Glissonean Pedicle Isolation Focusing on the Laennec’s Capsule for Minimally Invasive Anatomical Liver Resection
Abstract
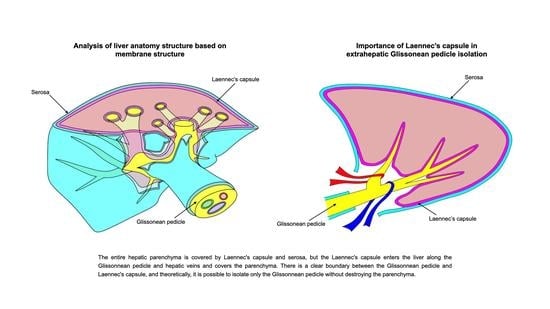
:1. Introduction
2. Material and Methods
2.1. Cadaver Simulation
2.2. Extrahepatic Glissonean Pedicle Isolation in LALR and RALR
2.3. Patient Characteristics
2.4. Peri- and Post-Operative Outcomes
2.5. Statistical Analysis
3. Results
3.1. Surgical Procedure
3.1.1. The Laparoscopic Extrahepatic Glissonean Approach and Pathologic Examination of the Laennec’s Capsule in Cadaveric Models (Supplementary File S1)
3.1.2. The Laparoscopic Extrahepatic Glissonean Approach and Pathologic Examination of the Laennec’s Capsule in a Live Body
3.1.3. The Robotic Extrahepatic Glissonean Approach and Pathologic Examination of the Laennec’s Capsule in a Live Body
3.2. Patient Characteristics (Table 1)
3.3. Types of Liver Resection (Table 2)
3.4. Operative Data (Table 3)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALR | Anatomical liver resection. |
| GPI | Glissonean pedicle isolation |
| LALR | Laparoscopic anatomic liver resection |
| RALR | Robotic anatomical liver resection |
| HCC | Hepatocellular carcinoma |
| ICGR15 | Indocyanine green retention rate at 15 min |
References
- Huang, X.; Lu, S. A Meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB 2017, 19, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Margonis, G.A.; Buettner, S.; Andreatos, N.; Sasaki, K.; Ijzermans, J.N.M.; van Vugt, J.L.A.; Pawlik, T.M.; Choti, M.A.M.; Cameron, J.L.; He, J.; et al. Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann. Surg. 2017, 266, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Tomassini, F.; Berardi, G.; Mori, Y.; Shirata, C.; Abu Hilal, M.; Asbun, H.J.; Cherqui, D.; Gotohda, N.; Han, H.; et al. Glissonean approach for hepatic inflow control in minimally invasive anatomic liver resection: A systematic review. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 51–65. [Google Scholar] [CrossRef]
- Sugioka, A.; Kato, Y.; Tanahashi, Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec’s capsule: Proposal of a novel comprehensive surgical anatomy of the liver. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasaki, K. Glissonean pedicle transection method for hepatic resection: A new concept of liver segmentation. J. Hepato-Biliary-Pancreat. Surg. 1998, 5, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Thiel, W. The preservation of the whole corpse with natural color. Ann. Anat. Anat. Anz. 1992, 174, 185–195. [Google Scholar] [CrossRef]
- Thiel, W. An arterial substance for subsequent injection during the preservation of the whole corpse. Ann. Anat. Anat. Anz. 1992, 174, 197–200. [Google Scholar] [CrossRef]
- Thiel, W. Supplement to the conservation of an entire cadaver according to W. Thiel. Ann. Anat. 2002, 184, 267–269. [Google Scholar] [CrossRef]
- Strasberg, S.; Belghiti, J.; Clavien, P.-A.; Gadzijev, E.; Garden, J.; Lau, W.-Y.; Makuuchi, M.; Strong, R. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB 2000, 2, 333–339. [Google Scholar] [CrossRef]
- Wakabayashi, G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg. Nutr. 2016, 5, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Han, H.-S.; Wakabayashi, G.; Soubrane, O.; Geller, D.; O’Rourke, N.; Buell, J.; Cherqui, D. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg. Oncol. 2018, 27, A5–A9. [Google Scholar] [CrossRef]
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative short- term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann. Surg. 2016, 263, 761–777. [Google Scholar]
- Jin, B.; Chen, M.-T.; Fei, Y.-T.; Du, S.-D.; Mao, Y.-L. Safety and efficacy for laparoscopic versus open hepatectomy: A meta-analysis. Surg. Oncol. 2018, 27, A26–A34. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Guo, H.-B.; Kan, J.-L.; Liu, S.-G.; Lv, H.-T.; Liu, J.-H.; Bian, W. Clinical outcome of open surgery versus laparoscopic surgery for cirrhotic hepatocellular carcinoma patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Yamamoto, H.; Kainuma, O.; Souda, H.; Ikeda, A.; Takiguchi, N.; Nagata, M. Safe and feasible extrahepatic Glissonean access in laparoscopic anatomical liver resection. Surg. Endosc. 2010, 25, 1333–1336. [Google Scholar] [CrossRef]
- Majno, P.; Mentha, G.; Toso, C.; Morel, P.; Peitgen, H.O.; Fasel, J.H. Anatomy of the liver: An outline with three levels of complexi-ty--a further step towards tailored territorial liver resections. J. Hepatol. 2014, 60, 654–662. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Up-dates of the Brisbane 2000 system. J. Hepatobiliary Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, J.; Wang, S.; Zhang, W.; Sun, X.; Sun, B.; Yu, D. Laennec’s approach for laparoscopic anatomic hepatectomy based on Laennec’s cap-sule. BMC Gastroenterol. 2019, 19, 194. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Zhang, G.; Chen, M.; Zhong, C.; Li, M.; Sun, X.; Li, K.; Wang, Z. Laennec’s approach for laparoscopic anatomical hemihepatectomy. World J. Surg. Oncol. 2021, 19, 295. [Google Scholar] [CrossRef]
- Laennec, R.T.H. Lettre sur des Tuniques qui enveloppent certains Visceres, et fournissentdes gaines membraneuses a leurs vais-seaux. Journ De Med Chir et Pharm Vendemiaire XI 1802, 1802, 539–575. [Google Scholar]
- Couinaud, C. Liver lobes and segments: Notes on the anatomical architecture and surgery of the liver. Presse Med. 1954, 62, 709–712. [Google Scholar]
- Couinaud, C. The Vasculo-Biliary Sheath. Surgical Anatomy of the Liver Revisited; Pers Ed: Paris, France, 1989; pp. 29–39. [Google Scholar]
- Hayashi, S.; Murakami, G.; Ohtsuka, A.; Itoh, M.; Nakano, T.; Fukuzawa, Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J. Hepato-Biliary-Pancreat. Surg. 2008, 15, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Giulianotti, P.C.; Sbrana, F.; Coratti, A.; Bianco, F.M.; Addeo, P.; Buchs, N.C.; Ayloo, S.M.; Benedetti, E. Totally robotic right hepatectomy: Surgical technique and outcomes. Arch. Surg. 2011, 146, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Sucandy, I.; Giovannetti, A.; Ross, S.; Rosemurgy, A. Institutional first 100 case experience and outcomes of robotic hepatectomy for liver tumors. Am. Surg. 2020, 86, 200–207. [Google Scholar] [CrossRef]
- Luberice, K.; Sucandy, I.; Modasi, A.; Castro, M.; Krill, E.; Ross, S.; Rosemurgy, A. Applying IWATE criteria to robotic hepatectomy: Is there a “robotic effect”? HPB 2020, 23, 899–906. [Google Scholar] [CrossRef]
- Machado, M.A.; Mattos, B.H.; Filho, M.M.L.; Makdissi, F.F. Robotic Right Hepatectomy with Portal Vein Thrombectomy for Colorectal Liver Metastasis (with Video). J. Gastrointest. Surg. 2021, 25, 1932–1935. [Google Scholar] [CrossRef]
- Machado, M.A.; Mattos, B.V.; Filho, M.M.L.; Makdissi, F. Robotic Resection of Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 4166–4170. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, D.H.; Jang, D.-S.; Choi, G.H.; Choi, J.S. Robotic extrahepatic Glissonean pedicle approach for anatomic liver resection in the right liver: Techniques and perioperative outcomes. Surg. Endosc. 2016, 30, 3882–3888. [Google Scholar] [CrossRef]
- Kato, Y.; Sugioka, A.; Kojima, M.; Kiguchi, G.; Mii, S.; Uchida, Y.; Takahara, T.; Uyama, I. Initial experience with robotic liver resection: Audit of 120 consecutive cases at a single center and comparison with open and laparoscopic approaches. J. Hepato-Biliary-Pancreat. Sci. 2023, 30, 72–90. [Google Scholar] [CrossRef]
- Bartoș, A.; Iancu, I.; Ciobanu, L.; Badea, R.; Spârchez, Z.; Bartoș, D.M. Intraoperative ultrasound in liver and pancreatic surgery. Med. Ultrason. 2021, 23, 319–328. [Google Scholar] [CrossRef] [PubMed]



| Lap (n = 60) | Robot (n = 39) | ||
|---|---|---|---|
| Age; mean ± SD (years) | 69.3 ± 10.5 | 69.6 ± 9.5 | N. S |
| Gender (male/female) | 34/26 | 23/16 | N. S |
| BMI; mean ± SD (kg/m2) | 23.4 ± 3.9 | 22.6 ± 3.5 | N. S |
| Liver cirrhosis; n (%) | 15 (25) | 9 (23) | N. S |
| ICG R15, % | 10.1 ± 4.6 | 12.3 ± 5.7 | N. S |
| Indication for liver resection, n (%) | |||
| Hepatocellular carcinoma | 29 | 18 | N. S |
| Intrahepatic cholangiocarcinoma | 6 | 4 | |
| Symptomatic cyst | 2 | 0 | |
| Hemangioma | 1 | 0 | |
| Colorectal metastasis to liver | 22 | 17 | |
| Previous liver resection | 8 (13%) | 5 (13%) | |
| Open surgery; n (%) | 3 | 3 | N. S |
| Laparoscopic surgery; n (%) | 4 | 1 | |
| Robotic surgery; n (%) | 1 | 1 |
| Lap (n = 60) | Robot (n = 39) | ||
|---|---|---|---|
| Left lateral sectionectomy | 5 | 5 | N. S |
| Segmentectomy | |||
| Segmentectomy 1 | 3 | 2 | N. S |
| Segmentectomy 2 | 3 | 3 | |
| Segmentectomy 3 | 1 | 3 | |
| Segmentectomy 4a | 1 | 1 | |
| Segmentectomy 4b | 2 | 4 | |
| Segmentectomy 5 | 2 | 5 | |
| Segmentectomy 6 | 4 | 3 | |
| Segmentectomy 7 | 7 | 0 | |
| Segmentectomy 8 | 5 | 3 | |
| Left medial sectionectomy | 1 | 2 | N. S |
| Right anterior sectionnectomy | 2 | 0 | N. S |
| Right posterior sectionectomy | 4 | 1 | N. S |
| Left hepatectomy | 12 | 5 | N. S |
| Right hepatectomy | 8 | 2 | N. S |
| Lap (n = 60) | Robot (n = 39) | ||
|---|---|---|---|
| Operative time; mean ± SD (min) | 399.8 ± 109.5 | 366.1 ± 98.3 | N. S |
| Duration of Glissonean pedicle isolation; mean ± SD (min) | 32.9 ± 11.6 | 27.2 ± 9.3 | p < 0.05 |
| Measured blood loss; mean ± SD (mL) | 289.7 ± 303.8 | 131.6 ± 102.2 | p < 0.05 |
| Iwate difficulty score; mean ± SD | 7.7 ± 1.9 | 7.4 ± 2.1 | N. S |
| Difficulty level; n (%) | |||
| Intermediate | 20 (33%) | 12 (31%) | N. S |
| Advanced | 32 (53%) | 20 (51%) | N. S |
| Expert | 8 (13%) | 7 (18%) | N. S |
| Morbidity Clavien-Dindo ≥grade Ⅲa; n (%) | 2 (3.3%) | 2 (5.1%) | N. S |
| Bile leakage; n (%) | 0 | 1 | |
| Abdominal abscess; n (%) | 2 | 1 | |
| Mortality; n (%) | 0 | 0 | |
| Postoperative hospital stay; mean ± SD (day) | 12.8 ± 8.5 | 11.3 ± 3.9 | N. S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, M.; Matsuo, Y.; Nonoyama, K.; Denda, Y.; Murase, H.; Kato, T.; Imafuji, H.; Saito, K.; Takiguchi, S. Glissonean Pedicle Isolation Focusing on the Laennec’s Capsule for Minimally Invasive Anatomical Liver Resection. J. Pers. Med. 2023, 13, 1154. https://doi.org/10.3390/jpm13071154
Morimoto M, Matsuo Y, Nonoyama K, Denda Y, Murase H, Kato T, Imafuji H, Saito K, Takiguchi S. Glissonean Pedicle Isolation Focusing on the Laennec’s Capsule for Minimally Invasive Anatomical Liver Resection. Journal of Personalized Medicine. 2023; 13(7):1154. https://doi.org/10.3390/jpm13071154
Chicago/Turabian StyleMorimoto, Mamoru, Yoichi Matsuo, Keisuke Nonoyama, Yuki Denda, Hiromichi Murase, Tomokatsu Kato, Hiroyuki Imafuji, Kenta Saito, and Shuji Takiguchi. 2023. "Glissonean Pedicle Isolation Focusing on the Laennec’s Capsule for Minimally Invasive Anatomical Liver Resection" Journal of Personalized Medicine 13, no. 7: 1154. https://doi.org/10.3390/jpm13071154