Influence of Clinical Factors on the Quality of Life in Romanian People with Epilepsy—A Follow-Up Study in Real-Life Clinical Practice
Abstract
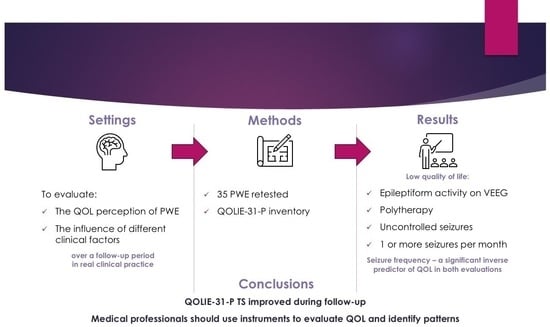
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Instruments
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Berto, P. Quality of Life in Patients with Epilepsy and Impact of Treatments. Pharmacoeconomics 2002, 20, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Tombini, M.; Assenza, G.; Quintiliani, L.; Ricci, L.; Lanzone, J.; Di Lazzaro, V. Epilepsy and quality of life: What does really matter? Neurol. Sci. 2021, 42, 3757–3765. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Aledo-Serrano, A.; Coppola, A.; Didelot, A.; Bates, E.; Sainz-Fuertes, R.; Lawthom, C. The impact of epilepsy on quality of life: Findings from a European survey. Epilepsy Behav. 2023, 142, 109179. [Google Scholar] [CrossRef]
- Baranowski, C.J. The quality of life of older adults with epilepsy: A systematic review. Seizure 2018, 60, 190–197. [Google Scholar] [CrossRef]
- Mehta, S.; Tyagi, A.; Tripathi, R.; Kumar, M. Study of inter-relationship of depression, seizure frequency and quality of life of people with epilepsy in India. Ment. Illn. 2014, 6, 5169. [Google Scholar] [CrossRef]
- Piperidou, C.; Karlovasitou, A.; Triantafyllou, N.; Dimitrakoudi, E.; Terzoudi, A.; Mavraki, E.; Trypsianis, G.; Vadikolias, K.; Heliopoulos, I.; Vassilopoulos, D.; et al. Association of demographic, clinical and treatment variables with quality of life of patients with epilepsy in Greece. Qual. Life Res. 2008, 17, 987–996. [Google Scholar] [CrossRef]
- Edefonti, V.; Bravi, F.; Turner, K.; Beghi, E.; Canevini, M.P.; Ferraroni, M.; Piazzini, A. Health-related quality of life in adults with epilepsy: The effect of age, age at onset and duration of epilepsy in a multicentre Italian study. BMC Neurol. 2011, 11, 33. [Google Scholar] [CrossRef]
- Bujan Kovač, A. Quality of life in patients with epilepsy—Single centre experience. Acta Clin. Croat. 2021, 60 (Suppl. S3), 16–23. [Google Scholar] [CrossRef]
- Grinalds, M.S.; Yoder, C.; Krauss, Z.; Chen, A.M.; Rhoney, D.H. Scoping review of rational polytherapy in patients with drug-resistant epilepsy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 53–84. [Google Scholar] [CrossRef]
- Cioriceanu, I.H.; Constantin, D.A.; Marceanu, L.G.; Anastasiu, C.V.; Serbanica, A.N.; Rogozea, L. Impact of Clinical and Socio-Demographic Factors on the Quality of Life in Romanian People with Epilepsy. Healthcare 2022, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Van Hammée, G.; N132 Study Group. Maintenance of improvement in health-related quality of life during long-term treatment with levetiracetam. Epilepsy Behav. 2003, 4, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.; The QOLIE Development Group. Scoring Manual for the QOLIE-31-P: Patient-Weighted Quality of Life in Epilepsy; The QOLIE Development Group: Houston, TX, USA, 2013. [Google Scholar]
- Zadeh, W.W.; Escartin, A.; Byrnes, W.; Tennigkeit, F.; Borghs, S.; Li, T.; Dedeken, P.; De Backer, M.; SP0954 Study Group. Efficacy and safety of lacosamide as first add-on or later adjunctive treatment for uncontrolled partial-onset seizures: A multicentre open-label trial. Seizure 2015, 31, 72–79. [Google Scholar] [CrossRef]
- Klein, P.; Schiemann, J.; Sperling, M.R.; Whitesides, J.; Liang, W.; Stalvey, T.; Brandt, C.; Kwan, P. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia 2015, 56, 1890–1898. [Google Scholar] [CrossRef]
- Koh, M.Y.; Lim, K.S.; Fong, S.L.; Khor, S.B.; Tan, C.T. Impact of COVID-19 on quality of life in people with epilepsy, and a multinational comparison of clinical and psychological impacts. Epilepsy Behav. 2021, 117, 107849. [Google Scholar] [CrossRef]
- Strizović, S.; Vojvodić, N.; Kovačević, M.; Pejović, A.; Bukumirić, Z.; Sokić, D.; Ristić, A.J. Influence of COVID-19 pandemic on quality of life in patients with epilepsy—Follow-up study. Epilepsy Behav. 2021, 121, 108026. [Google Scholar] [CrossRef]
- Tlusta, E.; Zarubova, J.; Simko, J.; Hojdikova, H.; Salek, S.; Vlcek, J. Clinical and demographic characteristics predicting QOL in patients with epilepsy in the Czech Republic: How this can influence practice. Seizure 2009, 18, 85–89. [Google Scholar] [CrossRef]
- Cramer, J.A.; Hammer, A.E.; Kustra, R.P. Quality of life improvement with conversion to lamotrigine monotherapy. Epilepsy Behav. 2004, 5, 224–230. [Google Scholar] [CrossRef]
- Birbeck, G.L.; Hays, R.D.; Cui, X.; Vickrey, B.G. Seizure Reduction and Quality of Life Improvements in People with Epilepsy. Epilepsia 2002, 43, 535–538. [Google Scholar] [CrossRef]
- Lotfinia, M.; Maloumeh, E.N.; Asaadi, S.; Omidbeigi, M.; Sharifi, G.; Asadi, B. Health-related quality of life after epilepsy surgery: A prospective, controlled follow-up on the Iranian population. Sci. Rep. 2019, 9, 7875. [Google Scholar] [CrossRef] [PubMed]
- Nagarathnam, M.; Vengamma, B.; Shalini, B.; Latheef, S. Stigma and Polytherapy: Predictors of Quality of Life in Patients with Epilepsy from South India. Ann. Indian Acad. Neurol. 2017, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Tiwari, P.; Pahuja, M.; Dada, R.; Tripathi, M. Anti-seizure medications and quality of life in person with epilepsy. Heliyon 2022, 8, e11073. [Google Scholar] [CrossRef]
- Guekht, A.B.; Mitrokhina, T.V.; Lebedeva, A.V.; Dzugaeva, F.K.; Milchakova, L.E.; Lokshina, O.B.; Feygina, A.A.; Gusev, E.I. Factors influencing on quality of life in people with epilepsy. Seizure 2007, 16, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, A.; Ramaglia, G.; Turner, K.; Chifari, R.; El Kiky, E.; Canger, R.; Canevini, M.P. Coping strategies in epilepsy: 50 drug-resistant and 50 seizure-free patients. Seizure 2007, 16, 211–217. [Google Scholar] [CrossRef]
- Taylor, R.S.; Sander, J.W.; Taylor, R.J.; Baker, G.A. Predictors of health-related quality of life and costs in adults with epilepsy: A systematic review. Epilepsia 2011, 52, 2168–2180. [Google Scholar] [CrossRef]
- World Health Organization (COVID-19) Dashboard. Available online: https://covid19.who.int/region/euro/country/ro/measures (accessed on 19 January 2023).
- Coman, C.; Bularca, M.C.; Repanovici, A.; Rogozea, L. Misinformation about medication during the COVID–19 pandemic: A perspective of medical staff. PLoS ONE 2022, 17, e0276693. [Google Scholar] [CrossRef]
- Grigorescu, S.; Cazan, A.M.; Rogozea, L.; Grigorescu, D.O. Predictive Factors of the Burnout Syndrome Occurrence in the Healthcare Workers During the COVID-19 Pandemic. Front. Med. 2022, 9, 842457. [Google Scholar] [CrossRef]
- Rogozea, L.M.; Sechel, G.; Bularca, M.C.; Coman, C.; Cocuz, M.E. Who’s Getting Shots First? Dealing with the Ethical Responsibility for Prioritizing Population Groups in Vaccination. Am. J. Ther. 2021, 28, e478–e487. [Google Scholar] [CrossRef]
- Sahin, S.; Karsidag, S.; Cinar, N.; Ates, M.F.; Demir, S.; Eren, F.; Neyal, A.; Ak, A.K.; Tokcaer, A.B.; Ataoglu, E.E.; et al. The Impact of the COVID-19 Lockdown on the Quality of Life in Chronic Neurological Diseases: The Results of a COVQoL-CND Study. Eur. Neurol. 2021, 84, 450–459. [Google Scholar] [CrossRef]
- French, J.A.; Brodie, M.J.; Caraballo, R.; Devinsky, O.; Ding, D.; Jehi, L.; Jette, N.; Kanner, A.; Modi, A.C.; Newton, C.R.; et al. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology 2020, 94, 1032–1037. [Google Scholar] [CrossRef]
| Total Score | p | ||
|---|---|---|---|
| Initial evaluation | |||
| Age | 18–44 (n = 23)/≥45 (n = 12) | 69.54 (±14.79) vs. 66.61 (±18.35) | 0.6119 |
| Sex | Female (n = 21)/Male (n = 14) | 68.18 (±15.50) vs. 69.08 (±17.02) | 0.8724 |
| Environment | Urban (n = 28)/Rural (n = 7) | 68.74 (±16.26) vs. 67.74 (±15.47) | 0.8842 |
| Marital Status | Non-married (n = 16)/Married (n = 19) | 70.01 (±15.07) vs. 67.29 (±16.85) | 0.6211 |
| Employment status | Employed (n = 19)/Unemployed (n = 16) | 71.09 (±14.73) vs. 65.51 (±17.14) | 0.3077 |
| Education | University (n = 12)/Other (n = 23) | 71.46 (±13.81) vs. 67.01 (±16.96) | 0.4398 |
| Age of onset (years) | <18 (n = 12)/>18 (n = 23) | 67.78 (±15.74) vs. 68.93 (±16.30) | 0.8424 |
| Seizure type | With (n = 32)/Without motor tonic-clonic (n = 3) | 69.50 (±15.84) vs. 58.22 (±15.01) | 0.2452 |
| Seizures in sleep | Yes (n = 6)/No (n = 29) | 77.72 (±12.88) vs. 66.64 (±15.97) | 0.1215 |
| Epilepsy type (onset) | Focal (n = 33)/Generalized (n = 2) | 68.17 (±16.02) vs. 74.59 (±16.91) | 0.5865 |
| Etiology | Unknown (n = 14)/Structural (n = 20)/Genetic (n = 1) | 69.16 (±18.64) vs. 68.40 (±14.56) vs. 63.63 (±0.00) | 0.9268 |
| Presence of aura | Yes (n = 16)/No (n = 19) | 64.16 (±15.78) vs. 72.22 (±15.43) | 0.1371 |
| Epileptiform activity | With (n = 32)/Missing (n = 3) | 67.59 (±16.31) vs. 78.61 (±1.160) | 0.0007 |
| Seizure control | Controlled (n = 5)/Uncontrolled (n = 30) | 77.27 (±5.187) vs. 67.08 (±16.64) | 0.0145 |
| Seizure frequency | One or more seizures per month (n = 12)/other (n = 23) | 54.20 (±14.53) vs. 76.02 (±10.67) | <0.0001 |
| Number of ASM taken | One (n = 16)/≥2 (n = 14)/Without (n = 5) | 75.39 (±12.29) vs. 55.99 (±13.63) vs. 81.72 (±4.385) | <0.0001 |
| Final evaluation | |||
| Age | 18–44 (n = 19)/≥45 (n = 16) | 77.43 (±14.22) vs. 70.26 (±19.73) | 0.2212 |
| Presence of aura | Yes (n = 16)/No (n = 19) | 66.20 (±17.28) vs. 80.85 (14.12) | 0.0093 |
| Epileptiform activity | With (n = 32)/Missing (n = 3) | 73.23 (±17.55) vs. 84.00 (±5.446) | 0.0433 |
| Seizure control | Controlled (n = 21)/Uncontrolled (n = 14) | 81.91 (±12.13) vs. 62.52 (±17.16) | 0.0004 |
| Seizure frequency | One or more seizures per month (n = 7)/Other (n = 28) | 51.31 (±17.48) vs. 79.86 (±11.44) | <0.0001 |
| Number of ASM taken | One (n = 18)/≥2 (n = 15)/Without (n = 2) | 79.94 (±9.575) vs. 62.13 (±19.12) vs. 89.30 (±4.767) | 0.0001 |
| Initial | Final | Difference Mean Values | |
|---|---|---|---|
| Energy | 37.41 (±28.78) | 45.24 (±31.58) | 7.83 |
| Mood | 38.33 (±25.49) | 47.53 (±29.86 | 9.20 |
| Daily Activities | 49.17 (±35.13) | 56.08 (±34.67) | 6.91 |
| Cognition | 51.71 (±35.40) | 66.39 (±32.92) | 14.68 |
| Medication Effects | 44.97 (±30.96) | 60.40 (±35.75) | 15.43 |
| Seizure Worry | 32.43 (±31.28) | 47.46 (±35.11) | 15.03 |
| Overall Quality of Life | 42.34 (±25.47) | 55.31 (±27.71) | 12.97 |
| Estimate | Standard Error | 95% CI | |t| | p Value | |
|---|---|---|---|---|---|
| Parameter estimates | |||||
| Initial | |||||
| Seizure frequency | −17.11 | 5.568 | −28.60 to −5.619 | 3.073 | 0.0052 |
| Number of ASM taken | −15.95 | 7.139 | −30.69 to −1.220 | 2.235 | 0.0350 |
| Final | |||||
| Seizure frequency | −18.94 | 7.939 | −35.32 to −2.550 | 2.385 | 0.0253 |
| Number of ASM taken | −13.73 | 10.69 | −35.80 to 8.344 | 1.284 | 0.2115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioriceanu, I.-H.; Constantin, D.-A.; Bobescu, E.; Marceanu, L.G.; Rogozea, L. Influence of Clinical Factors on the Quality of Life in Romanian People with Epilepsy—A Follow-Up Study in Real-Life Clinical Practice. J. Pers. Med. 2023, 13, 752. https://doi.org/10.3390/jpm13050752
Cioriceanu I-H, Constantin D-A, Bobescu E, Marceanu LG, Rogozea L. Influence of Clinical Factors on the Quality of Life in Romanian People with Epilepsy—A Follow-Up Study in Real-Life Clinical Practice. Journal of Personalized Medicine. 2023; 13(5):752. https://doi.org/10.3390/jpm13050752
Chicago/Turabian StyleCioriceanu, Ionut-Horia, Dan-Alexandru Constantin, Elena Bobescu, Luigi Geo Marceanu, and Liliana Rogozea. 2023. "Influence of Clinical Factors on the Quality of Life in Romanian People with Epilepsy—A Follow-Up Study in Real-Life Clinical Practice" Journal of Personalized Medicine 13, no. 5: 752. https://doi.org/10.3390/jpm13050752