Trastuzumab-Mediated Cardiotoxicity and Its Preventive Intervention by Zingerone through Antioxidant and Inflammatory Pathway in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Chemicals
2.3. Experimental Protocol
2.4. Dissection and Homogenization
2.5. Biochemical Estimations
2.5.1. Determination of AST
2.5.2. Determination of CK
2.5.3. LDH Assay
2.5.4. LPO Assay
2.5.5. GSH Assay
2.5.6. GPx Assay
2.5.7. GR Assay
2.5.8. GST Assay
2.5.9. SOD Assay
2.5.10. CAT Assay
2.5.11. Inflammatory Cytokine (IL-2 and TNF-α) Assay
2.5.12. Histopathological Examination
2.5.13. Statistical Analysis
3. Results
3.1. Action of Zingerone on Serum Marker
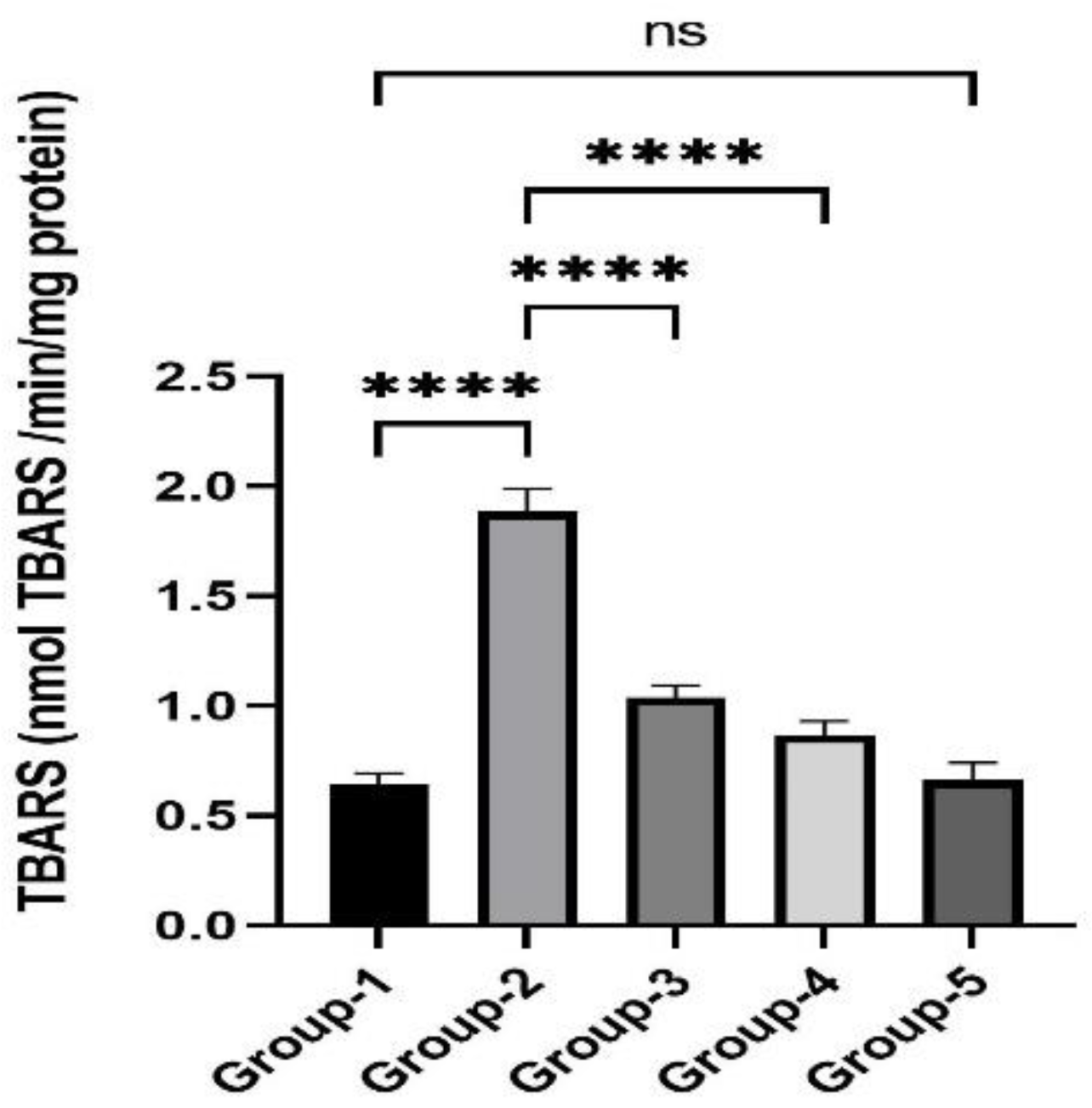
3.2. Action of Zingerone on TBARS
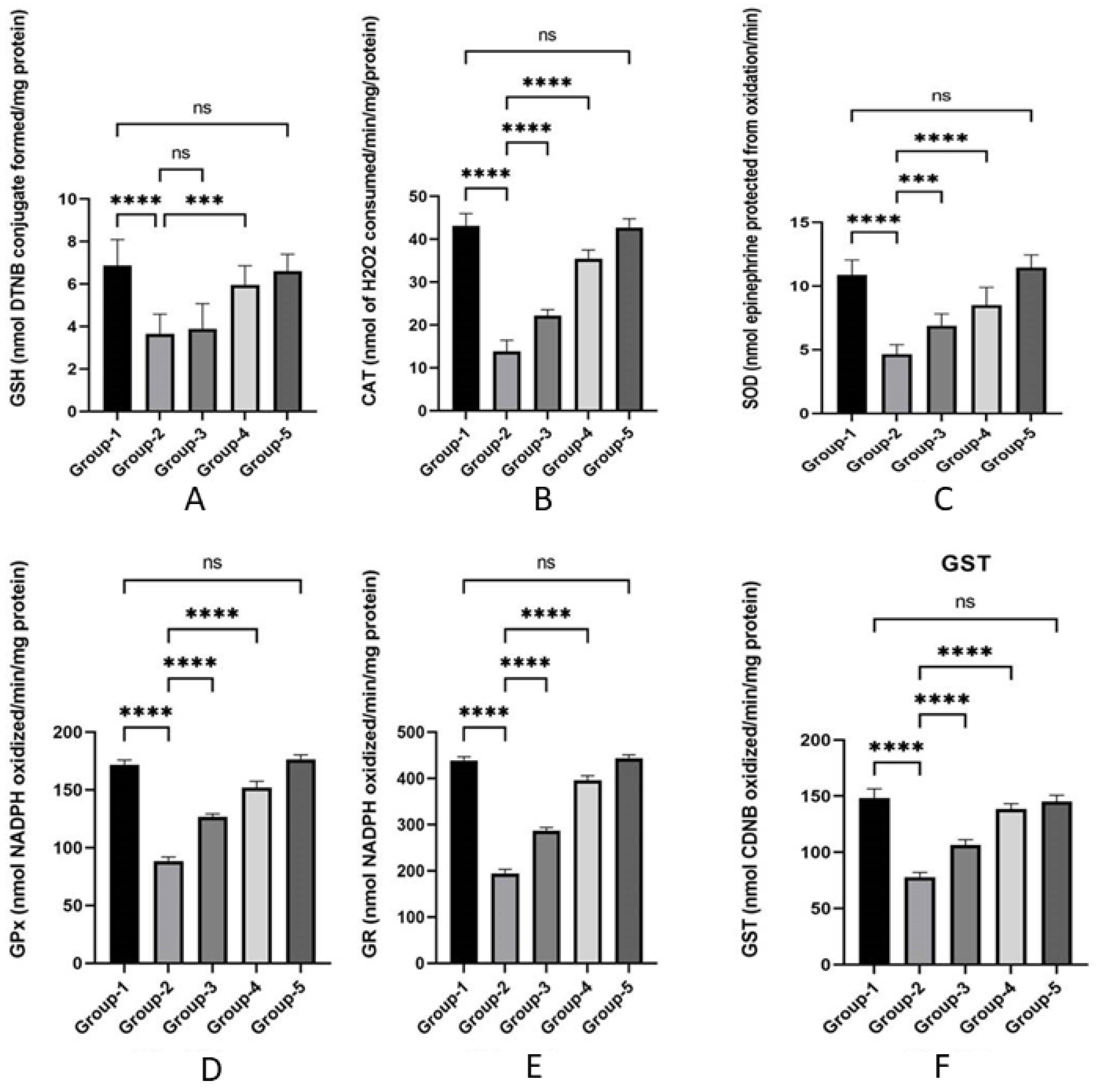
3.3. Action of Zingerone on Antioxidant
3.4. Action of Zingerone on Inflammatory Cytokines
3.5. Action of Zingerone on Histopathological Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline mediated cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Steinhertz, L.J.; Steinhertz, P.G.; Tan, C.T.; Heller, G.; Murphy, M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991, 266, 1672–1677. [Google Scholar] [CrossRef]
- Cadeddu, D.C.; Deidda, M.M.D.; Bassareo, P.P.; Esposito, R.S.C.; Lembo, M.G.M.; Mercuro, G. Chemotherapy-induced cardiotoxicity: New insights into mechanisms, monitoring, and prevention. J. Cardiovasc. Med. 2018, 19, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug mediated mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Cross, M.J.; Berridge, B.R.; Clement, P.J.M.; Cove-Smith, L.; Force, T.L.; Hoffman, P.; Holbrook, A.R.; Lyon, A.R.; Mellor, H.R.; Norris, A.A.; et al. Physiological, pharmacological and toxicological consideration of drug- mediated structural cardiac injury. Br. J. Pharmacol. 2015, 172, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Nebija, D.; Noe, C.R.; Urban, E.; Lachmann, B. Quality control and stability studies with the monoclonal antibody, trastuzumab: Application of 1D- vs. 2D-gel electrophoresis. Int. J. Mol. Sci. 2014, 15, 6399–6411. [Google Scholar] [CrossRef]
- Negro, A.; Brar, B.K.; Gu, Y.; Peterson, K.L.; Vale, W.; Lee, K.F. erbB2 is required for G protein-coupled receptor signaling in the heart. Proc. Natl. Acad. Sci. USA 2006, 103, 15889–15893. [Google Scholar] [CrossRef]
- Zeglinski, M.; Ludke, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A ‘dual-hit’. Exp. Clin. Cardiol. 2011, 16, 70–74. [Google Scholar]
- Negro, A.; Brar, B.K.; Lee, K.F. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog. Horm. Res. 2004, 59, 1–12. [Google Scholar] [CrossRef]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef]
- Madonna, R.; Cadeddu, C.; Deidda, M.; Mele, D.; Monte, I.; Nove, G.; Pagliaro, P.; Pepe, A.; Spallarossa, P.; Tocchetti, C.G.; et al. Improving the potential models for the study of chemotherapy- mediated cardiotoxicity: A position paper of the Italian working group on drug cardiotoxicity and cardioprotection. Heart Fail. Rev. 2015, 20, 621–631. [Google Scholar] [CrossRef]
- Badreldin, H.A.; Gerald, B.; Musbah, O.; Tanira, A.N. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar]
- Wang, S.; Zhang, C.; Yang, G.; Yang, Y. Biological properties of 6-gingerol: A brief review. Nat. Prod. Commun. 2014, 9, 1027–1030. [Google Scholar] [CrossRef]
- Rao, B.N.; Rao, B.S. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-mediated cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis 2010, 25, 577–587. [Google Scholar]
- Zhang, Y.X.; Li, J.; Chen, L.; Peng, W.; Cai, B. Simultaneous determination of five gingerols in raw and processed ginger by HPLC. Chin. Pharm. J. 2012, 47, 471–474. [Google Scholar]
- Kim, K.M.; Chung, S.W.; Kim, D.H.; Kim, J.M.; Lee, E.K.; Kim, J.Y.; Ha, Y.M.; Kim, Y.H.; No, J.-K.; Chung, H.S.; et al. Modulation of age-related NF-KappaB activation by Zingerone via MAPK pathway. Exp. Gerontol. 2010, 45, 419–426. [Google Scholar] [CrossRef]
- Alam, M.F.; Safhi, M.M.; Anwer, T.; Siddiqui, R.; Khan, G.; Moni, S.S. Therapeutic potential of Vanillylacetone against CCl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in Swiss albino mice. Exp. Mol. Pathol. 2018, 105, 81–88. [Google Scholar] [CrossRef]
- Alam, M.F.; Alshahrani, S.; Alamer, E.A.; Alhazmi, M.A.; Anwer, T.; Khan, G.; Khan, A.; Tanweer, K.T.; Moni, S.S. Nephroprotective effects of 4-4(hydroxyl-3 methoxyphenyl)-2-butane against sodium tellurite induced acute kidney dysfunction by attenuating oxidative stress and inflammatory cytokine in rats. Arab. J. Chem. 2022, 15, 103857. [Google Scholar] [CrossRef]
- Ayman, M.M.; Khaled, E.A.S.; Fahad, A.A.; Ahmad, A.; Khaled, S.A.; Mohammad, A.A.; Faris, A.; Mohammad, A.; Ameen, S.S.A.; Ahmed, A.A.; et al. Could allicin alleviate trastuzumab- mediated cardiotoxicity in a rat model through antioxidant, anti-inflammatory, and antihyperlipidemic properties? Life Sci. 2022, 302, 120656. [Google Scholar]
- Reitman, S.; Frankel, A.S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- David, J.R.; Robert, H.C. Creatine kinase and its CK-MB isoenzyme: The conventional marker for the diagnosis of acute myocardial infarction. J. Emerg. Med. 1999, 7, 95–104. [Google Scholar]
- Lum, G.; Gambino, S.R. A comparison of serum versus heparinized plasma for routine chemistry tests. Am. J. Clin. Pathol. 1974, 61, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurements with the Folin’s reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984, 44, 5086–5091. [Google Scholar]
- Haque, R.; Bin-Hafeez, B.; Parvez, S.; Pandey, S.; Saeed, I.; Ali, M.; Raisuddin, S. Aqueous extract of walnut (Juglansregia L.) protects mice against cyclophosphamide- mediated biomedical toxicity. Hum. Exp. Toxicol. 2003, 22, 473. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Alam, M.F.; Ajeibi, A.O.; Safhi, M.H.; Alabdly, A.J.A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Jali, A.M.; Alqahtani, S.; Nomier, Y.; et al. Therapeutic Potential of Capsaicin against Cyclophosphamide-Induced Liver Damage. J. Clin. Med. 2023, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, C.; Huang, Y.; He, M.; Xu, W.W.; Li, B. Molecular mechanism of chemo-and radiotherapy resistance and the potential implications for cancer treatment. Med. Comm. 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Keefe, D.L. Trastuzumab-associated cardiotoxicity. Cancer 2002, 95, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Olorundare, O.; Adeneya, A.; Akinsola, A.; Soyemi, S.; Mgbehoma, A.; Okoye, I.; Ntambi, J.M.; Mukhtar, H. African Vegetable (Clerodebdrumvolibile leaf and irvingiagabonensis seed extract) effectively mitigate trastuzumab-mediated cardiotoxicity in wistar rats. Oxidative Med. Cell. Longev. 2020, 15, 9535426. [Google Scholar]
- Zhang, S.; Meng, T.; Liu, J.; Zhang, X.; Jhang, J. Cardioprotective effect of dexrazoxane on animal cardiotoxicity model mediated by anthracycline combine with trastuzumab is associated with upregulation of calpain-2. Medicine 2015, 94, e445. [Google Scholar] [CrossRef]
- Yavas, G.; Celik, E.; Yavas, C.; Elsurer, C.; Afsar, R.E. Spironolactone ameliorates the cardiovascular toxicity mediated by concomitant trastuzumab and thoracic radiotheraoy. Rep. Pract. Oncol. Radiother. 2017, 22, 295–302. [Google Scholar] [CrossRef]
- Khan, G.; Haque, S.E.; Anwer, T.; Ahsan, M.N.; Safhi, M.M.; Alam, M.F. Cardioprotective effect of green tea extract on doxorubicin-mediated cardiotoxicity in rats. Acta Pol. Pharm. Drug Res. 2014, 71, 861–868. [Google Scholar]
- Alam, M.F.; Khan, G.; Safhi, M.M.; Alshahrani, S.; Siddiqui, R.; Moni, S.S.; Anwer, T. Thymoquinone ameliorates doxorubicin-mediated cardiotoxicity in Swiss albino rats by modulating oxidative damage and cellular Inflammation. Cardiol. Res. Pract. 2018, 2018, 1483041. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, Z.; Segers, V.F.; De Keulenaer, G.W. Signaling at the crossing between heart failure and cancer. Basic Res. Cardiol. 2016, 111, 60. [Google Scholar] [CrossRef]
- Rupert, C.E.; Coulombe, K.L. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Lemmens, K.; Doggen, K.; De Keulenaer, G.W. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: Implictions for therapy of heart failure. Circulation 2007, 116, 954–960. [Google Scholar] [CrossRef]
- Gordon, L.I.; Burke, M.A.; Singh, A.T.; Prachand, S.; Lieberman, E.D.; Sun, L. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J. Biol. Chem. 2009, 284, 2080–2087. [Google Scholar] [CrossRef]
- Liu, F.; Yang, X.; Geng, M.; Huang, M. Targeting ERK, an Achilles’ heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B 2018, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Yousif, N.G.; Al-amran, F.G. Novel toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) mediated injury in mice. BMC Cardiovasc. Disord. 2011, 11, 62. [Google Scholar] [CrossRef]
- Ma, W.; Wei, S.; Zhang, B.; Li, W. Molecular mechanism of cardiomyocyte death in drug-mediated cardiotoxicity. Front. Cell Dev. Biol. 2020, 8, 434. [Google Scholar] [CrossRef]
- Jobard, E.; Tredan, O.; Bachelot, T.; Vigneron, A.M.; Ait-Oukhatar, C.M.; Arnedos, M.; Rios, M.; Bonneterre, J.; Dieras, V.; Jimenes, M.; et al. Longituidinal serum metabolomics evaluation of trastuzumab and everolimus combination as pre-operative treatment for HER-2positive breast cancer patients. Oncotarge 2017, 8, 83570–83584. [Google Scholar] [CrossRef]
- Patel, R.; Baker, S.S.; Liu, W.; Desai, S.; Alkhouri, R.; Kozielski, R.; Mastrandrea, L.; Sarfraz, A.; Cai, W.; Vlassara, H.; et al. Effect of dietary advanced glycation end products on mouse liver. PLoS ONE 2012, 7, e35143. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Costyn, L.J.; Nagy, T.; Cowan, E.A.; Oldham, C.D.; May, S.W.; Arnold, R.D. The antioxidant phenylaminoethylselenide reduces doxorubicinmediated cardiotoxicity in xenograft model of human prostate cancer. Arch. Biochem. Biophys. 2011, 515, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Robert, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Dringen, R.; Hirrlinger, J. Glutathione pathways in the brain. Biol. Chem. 2003, 384, 505–516. [Google Scholar] [CrossRef]
- Gonzalez, F.M.E.; Blanco, L.; Fernandez, C.I.; Lorigados, L.; Serrano, T.; Fernandez, J.L. Glutathione depletion: Starting point of brain metabolic stress, neuro-inflammation and cognitive impairment in rats. Brain Res. Bull. 2018, 137, 120–131. [Google Scholar] [CrossRef]
- Fernandez, S.F.; Almeida, A.; Bolanos, J.P. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 2012, 443, 3–11. [Google Scholar] [CrossRef]
- Alam, M.F.; Hijri, S.I.; Alshahrani, S.; Alqahtani, S.S.; Jali, A.M.; Ahmed, R.A.; Adawi, M.M.; Algassmi, S.M.; Shaheen, E.S.; Moni, S.S.; et al. Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network. Int. J. Mol. Sci. 2022, 23, 15617. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, E.I.; Fukazawa, R.; Kanbe, M.; Watanabe, M.; Abe, M.; Watanabe, M.; Kamisago, M.; Hajikano, M.; Katsube, Y.; Ogawa, S. Edaravone, a potent free radical scavenger, prevents Anthracycline-mediated myocardial cell death. Circ. J. 2007, 71, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Jiang, J.; Dokmanovic, M.; Wu, W.J. Trastuzumab-mediated cardiotoxicity: Current understanding, challenges, and frontiers. Antib Ther. 2018, 1, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; De, A.A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018, 18, 7582730. [Google Scholar] [CrossRef]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef] [PubMed]
- Simoes, R.; Silva, L.M.; Cruz, A.L.V.M.; Fraga, V.G.; de Paula, S.A.; Gomes, K.B. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: A narrative review. Biomed. Pharmacother. 2018, 107, 989–996. [Google Scholar] [CrossRef]
- Altundag, K. More predictive markers were identified for trastuzumab-induced cardiotoxicity. Med. Oncol. 2017, 35, 8. [Google Scholar] [CrossRef]


| Groups | AST (mg/dL) | CK-MB (µ/L) | LDH (µ/L) |
|---|---|---|---|
| Group 1 | 60.93 ± 5.62 | 689.71 ± 4.51 | 490.66 ± 7.93 |
| Group 2 | 265.12 ± 3.3 # | 3975.43 ± 8.32 # | 3395.57 ± 5.08 ## |
| Group 3 | 115.05 ± 3.10 * | 3194.48 ± 6.97 * | 1945.56 ± 5.38 ** |
| Group 4 | 85.39 ± 5.57 *** | 1790.97 ± 5.89 ** | 1465.41 ± 4.31 *** |
| Group 5 | 59.79 ± 6.03 ns | 695.99 ± 6.60 ns | 484.6 ± 4.08 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, G.; Alam, M.F.; Alshahrani, S.; Almoshari, Y.; Jali, A.M.; Alqahtani, S.; Khalid, M.; Mir Najib Ullah, S.N.; Anwer, T. Trastuzumab-Mediated Cardiotoxicity and Its Preventive Intervention by Zingerone through Antioxidant and Inflammatory Pathway in Rats. J. Pers. Med. 2023, 13, 750. https://doi.org/10.3390/jpm13050750
Khan G, Alam MF, Alshahrani S, Almoshari Y, Jali AM, Alqahtani S, Khalid M, Mir Najib Ullah SN, Anwer T. Trastuzumab-Mediated Cardiotoxicity and Its Preventive Intervention by Zingerone through Antioxidant and Inflammatory Pathway in Rats. Journal of Personalized Medicine. 2023; 13(5):750. https://doi.org/10.3390/jpm13050750
Chicago/Turabian StyleKhan, Gyas, Mohammad Firoz Alam, Saeed Alshahrani, Yosif Almoshari, Abdulmajeed M. Jali, Saud Alqahtani, Mohammad Khalid, Shehla Nasar Mir Najib Ullah, and Tarique Anwer. 2023. "Trastuzumab-Mediated Cardiotoxicity and Its Preventive Intervention by Zingerone through Antioxidant and Inflammatory Pathway in Rats" Journal of Personalized Medicine 13, no. 5: 750. https://doi.org/10.3390/jpm13050750





