Early MRI Predictors of Relapse in Primary Central Nervous System Lymphoma Treated with MATRix Immunochemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Acquisition and Post Processing
2.3. Consensus Response Evaluation
2.4. Statistical Analysis
3. Results
3.1. Overview
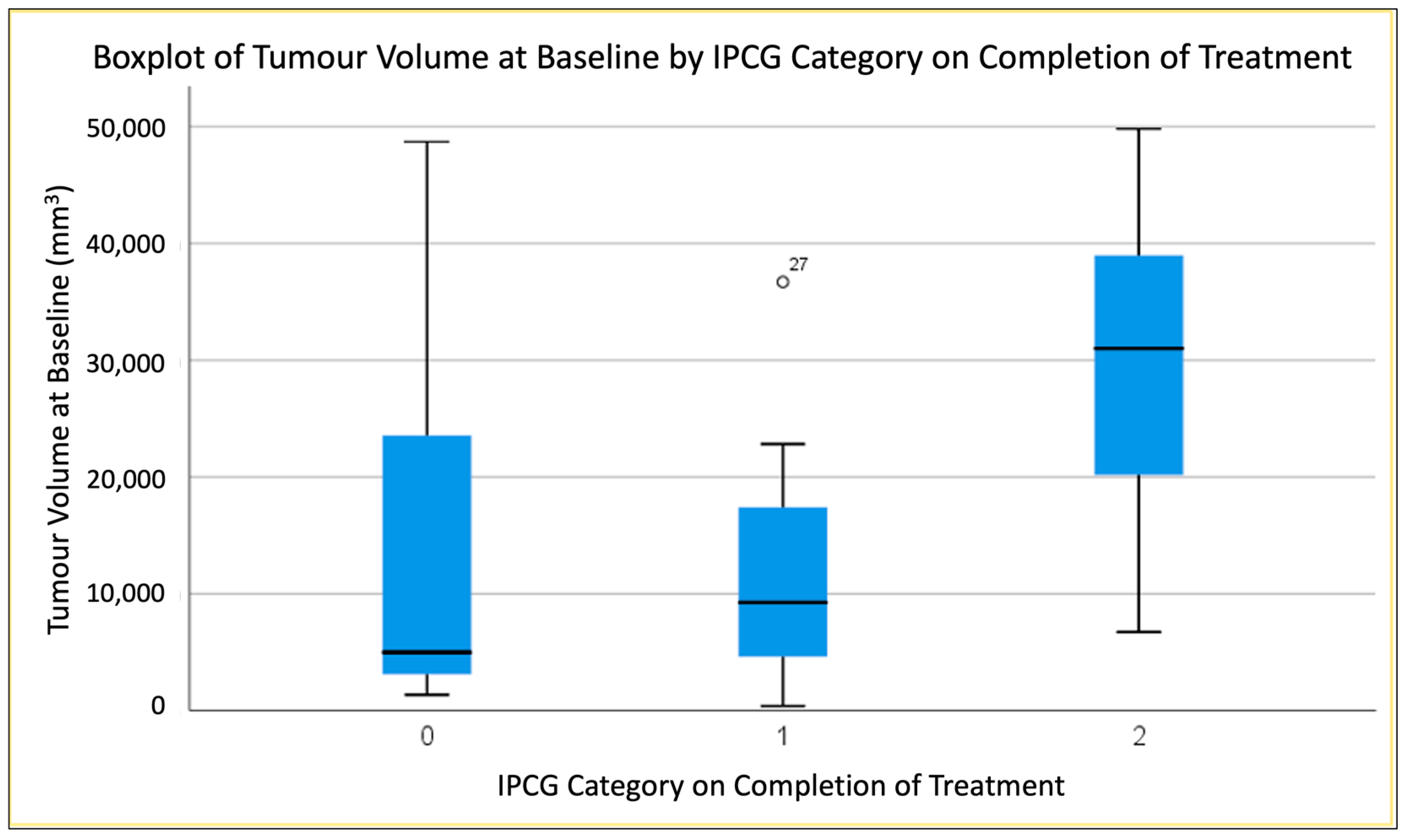
3.2. Predicting End of Treatment Response by T1CE Baseline Tumour Volume
3.3. Predicting End of Treatment Response by T1CE Percentage Volume Change (Baseline to 2 MATRix Cycles)
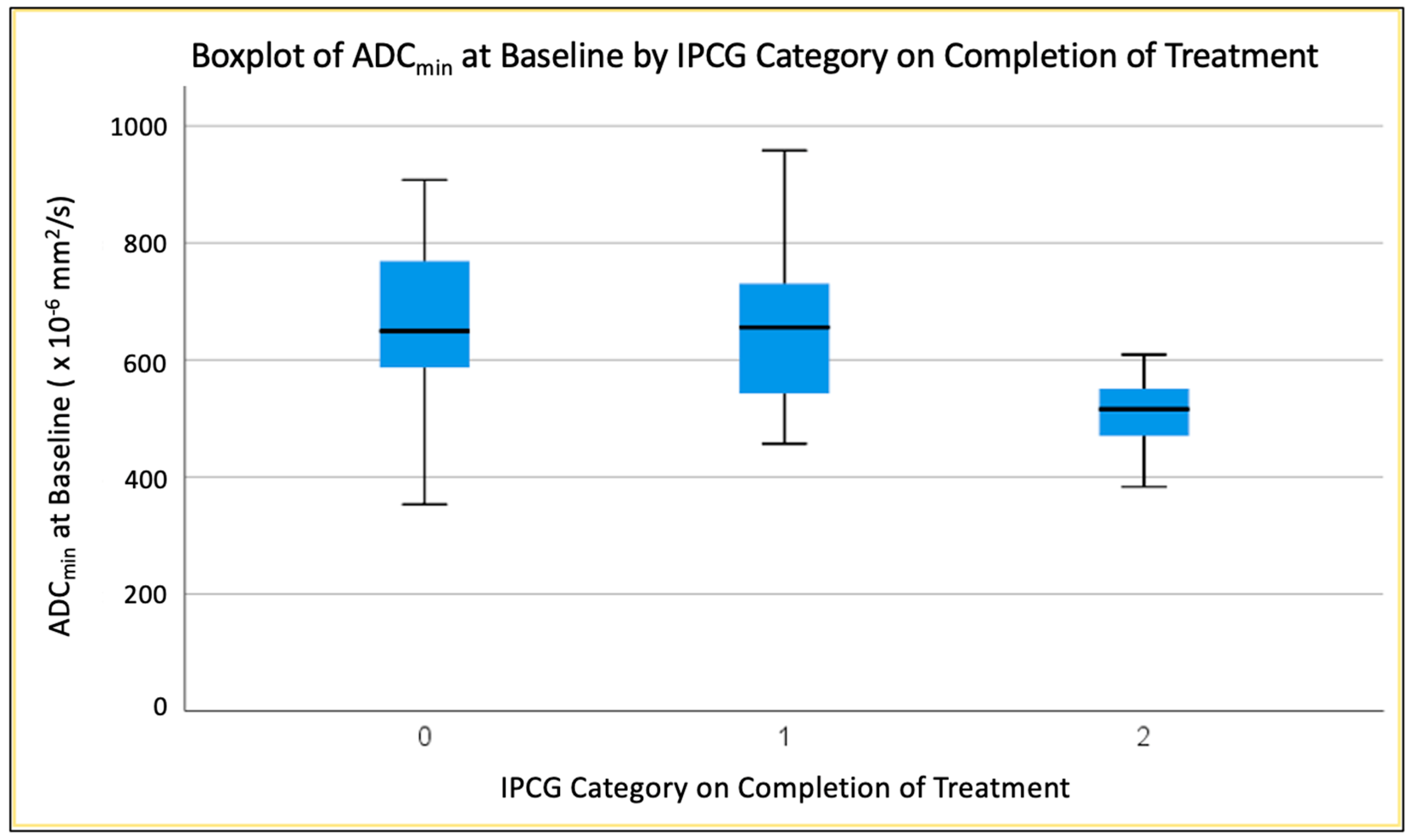
3.4. Predicting End of Treatment Response by ADC Values at Baseline
3.4.1. Second-Percentile ADC (ADC2nd) at Baseline
3.4.2. Twenty-Fifth-Percentile ADC (ADC25th) at Baseline
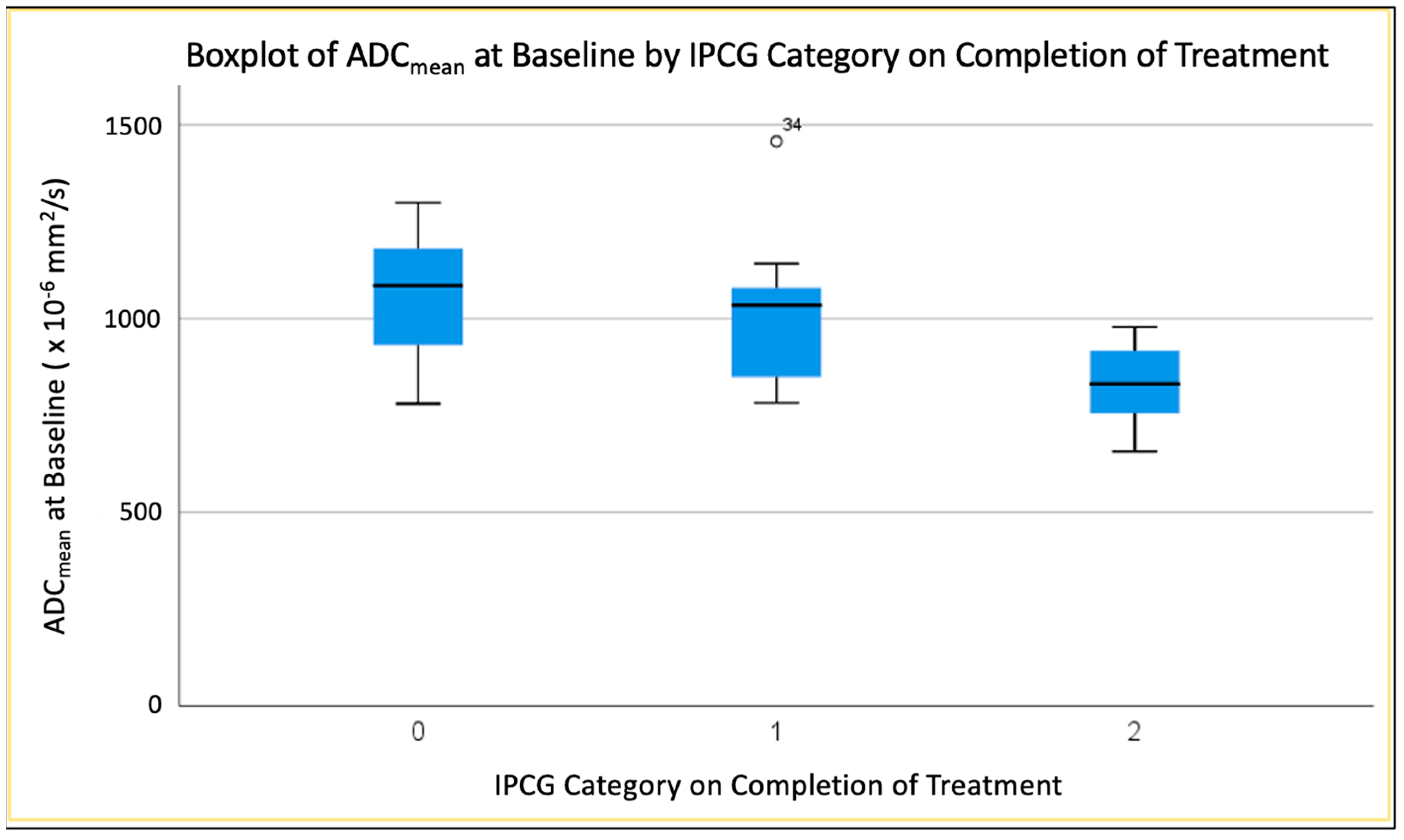
3.4.3. Mean ADC (ADCmean) at Baseline
3.5. Radiologist Early (after 2 Cycles) Compared to Later (after 4 Cycles) Assessment by IPCG Criteria
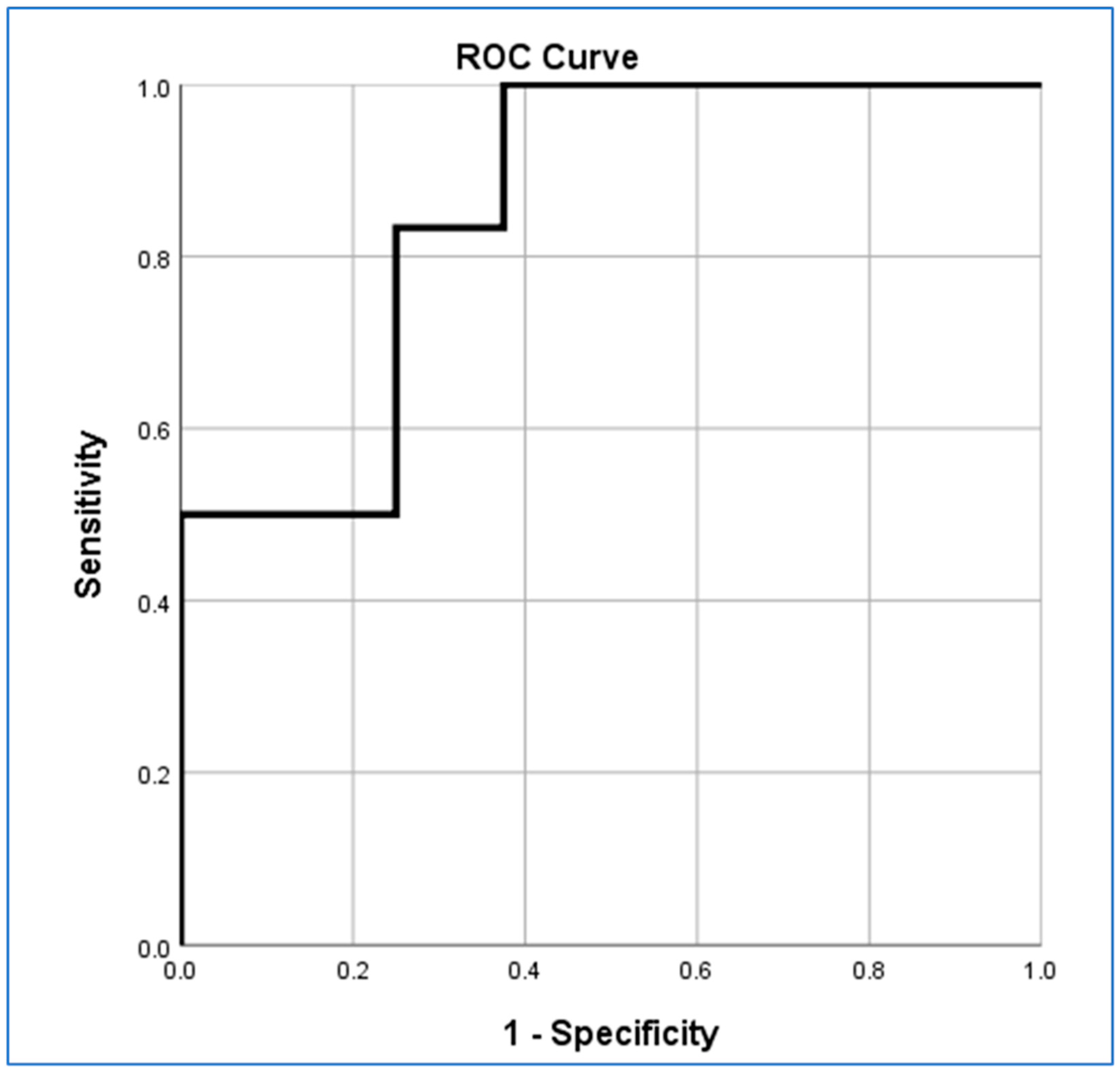
3.6. Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrey, L.E.; Batchelor, T.T.; Ferreri, A.J.M.; Gospodarowicz, M.; Pulczynski, E.J.; Zucca, E.; Smith, J.R.; Korfel, A.; Soussain, C.; DeAngelis, L.M.; et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J. Clin. Oncol. 2005, 23, 5034–5043. [Google Scholar] [CrossRef]
- Brandão, L.A.; Castillo, M. Lymphomas–Part 1. Neuroimaging Clin. N. Am. 2016, 26, 511–536. [Google Scholar] [CrossRef]
- Dandachi, D.; Ostrom, Q.T.; Chong, I.; Serpa, J.A.; Giordano, T.P.; Kruchko, C.; Barnholtz-Sloan, J.S.; Fowler, N.; Colen, R.R.; Morón, F.E. Primary Central Nervous System Lymphoma in Patients with and without HIV Infection: A Multicenter Study and Comparison with U.S National Data. Cancer Causes Control 2019, 30. [Google Scholar] [CrossRef]
- Huang, W.Y.; Wen, J.B.; Wu, G.; Yin, B.; Li, J.J.; Geng, D.Y. Diffusion-Weighted Imaging for Predicting and Monitoring Primary Central Nervous System Lymphoma Treatment Response. Am. J. Neuroradiol. 2016, 37, 2010–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, E.H.; Fox, C.P.; Cwynarski, K. Primary CNS Lymphoma. Curr. Hematol. Malig. Rep. 2014, 9, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.J.; Ponzoni, M.; Deckert, M.; Politi, L.S.; Torri, V.; Fox, C.P.; Rosée, P.L.; Schorb, E.; et al. Chemoimmunotherapy with Methotrexate, Cytarabine, Thiotepa, and Rituximab (MATRix Regimen) in Patients with Primary CNS Lymphoma: Results of the First Randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) Phase 2 Trial. Lancet Haematol. 2016, 3, e217–e227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schorb, E.; Fox, C.P.; Kasenda, B.; Linton, K.M.; Martinez-Calle, N.; Calimeri, T.; Ninkovic, S.; Eyre, T.A.; Cummin, T.; Smith, J.; et al. Induction Therapy with the MATRix Regimen in Patients with Newly Diagnosed Primary Diffuse Large B-Cell Lymphoma of the Central Nervous System—an International Study of Feasibility and Efficacy in Routine Clinical Practice. Br. J. Haematol. 2020, 189, 879–887. [Google Scholar] [CrossRef]
- Langner-Lemercier, S.; Houillier, C.; Soussain, C.; Ghesquières, H.; Chinot, O.; Taillandier, L.; Soubeyran, P.; Lamy, T.; Morschhauser, F.; Benouaich-Amiel, A.; et al. Primary CNS Lymphoma at First Relapse/Progression: Characteristics, Management, and Outcome of 256 Patients from the French LOC Network. Neuro. Oncol. 2016, 18, 1297–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Meulen, M.; Postma, A.A.; Smits, M.; Bakunina, K.; Minnema, M.C.; Seute, T.; Cull, G.; Enting, R.H.; Van Der Poel, M.; Stevens, W.B.C.; et al. Extent of Radiological Response Does Not Reflect Survival in Primary Central Nervous System Lymphoma. Neuro-Oncol. Adv. 2021, 3. [Google Scholar] [CrossRef]
- Barajas, R.F.; Politi, L.S.; Anzalone, N.; Schöder, H.; Fox, C.P.; Boxerman, J.L.; Kaufmann, T.J.; Quarles, C.C.; Ellingson, B.M.; Auer, D.; et al. Consensus Recommendations for MRI and PET Imaging of Primary Central Nervous System Lymphoma: Guideline Statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro. Oncol. 2021, 23, 1056–1071. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wang, X.X.; Deng, X.F.; Zhu, Y.Z. Value of Pretherapeutic DWI in Evaluating Prognosis and Therapeutic Effect in Immunocompetent Patients with Primary Central Nervous System Lymphoma given High-Dose Methotrexate-Based Chemotherapy: ADC-Based Assessment. Clin. Radiol. 2016, 71, 1018–1029. [Google Scholar] [CrossRef]
- Guo, A.C.; Cummings, T.J.; Dash, R.C.; Provenzale, J.M. Lymphomas and High-Grade Astrocytomas: Comparison of Water Diffusibility and Histologic Characteristics. Radiology 2002, 224, 177–183. [Google Scholar] [CrossRef]
- Lu, S.S.; Kim, S.J.; Kim, N.; Kim, H.S.; Choi, C.G.; Lim, Y.M. Histogram Analysis of Apparent Diffusion Coefficient Maps for Differentiating Primary CNS Lymphomas from Tumefactive Demyelinating Lesions. Am. J. Roentgenol. 2015, 204, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.; Rabiei, P.; LugoAndrea Lugo, A.; Arevalo, O.; Ocasio, L.; Syed, M.; Riascos, R.; Zhu, J.-J.; Cai, C.; Kamali, A. Use of Quantitative Diffusion-Weighted MR Imaging (DWI) in Differentiating between Glioblastoma and Primary Central Nervous System Lymphoma in Real-Time Exam Interpretation. Neurol. Neurosurg. 2019, 2. [Google Scholar] [CrossRef]
- Lin, X.; Lee, M.; Buck, O.; Woo, K.M.; Zhang, Z.; Hatzoglou, V.; Omuro, A.; Arevalo-Perez, J.; Thomas, A.A.; Huse, J.; et al. Diagnostic Accuracy of T1-Weighted Dynamic Contrast-Enhanced-MRI and DWI-ADC for Differentiation of Glioblastoma and Primary CNS Lymphoma. AJNR. Am. J. Neuroradiol. 2017, 38, 485–491. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Gerig, G. ITK-SNAP: An Intractive Medical Image Segmentation Tool to Meet the Need for Expert-Guided Segmentation of Complex Medical Images. IEEE Pulse 2017, 8, 54–57. [Google Scholar] [CrossRef]
- Pipe, J. Pulse Sequences for Diffusion-Weighted MRI. In Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy; Academic Press: Cambridge, MA, USA, 2014; pp. 11–34. ISBN 9780123964601. [Google Scholar]
- Grech-Sollars, M.; Hales, P.W.; Miyazaki, K.; Raschke, F.; Rodriguez, D.; Wilson, M.; Gill, S.K.; Banks, T.; Saunders, D.E.; Clayden, J.D.; et al. Multi-Centre Reproducibility of Diffusion MRI Parameters for Clinical Sequences in the Brain. NMR Biomed. 2015, 28, 468–485. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, M.; Smith, S. A Global Optimisation Method for Robust Affine Registration of Brain Images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F.; Rubenstein, J.L.; Chang, J.S.; Hwang, J.; Cha, S. Diffusion-Weighted MR Imaging Derived Apparent Diffusion Coefficient Is Predictive of Clinical Outcome in Primary Central Nervous System Lymphoma. Am. J. Neuroradiol. 2010, 31, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Valles, F.; Issa, S.; Behler, C.M.; Hwang, J.; McDermott, M.; Treseler, P.; O’Brien, J.; Shuman, M.A.; Cha, S.; et al. Immunochemotherapy with Intensive Consolidation for Primary CNS Lymphoma: A Pilot Study and Prognostic Assessment by Diffusion-Weighted MRI. Clin. Cancer Res. 2012, 18, 1146–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Modified Criteria for Radiographic Response Assessment in Glioblastoma Clinical Trials. Neurotherapeutics 2017, 14, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingson, B.M.; Kim, G.H.J.; Brown, M.; Lee, J.; Salamon, N.; Steelman, L.; Hassan, I.; Pandya, S.S.; Chun, S.; Linetsky, M.; et al. Volumetric Measurements Are Preferred in the Evaluation of Mutant IDH Inhibition in Non-Enhancing Diffuse Gliomas: Evidence from a Phase I Trial of Ivosidenib. Neuro. Oncol. 2022, 24, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Citterio, G.; Reni, M.; Gatta, G.; Ferreri, A.J.M. Primary Central Nervous System Lymphoma. Crit. Rev. Oncol. Hematol. 2017, 113, 97–110. [Google Scholar] [CrossRef]
- Fu, F.; Sun, X.; Li, Y.; Liu, Y.; Shan, Y.; Ji, N.; Wang, X.; Lu, J.; Sun, S. Dynamic Contrast-Enhanced Magnetic Resonance Imaging Biomarkers Predict Chemotherapeutic Responses and Survival in Primary Central-Nervous-System Lymphoma. Eur. Radiol. 2021, 31, 1863–1871. [Google Scholar] [CrossRef]
- Schob, S.; Meyer, J.; Gawlitza, M.; Frydrychowicz, C.; Müller, W.; Preuss, M.; Bure, L.; Quäschling, U.; Hoffmann, K.T.; Surov, A. Diffusion-Weighted MRI Reflects Proliferative Activity in Primary CNS Lymphoma. PLoS One 2016, 11. [Google Scholar] [CrossRef]
- Maciver, C.L.; Al Busaidi, A.; Ganeshan, B.; Maynard, J.A.; Wastling, S.; Hyare, H.; Brandner, S.; Markus, J.E.; Lewis, M.A.; Groves, A.M.; et al. Filtration-Histogram Based Magnetic Resonance Texture Analysis (MRTA) for the Distinction of Primary Central Nervous System Lymphoma and Glioblastoma. J. Pers. Med. 2021, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Wiestler, B.; Sahm, F.; Heiland, S.; Roethke, M.; Schlemmer, H.P.; Wick, W.; Bendszus, M.; Radbruch, A. Primary Central Nervous System Lymphoma and Atypical Glioblastoma: Multiparametric Differentiation by Using Diffusion-, Perfusion-, and Susceptibility-Weighted MR Imaging. Radiology 2014, 272, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Africa, E.; Pauciulo, A.; Vadalà, R.; Santa Lorusso, V.; Zecca, I.; Tartaglione, T. Primary Central Nervous System Lymphoma: Role of DWI in the Differential Diagnosis. Rays 2005, 30, 221–226. [Google Scholar] [PubMed]
- Chenevert, T.L.; Stegman, L.D.; Taylor, J.M.G.; Robertson, P.L.; Greenberg, H.S.; Rehemtulla, A.; Ross, B.D. Diffusion Magnetic Resonance Imaging: An Early Surrogate Marker of Therapeutic Efficacy in Brain Tumors. J. Natl. Cancer Inst. 2000, 92, 2029–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffat, B.A.; Chenevert, T.L.; Lawrence, T.S.; Meyer, C.R.; Johnson, T.D.; Dong, Q.; Tsien, C.; Mukherji, S.; Quint, D.J.; Gebarski, S.S.; et al. Functional Diffusion Map: A Noninvasive MRI Biomarker for Early Stratification of Clinical Brain Tumor Response. Proc. Natl. Acad. Sci. USA 2005, 102, 5524–5529. [Google Scholar] [CrossRef] [PubMed]
- Valles, F.E.; Perez-Valles, C.L.; Regalado, S.; Barajas, R.F.; Rubenstein, J.L.; Cha, S. Combined Diffusion and Perfusion MR Imaging as Biomarkers of Prognosis in Immunocompetent Patients with Primary Central Nervous System Lymphoma. AJNR. Am. J. Neuroradiol. 2013, 34, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Sugawara, H.; Kunimatsu, N.; Abe, O. Texture Analysis in Brain Tumor MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; Maynard, J.A.; Benenati, M.; Wastling, S.J.; Mancini, L.; Jaunmuktane, Z.; Brandner, S.; Jäger, H.R. Regional and Volumetric Parameters for Diffusion-Weighted WHO Grade II and III Glioma Genotyping: A Method Comparison. AJNR. Am. J. Neuroradiol. 2021, 42, 441–447. [Google Scholar] [CrossRef]
- Chong, I.; Ostrom, Q.; Khan, B.; Dandachi, D.; Garg, N.; Kotrotsou, A.; Colen, R.; Morón, F. Whole Tumor Histogram Analysis Using DW MRI in Primary Central Nervous System Lymphoma Correlates with Tumor Biomarkers and Outcome. Cancers 2019, 11, 1506. [Google Scholar] [CrossRef] [Green Version]
- Anjari, M.; Guha, A.; Burd, C.; Varela, M.; Goh, V.; Connor, S. Apparent Diffusion Coefficient Agreement and Reliability Using Different Region of Interest Methods for the Evaluation of Head and Neck Cancer Post Chemo-Radiotherapy. Dentomaxillofacial Radiol. 2021, 50, 20200579. [Google Scholar] [CrossRef]
- Pennig, L.; Hoyer, U.C.I.; Goertz, L.; Shahzad, R.; Persigehl, T.; Thiele, F.; Perkuhn, M.; Ruge, M.I.; Kabbasch, C.; Borggrefe, J.; et al. Primary Central Nervous System Lymphoma: Clinical Evaluation of Automated Segmentation on Multiparametric MRI Using Deep Learning. J. Magn. Reson. Imaging 2021, 53, 259–268. [Google Scholar] [CrossRef]
- Destito, M.; Marzullo, A.; Leone, R.; Zaffino, P.; Steffanoni, S.; Erbella, F.; Calimeri, F.; Anzalone, N.; Momi, E.D.; Ferreri, A.J.M.; et al. Radiomics-Based Machine Learning Model for Predicting Overall and Progression-Free Survival in Rare Cancer: A Case Study for Primary CNS Lymphoma Patients. Bioengineering 2023, 10, 285. [Google Scholar] [CrossRef] [PubMed]


| Response | Brain Imaging |
|---|---|
| CR | Disappearance of all enhancing abnormalities on gadolinium-enhancing MRI |
| CRu | Small but persistent abnormality on MRI related to biopsy or focal haemorrhage, found to represent scar tissue on serial scans |
| PR | ≥50% decrease in contrast-enhancing lesion seen on MRI as compared with baseline imaging |
| PD | >25% increase in contrast-enhancing lesion on MRI compared with baseline or best response |
| Subject | Manufacturer | Model | Field Strength (T) | Series | TE (ms) | TR (ms) | Slice Thickness (mm) | Slice Spacing (mm) | In Plane Resolution (mm × mm) |
|---|---|---|---|---|---|---|---|---|---|
| NHNN_001a | Siemens | SymphonyTim | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2 | 106 | 4400 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_001b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3734 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_002a | Siemens | Avanto | 1.5 | 00004-MR-ep2d_diff_3scan_trace_p2 | 102 | 3723 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_002b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_003a | Siemens | Espree | 1.5 | 00007-MR-ep2d_diff_3scan_trace_aa | 100 | 4000 | 5 | 6.5 | 1.6 × 1.6 |
| NHNN_003b | Siemens | SymphonyTim | 1.5 | 00007-MR-DWI_Tra | 84 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_003c | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3348 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_004a | Siemens | Avanto | 1.5 | 00006-MR-ep2d_diff_3scan_trace | 102 | 4300 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_004b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_004c | Philips | Achieva | 3 | 00401-MR-dwi_1000 | 95 | 2900 | 5 | 6 | 0.9 × 0.9 |
| NHNN_005b | Siemens | SymphonyTim | 1.5 | 00009-MR-DWI_Tra | 84 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_005c | Philips | Achieva | 3 | 00401-MR-dwi_1000 | 96 | 2934 | 5 | 6 | 0.9 × 0.9 |
| NHNN_006a | Philips | Achieva | 3 | 00401-MR-dwi_1000 | 96 | 2934 | 5 | 6 | 0.9 × 0.9 |
| NHNN_006b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3348 | 5 | 6.5 | 2.0 × 2.0 |
| NHNN_007a | Siemens | Avanto | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_aa | 89 | 3600 | 5 | 6.5 | 1.3 × 1.3 |
| NHNN_008a | Philips | Achieva | 1.5 | 00501-MR-sDW_SSh | 90 | 3174 | 5 | 6 | 1.8 × 1.8 |
| NHNN_009a | GE Medical Systems | SIGNA Excite | 1.5 | 00004-MR-Ax_DWI_1000b | 100 | 9000 | 4 | 4.4 | 1.2 × 1.2 |
| NHNN_009b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_010a | GE Medical Systems | Discovery MR450 | 1.5 | 00008-MR-Ax_DWI_(NEW) | 83 | 8000 | 5 | 6 | 0.9 × 0.9 |
| NHNN_010b | Siemens | Avanto | 1.5 | 00008-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_011a | Siemens | Avanto | 1.5 | 00010-MR-ep2d_diff_3scan_trace_p2_aa | 89 | 3600 | 5 | 6.5 | 1.3 × 1.3 |
| NHNN_011b | Siemens | Avanto | 1.5 | 00005-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_012a | Siemens | Espree | 1.5 | 00005-MR-DWI_3scanTrace_2.5iso | 87 | 6800 | 2.5 | 2.5 | 2.5 × 2.5 |
| NHNN_012b | Siemens | Avanto | 1.5 | 00008-MR-ep2d_diff_3scan | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_012c | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 76 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_013a | Siemens | Prisma_fit | 3 | 00007-MR-resolve_4scan_trace_ p2_192_TRACEW | 55 | 3700 | 5 | 6.5 | 1.1 × 1.1 |
| NHNN_013b | Philips | Achieva | 3 | 00401-MR-dwi_1000 | 96 | 2933 | 5 | 6 | 0.9 × 0.9 |
| NHNN_013c | Philips | Ingenia | 3 | 00702-MR-Reg_-_DWI_3b_Tra_SENSE | 99 | 3961 | 5 | 5.5 | 1.0 × 1.0 |
| NHNN_014a | Siemens | Avanto | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2 | 102 | 3400 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_014b | Philips | Achieva | 3 | 00401-MR-dwi_1000 | 96 | 2933 | 5 | 6 | 0.9 × 0.9 |
| NHNN_015a | Siemens | Skyra | 3 | 00009-MR-ep2d_diff_3scan_trace_p2_aa_TRACEW | 98 | 6000 | 5 | 6.5 | 1.1 × 1.1 |
| NHNN_015b | Siemens | Avanto | 1.5 | 00009-MR-DWI_Tra | 81 | 3900 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_016a | Siemens | Avanto | 1.5 | 00010-MR-ep2d_diff_3scan_trace_p2_aa | 89 | 3600 | 5 | 6.5 | 1.3 × 1.3 |
| NHNN_016b | Siemens | SymphonyTim | 1.5 | 00007-MR-DWI_Tra | 84 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_016c | Philips | Ingenia | 3 | 00801-MR-DWI_3b_ax_cc | 101 | 4392 | 5 | 5.5 | 1.0 × 1.0 |
| NHNN_017a | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-Ax_DWI_1000b | 82 | 8000 | 4 | 4.5 | 1.1 × 1.1 |
| NHNN_017b | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-Ax_DWI_1000b | 82 | 8650 | 4 | 4.5 | 1.1 × 1.1 |
| NHNN_018a | Siemens | Avanto | 1.5 | 00004-MR-ep2d_diff_3scan_trace_p2_aa | 89 | 3600 | 5 | 6.5 | 1.3 × 1.3 |
| NHNN_020a | Siemens | Prisma_fit | 3 | 00007-MR-resolve_4scan_trace_ p2_192_TRACEW | 55 | 3700 | 4 | 5 | 1.1 × 1.1 |
| NHNN_020b | Philips | Ingenia | 3 | 00801-MR-DWI_3b_ax_cc | 99 | 3917 | 5 | 5.5 | 1.0 × 1.0 |
| NHNN_020c | Siemens | Avanto | 1.5 | 00008-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_021a | Siemens | Avanto | 1.5 | 00007-MR-ep2d_diff_3scan_trace_p2 | 102 | 3200 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_021b | Philips | Achieva | 3 | 00501-MR-dwi_1000 | 95 | 2939 | 5 | 6 | 0.9 × 0.9 |
| NHNN_022b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_023a | Siemens | Skyra | 3 | 00012-MR-ep2d_diff_3scan_trace_p2_aa_TRACEW | 98 | 6000 | 5 | 6.5 | 1.1 × 1.1 |
| NHNN_024a | Siemens | Avanto | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_aa | 89 | 3600 | 5 | 6.5 | 1.3 × 1.3 |
| NHNN_025a | Siemens | Avanto | 1.5 | 00006-MR-ep2d_diff_3scan_trace_p2 | 102 | 4100 | 5 | 6.5 | 1.2 × 1.2 |
| NHNN_026b | Siemens | Avanto | 1.5 | 00007-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_026c | Siemens | SymphonyTim | 1.5 | 00010-MR-DWI_Tra | 84 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| NHNN_028a | Siemens | Avanto | 1.5 | 00011-MR-DWI_Tra | 81 | 3200 | 5 | 6.5 | 1.8 × 1.8 |
| Subject | Manufacturer | Model | Field Strength (T) | Series | TE (ms) | TR (ms) | Slice Thickness (mm) | Slice Spacing (mm) | In Plane Resolution (mm × mm) |
|---|---|---|---|---|---|---|---|---|---|
| Nott_020a | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 81 | 8000 | 4 | 5 | 1.0 × 1.0 |
| Nott_026b | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 81 | 8000 | 4 | 5 | 1.0 × 1.0 |
| Nott_060a | Philips | Achieva | 3 | 00401-MR-DW_SSh_new_2012 | 55 | 2661 | 4 | 5 | 1.0 × 1.0 |
| Nott_060b | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 82 | 7000 | 4 | 5 | 1.0 × 1.0 |
| Nott_063a | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-Ax_DWI | 82 | 8000 | 4 | 5 | 0.9 × 0.9 |
| Nott_063b | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 82 | 7000 | 4 | 5 | 1.0 × 1.0 |
| Nott_063c | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 82 | 7000 | 4 | 5 | 1.0 × 1.0 |
| Nott_066a | Siemens | Avanto | 1.5 | 00006-MR-ep2d_diff_3scan_trace_p2 | 102 | 4100 | 5 | 6.5 | 1.2 × 1.2 |
| Nott_066b | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 81 | 7175 | 4 | 5 | 1.0 × 1.0 |
| Nott_066c | Siemens | Aera | 1.5 | 00007-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 6300 | 5 | 6.5 | 0.6 × 0.6 |
| Nott_067a | GE Medical Systems | Signa HDxt | 1.5 | 00004-MR-DWI | 81 | 7000 | 4 | 5 | 1.0 × 1.0 |
| Nott_067b | Siemens | Aera | 1.5 | 00007-MR-resolve_4scan_trace_tra_160_p2_TRACEW | 60 | 6150 | 4 | 4.96 | 1.4 × 1.4 |
| Nott_068b | Siemens | Aera | 1.5 | 00007-MR-resolve_4scan_trace_tra_160_p2_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_068c | Philips | Achieva | 3 | 00601-MR-DWI | 96 | 4176 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_070a | Philips | Achieva | 3 | 00601-MR-DWI | 96 | 4043 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_070b | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_070c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_072a | GE Medical Systems | Signa HDxt | 1.5 | 00006-MR-Ax_DWI | 82 | 8000 | 4 | 5 | 0.9 × 0.9 |
| Nott_072b | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6560 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_077a | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4008 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_077b | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_077c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_080a | GE Medical Systems | Signa HDxt | 1.5 | 00003-MR-Ax_DWI | 82 | 8000 | 4 | 5 | 0.9 × 0.9 |
| Nott_080b | Siemens | Aera | 1.5 | 00007-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_080c | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_081a | Philips | Achieva | 1.5 | 00501-MR-DWI | 89 | 4119 | 4 | 5 | 1.0 × 1.0 |
| Nott_081b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_082a | GE Medical Systems | Signa HDxt | 1.5 | 00007-MR-Ax_DWI | 82 | 8000 | 4 | 5 | 0.9 × 0.9 |
| Nott_082b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_082c | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8800 | 4 | 5 | 0.6 × 0.6 |
| Nott_083a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_083b | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4007 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_085a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8800 | 4 | 5 | 0.6 × 0.6 |
| Nott_085b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 9000 | 4 | 5 | 0.6 × 0.6 |
| Nott_085c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6780 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_086a | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4541 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_086b | Siemens | Aera | 1.5 | 00007-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6780 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_086c | Siemens | Aera | 1.5 | 00010-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 7010 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_087a | Philips | Intera | 1.5 | 00601-MR-sDW_SSh | 91 | 4727 | 4 | 5 | 2.0 × 2.0 |
| Nott_087b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_087c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6560 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_095a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 115 | 6700 | 4 | 4.4 | 1.3 × 1.3 |
| Nott_095b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_095c | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_099a | Philips | Achieva | 3 | 00401-MR-DWI | 96 | 4077 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_099b | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6780 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_099c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6330 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_100a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_100b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_100c | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4044 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_103a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_103b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8200 | 4 | 5 | 0.6 × 0.6 |
| Nott_103c | Siemens | Aera | 1.5 | 00006-MR-resolve_4scan_trace_tra_160_p2_DWI_TRACEW | 60 | 6780 | 4 | 4.96 | 1.6 × 1.6 |
| Nott_109a | GE Medical Systems | Discovery MR750w | 3 | 00003-MR-DWI_TRA_b1250 | 75 | 12025 | 3.6 | 3.9 | 0.9 × 0.9 |
| Nott_109b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 7800 | 4 | 5 | 0.6 × 0.6 |
| Nott_111a | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4442 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_111b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 7800 | 4 | 5 | 0.6 × 0.6 |
| Nott_111c | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8100 | 4 | 5 | 0.6 × 0.6 |
| Nott_116a | Philips | Achieva | 3 | 00601-MR-DWI | 95 | 4032 | 4 | 4.4 | 0.9 × 0.9 |
| Nott_116b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 8600 | 4 | 5 | 0.6 × 0.6 |
| Nott_120a | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 115 | 6700 | 4 | 4.4 | 1.3 × 1.3 |
| Nott_120b | Siemens | Aera | 1.5 | 00005-MR-ep2d_diff_3scan_trace_p2_TRACEW | 89 | 7800 | 4 | 5 | 0.6 × 0.6 |
| Nott_120c | Philips | Ingenia | 1.5 | 00401-MR-DWI | 90 | 3627 | 4 | 5 | 1.3 × 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornell, I.; Al Busaidi, A.; Wastling, S.; Anjari, M.; Cwynarski, K.; Fox, C.P.; Martinez-Calle, N.; Poynton, E.; Maynard, J.; Thust, S.C. Early MRI Predictors of Relapse in Primary Central Nervous System Lymphoma Treated with MATRix Immunochemotherapy. J. Pers. Med. 2023, 13, 1182. https://doi.org/10.3390/jpm13071182
Cornell I, Al Busaidi A, Wastling S, Anjari M, Cwynarski K, Fox CP, Martinez-Calle N, Poynton E, Maynard J, Thust SC. Early MRI Predictors of Relapse in Primary Central Nervous System Lymphoma Treated with MATRix Immunochemotherapy. Journal of Personalized Medicine. 2023; 13(7):1182. https://doi.org/10.3390/jpm13071182
Chicago/Turabian StyleCornell, Isabel, Ayisha Al Busaidi, Stephen Wastling, Mustafa Anjari, Kate Cwynarski, Christopher P. Fox, Nicolas Martinez-Calle, Edward Poynton, John Maynard, and Steffi C. Thust. 2023. "Early MRI Predictors of Relapse in Primary Central Nervous System Lymphoma Treated with MATRix Immunochemotherapy" Journal of Personalized Medicine 13, no. 7: 1182. https://doi.org/10.3390/jpm13071182





