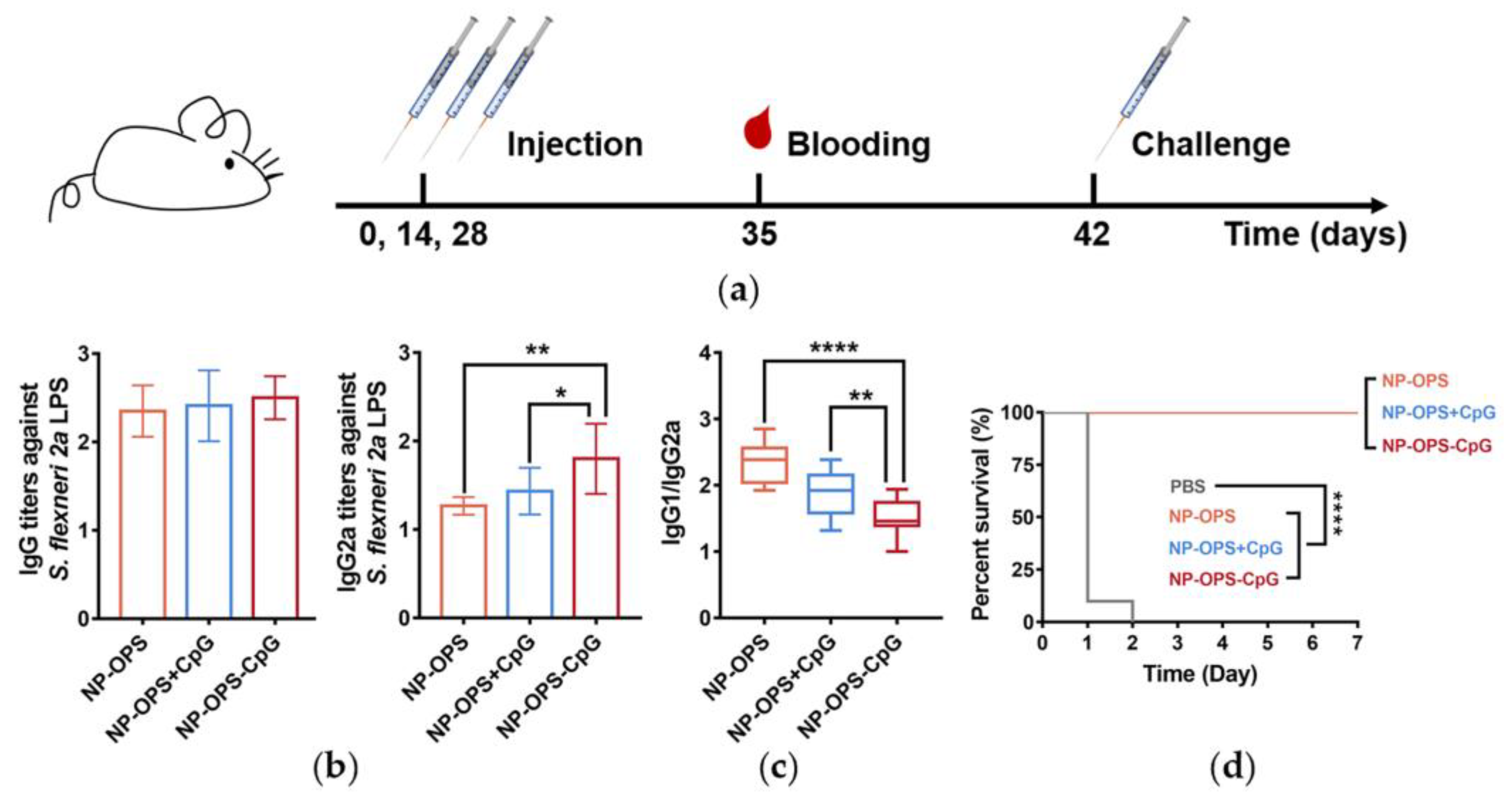
3.6. Antibody Response and Protective Effect in NP-OPS-CpG Immunized Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurt Yilmaz, N.; Schiffer, C.A. Introduction: Drug Resistance. Chem. Rev. 2021, 121, 3235–3237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv. Mater. 2020, 32, e1901255. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Chandrudu, S.; Skwarczynski, M.; Toth, I. The application of self-assembled nanostructures in peptide-based subunit vaccine development. Eur. Polym. J. 2017, 93, 670–681. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, C.; Wang, K.; Liu, Y.; Sun, Y.; Guo, Y.; Sun, P.; Wu, J.; Lu, Y.; Zhu, L.; et al. Construction of Orthogonal Modular Proteinaceous Nanovaccine Delivery Vectors Based on mSA-Biotin Binding. Nanomaterials 2022, 12, 734. [Google Scholar] [CrossRef]
- Pan, C.; Wu, J.; Qing, S.; Zhang, X.; Zhang, L.; Yue, H.; Zeng, M.; Wang, B.; Yuan, Z.; Qiu, Y.; et al. Biosynthesis of Self-Assembled Proteinaceous Nanoparticles for Vaccination. Adv. Mater. 2020, 32, e2002940. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, J.; Wang, K.; Li, X.; Sun, P.; Zhang, L.; Huang, J.; Liu, Y.; Hua, X.; Yu, Y.; et al. Production of a Promising Biosynthetic Self-Assembled Nanoconjugate Vaccine against Klebsiella Pneumoniae Serotype O2 in a General Escherichia Coli Host. Adv. Sci. 2021, 8, e2100549. [Google Scholar] [CrossRef]
- Koestler, B.J.; Fisher, C.R.; Payne, S.M. Formate Promotes Shigella Intercellular Spread and Virulence Gene Expression. mBio 2018, 9, e01777-18. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Zhou, Z.; Li, B.; Xiao, Y.; Li, M.; Zeng, H.; Guo, X.; Gu, G. The Mechanism of Facultative Intracellular Parasitism of Brucella. Int. J. Mol. Sci. 2021, 22, 3673. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Shan, P.; Wei, D.; Hao, S.; Zhang, Z.; Xu, J. Improved Aluminum Adjuvants Eliciting Stronger Immune Response When Mixed with Hepatitis B Virus Surface Antigens. ACS Omega 2022, 7, 34528–34537. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, I.; Suzuki, Y.; Oshiumi, H.; Begum, N.A.; Ebihara, T.; Matsumoto, M.; Hazeki, K.; Kodama, K.; Kashiwazaki, Y.; Seya, T. Recombinant interleukin-12 and interleukin-18 antitumor therapy in a guinea-pig hepatoma cell implant model. Cancer Sci. 2007, 98, 1936–1942. [Google Scholar] [CrossRef]
- Novick, D.; Kim, S.; Kaplanski, G.; Dinarello, C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013, 25, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Strbo, N.; Yamazaki, K.; Lee, K.; Rukavina, D.; Podack, E.R. Heat shock fusion protein gp96-Ig mediates strong CD8 CTL expansion in vivo. Am. J. Reprod. Immunol. 2002, 48, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Singh-Jasuja, H.; Scherer, H.U.; Hilf, N.; Arnold-Schild, D.; Rammensee, H.G.; Toes, R.E.; Schild, H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur. J. Immunol. 2000, 30, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Che, Y.; Zhao, Y.; Wang, X. Prevention and treatment of cervical cancer by a single administration of human papillomavirus peptide vaccine with CpG oligodeoxynucleotides as an adjuvant in vivo. Int. Immunopharmacol. 2019, 69, 279–288. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Klinman, D.M.; Klaschik, S.; Sato, T.; Tross, D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv. Drug Deliv. Rev. 2009, 61, 248–255. [Google Scholar] [CrossRef]
- Cooper, C.L.; Davis, H.L.; Angel, J.B.; Morris, M.L.; Elfer, S.M.; Seguin, I.; Krieg, A.M.; Cameron, D.W. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. Aids 2005, 19, 1473–1479. [Google Scholar] [CrossRef]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wu, D.; Zhao, S.; Shao, Y.; Xia, Y.; Ni, D.; Qiu, X.; Zhang, J.; Chen, J.; Meng, F.; et al. Immunotherapy of Malignant Glioma by Noninvasive Administration of TLR9 Agonist CpG Nano-Immunoadjuvant. Adv. Sci. 2022, 9, e2103689. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Gursel, I.; Ivins, B.E.; Singh, M.; O’Hagan, D.T.; Ulmer, J.B.; Klinman, D.M. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect. Immun. 2005, 73, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinman, D.M.; Xie, H.; Little, S.F.; Currie, D.; Ivins, B.E. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 2004, 22, 2881–2886. [Google Scholar] [CrossRef]
- Klinman, D.M.; Currie, D.; Lee, G.; Grippe, V.; Merkel, T. Systemic but not mucosal immunity induced by AVA prevents inhalational anthrax. Microbes Infect. 2007, 9, 1478–1483. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants. medRxiv 2020, 27, 1071–1078. [Google Scholar] [CrossRef]
- Chen, N.; Wei, M.; Sun, Y.; Li, F.; Pei, H.; Li, X.; Su, S.; He, Y.; Wang, L.; Shi, J.; et al. Self-assembly of poly-adenine-tailed CpG oligonucleotide-gold nanoparticle nanoconjugates with immunostimulatory activity. Small 2014, 10, 368–375. [Google Scholar] [CrossRef]
- Weiner, G.J.; Liu, H.M.; Wooldridge, J.E.; Dahle, C.E.; Krieg, A.M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc. Natl. Acad. Sci. USA 1997, 94, 10833–10837. [Google Scholar] [CrossRef] [Green Version]
- Mi, T.; Wang, T.; Xu, H.; Sun, P.; Hou, X.; Zhang, X.; Ke, Q.; Liu, J.; Hu, S.; Wu, J.; et al. Kappa-RBD produced by glycoengineered Pichia pastoris elicited high neutralizing antibody titers against pseudoviruses of SARS-CoV-2 variants. Virology 2022, 569, 56–63. [Google Scholar] [CrossRef]
- Chu, R.S.; McCool, T.; Greenspan, N.S.; Schreiber, J.R.; Harding, C.V. CpG Oligodeoxynucleotides Act as Adjuvants for Pneumococcal Polysaccharide-Protein Conjugate Vaccines and Enhance Antipolysaccharide Immunoglobulin G2a (IgG2a) and IgG3 Antibodies. Infect. Immun. 2000, 68, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.M.; Ajit, J.; Albin, T.J.; Kapoor, N.; Maroju, S.; Berges, A.; Pill, L.; Fairman, J.; Esser-Kahn, A.P. Site-specific antigen-adjuvant conjugation using cell-free protein synthesis enhances antigen presentation and CD8(+) T-cell response. Sci. Rep. 2021, 11, 6267. [Google Scholar] [CrossRef]
- Xi, X.; Zhang, L.; Lu, G.; Gao, X.; Wei, W.; Ma, G. Lymph Node-Targeting Nanovaccine through Antigen-CpG Self-Assembly Potentiates Cytotoxic T Cell Activation. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Lau, S.; Graham, B.; Cao, N.; Boyd, B.J.; Pouton, C.W.; White, P.J. Enhanced extravasation, stability and in vivo cardiac gene silencing via in situ siRNA-albumin conjugation. Mol. Pharm. 2012, 9, 71–80. [Google Scholar] [CrossRef]
- Chrysanthou, A.; Bosch-Fortea, M.; Gautrot, J.E. Co-Surfactant-Free Bioactive Protein Nanosheets for the Stabilization of Bioemulsions Enabling Adherent Cell Expansion. Biomacromolecules 2023. [Google Scholar] [CrossRef]
- Ji, W.; Smith, P.N.; Koepsel, R.R.; Andersen, J.D.; Baker, S.L.; Zhang, L.; Carmali, S.; Myerson, J.W.; Muzykantov, V.; Russell, A.J. Erythrocytes as carriers of immunoglobulin-based therapeutics. Acta Biomater. 2020, 101, 422–435. [Google Scholar] [CrossRef]
- Feige, M.J.; Braakman, I.; Hendershot, L.M. Disulfide Bonds in Protein Folding and Stability. In Oxidative Folding of Proteins: Basic Principles, Cellular Regulation and Engineering; Feige, M.J., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Jiang, X.; Hao, X.; Jing, L.; Wu, G.; Kang, D.; Liu, X.; Zhan, P. Recent applications of click chemistry in drug discovery. Expert Opin. Drug Discov. 2019, 14, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Boatner, L.M.; Desai, H.S.; Burton, N.R.; Armenta, E.; Chan, N.J.; Castellon, J.O.; Backus, K.M. Multiplexed CuAAC Suzuki-Miyaura Labeling for Tandem Activity-Based Chemoproteomic Profiling. Anal. Chem. 2021, 93, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Yasuda, K.; Ogawa, Y.; Nishikawa, M.; Takakura, Y. DNA and its cationic lipid complexes induce CpG motif-dependent activation of murine dendritic cells. Immunology 2007, 120, 295–302. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.A.; Gerlach, M.; Schneider, A.F.L.; Groneberg, C.; Ochtrop, P.; Boldt, S.; Schumacher, D.; Helma, J.; Leonhardt, H.; Christmann, M.; et al. N-Hydroxysuccinimide-Modified Ethynylphosphonamidates Enable the Synthesis of Configurationally Defined Protein Conjugates. Chembiochem 2020, 21, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, M.; Li, X.; Li, C.; Wang, K.; Li, S.; Guo, Y.; Sun, P.; Wu, J.; Lu, Y.; Pan, C.; et al. Enhancement of Immune Response of Bioconjugate Nanovaccine by Loading of CpG through Click Chemistry. J. Pers. Med. 2023, 13, 507. https://doi.org/10.3390/jpm13030507
Mo M, Li X, Li C, Wang K, Li S, Guo Y, Sun P, Wu J, Lu Y, Pan C, et al. Enhancement of Immune Response of Bioconjugate Nanovaccine by Loading of CpG through Click Chemistry. Journal of Personalized Medicine. 2023; 13(3):507. https://doi.org/10.3390/jpm13030507
Chicago/Turabian StyleMo, Mengting, Xiang Li, Caixia Li, Kangfeng Wang, Shulei Li, Yan Guo, Peng Sun, Jun Wu, Ying Lu, Chao Pan, and et al. 2023. "Enhancement of Immune Response of Bioconjugate Nanovaccine by Loading of CpG through Click Chemistry" Journal of Personalized Medicine 13, no. 3: 507. https://doi.org/10.3390/jpm13030507






