Effect of Radiation Therapy on Composition of Lymphocyte Populations in Patients with Primary Breast Cancer
Abstract
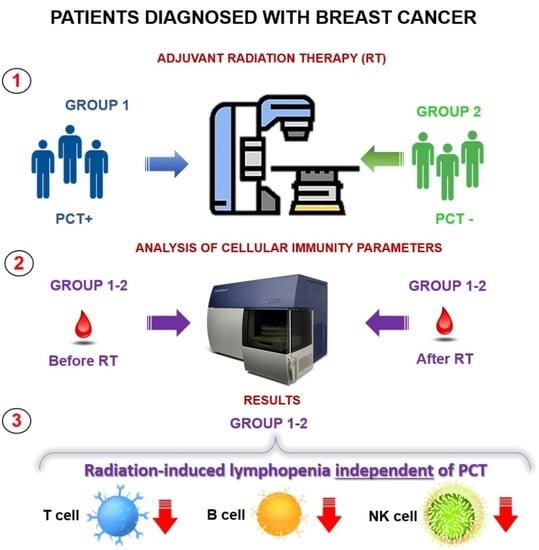
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessment of Indicators of Cellular Immunity
3. Results
Assessment of the Immune Status before the Start of Adjuvant External Beam Radiotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaprin, A.D.; Starinskij, V.V.; Petrova, G.V. The State of Oncological Care for the Population of Russia in 2021; M.: MNIOI im. P. A. Gercena—Filial FGBU «NMIC radiologii» Minzdrava Rossii: Moscow, Russia, 2022; ISBN 978-5-85502-275-9. (In Russian) [Google Scholar]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of Radiotherapy after Mastectomy and Axillary Surgery on 10-Year Recurrence and 20-Year Breast Cancer Mortality: Meta-Analysis of Individual Patient Data for 8135 Women in 22 Randomised Trials. Lancet Lond. Engl. 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Chen, F.; Yu, H.; Zhang, H.; Nong, Y.; Wang, Q.; Jing, H.; Han, Y.; Wu, J.; Zhou, Z.; Yang, L.; et al. Risk Factors for Radiation Induced Lymphopenia in Patients with Breast Cancer Receiving Adjuvant Radiotherapy. Ann. Transl. Med. 2021, 9, 1288. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Glasow, A.; Paape, D.; Hildebrandt, G. Effects of Ionizing Radiation on the Immune System with Special Emphasis on the Interaction of Dendritic and T Cells. Front. Oncol. 2012, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Ménétrier-Caux, C.; Ray-Coquard, I.; Blay, J.-Y.; Caux, C. Lymphopenia in Cancer Patients and Its Effects on Response to Immunotherapy: An Opportunity for Combination with Cytokines? J. Immunother. Cancer 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- So, T.H.; Chan, S.K.; Chan, W.L.; Choi, H.; Chiang, C.L.; Lee, V.; Lam, T.C.; Wong, I.; Law, S.; Kwong, D.; et al. Lymphopenia and Radiation Dose to Circulating Lymphocytes with Neoadjuvant Chemoradiation in Esophageal Squamous Cell Carcinoma. Adv. Radiat. Oncol. 2020, 5, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.T.; Ye, X.; Ellsworth, S.G.; Smith, J.A.; Narang, A.K.; Garg, T.; Campian, J.; Laheru, D.A.; Zheng, L.; Wolfgang, C.L.; et al. The Association Between Chemoradiation-Related Lymphopenia and Clinical Outcomes in Patients with Locally Advanced Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. 2015, 38, 259–265. [Google Scholar] [CrossRef]
- Tang, C.; Liao, Z.; Gomez, D.; Levy, L.; Zhuang, Y.; Gebremichael, R.A.; Hong, D.S.; Komaki, R.; Welsh, J.W. Lymphopenia Association with Gross Tumor Volume and Lung V5 and Its Effects on Non-Small Cell Lung Cancer Patient Outcomes. Int. J. Radiat. Oncol. 2014, 89, 1084–1091. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, B.; Chen, G.; Zhao, X.; Hu, Y.; Zhu, W.; Zeng, Z.; Chen, Y. Association Between Circulating Lymphocyte Populations and Outcome after Stereotactic Body Radiation Therapy in Patients with Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 896. [Google Scholar] [CrossRef]
- Jing, W.; Xu, T.; Wu, L.; Lopez, P.B.; Grassberger, C.; Ellsworth, S.G.; Mohan, R.; Hobbs, B.P.; Blumenschein, G.R.; Tu, J.; et al. Severe Radiation-Induced Lymphopenia Attenuates the Benefit of Durvalumab After Concurrent Chemoradiotherapy for NSCLC. JTO Clin. Res. Rep. 2022, 3, 100391. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Dussart, S.; Goillot, C.; Mayeur, D.; Debourdeau, P.; Ghesquieres, H.; Bachelot, T.; Le Cesne, A.; Anglaret, B.; Agostini, C.; et al. A Risk Model for Severe Anemia to Select Cancer Patients for Primary Prophylaxis with Epoetin α: A Prospective Randomized Controlled Trial of the ELYPSE Study Group. Ann. Oncol. 2009, 20, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Afghahi, A.; Purington, N.; Han, S.S.; Desai, M.; Pierson, E.; Mathur, M.B.; Seto, T.; Thompson, C.A.; Rigdon, J.; Telli, M.L.; et al. Higher Absolute Lymphocyte Counts Predict Lower Mortality from Early-Stage Triple-Negative Breast Cancer. Clin. Cancer Res. 2018, 24, 2851–2858. [Google Scholar] [CrossRef]
- Cho, O.; Chun, M.; Kim, S.W.; Jung, Y.S.; Yim, H. Lymphopenia as a Potential Predictor of Ipsilateral Breast Tumor Recurrence in Early Breast Cancer. Anticancer Res. 2019, 39, 4467–4474. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Mego, M.; Scarpi, E.; Giuliano, M.; Giordano, A.; Reuben, J.M.; Valero, V.; Ueno, N.T.; Hortobagyi, G.N.; Cristofanilli, M. Relationship Between Lymphocytopenia and Circulating Tumor Cells as Prognostic Factors for Overall Survival in Metastatic Breast Cancer. Clin. Breast Cancer 2012, 12, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.; Tredan, O.; Bachelot, T.; Clapisson, G.; Courtier, A.; Parmentier, G.; Rabeony, T.; Grives, A.; Perez, S.; Mouret, J.-F.; et al. Lymphopenia Combined with Low TCR Diversity (Divpenia) Predicts Poor Overall Survival in Metastatic Breast Cancer Patients. OncoImmunology 2012, 1, 432–440. [Google Scholar] [CrossRef]
- Sun, G.-Y.; Wang, S.-L.; Song, Y.-W.; Jin, J.; Wang, W.-H.; Liu, Y.-P.; Ren, H.; Fang, H.; Tang, Y.; Zhao, X.-R.; et al. Radiation-Induced Lymphopenia Predicts Poorer Prognosis in Patients with Breast Cancer: A Post Hoc Analysis of a Randomized Controlled Trial of Postmastectomy Hypofractionated Radiation Therapy. Int. J. Radiat. Oncol. 2020, 108, 277–285. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Wang, Q.; Zhou, M.; Nong, Y.; Jing, H.; Han, Y.; Liu, Y.; Hu, Y.; Yu, H.; et al. Chemotherapy Is a Risk Factor of Lymphopenia before Adjuvant Radiotherapy in Breast Cancer. Cancer Rep. 2022, 5, e1525. [Google Scholar] [CrossRef]
- Sage, E.K.; Schmid, T.E.; Sedelmayr, M.; Gehrmann, M.; Geinitz, H.; Duma, M.N.; Combs, S.E.; Multhoff, G. Comparative Analysis of the Effects of Radiotherapy versus Radiotherapy after Adjuvant Chemotherapy on the Composition of Lymphocyte Subpopulations in Breast Cancer Patients. Radiother. Oncol. 2016, 118, 176–180. [Google Scholar] [CrossRef]
- Petrini, B.; Wasserman, J.; Blomgren, H.; Baral, E.; Strender, L.E.; Wallgren, A. Blood Lymphocyte Subpopulations in Breast Cancer Patients Following Post-Operative Adjuvant Chemotherapy or Radiotherapy. Clin. Exp. Immunol. 1979, 38, 361–365. [Google Scholar]
- Petrini, B.; Wasserman, J.; Blomgren, H.; Baral, E. Blood Lymphocyte Subpopulations in Breast Cancer Patients Following Radiotherapy. Clin. Exp. Immunol. 1977, 29, 36–42. [Google Scholar]
- Chen, F.; Jin, J.-Y.; Hui, T.S.K.; Jing, H.; Zhang, H.; Nong, Y.; Han, Y.; Wang, W.; Ma, L.; Yi, F.; et al. Radiation Induced Lymphopenia is Associated with the Effective Dose to the Circulating Immune Cells in Breast Cancer. Front. Oncol. 2022, 12, 768956. [Google Scholar] [CrossRef] [PubMed]
- Emile, G.; Penager, S.; Levy, C.; Johnson, A.; Allouache, D.; Lequesne, J.; Hrab, I.; Segura, C.; Morel, A.; Gunzer, K.; et al. Baseline Lymphopenia as Prognostic Factor in Patients with Metastatic Breast Cancer Treated with Palbociclib. Oncol. Lett. 2021, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A Phase 2 Clinical Trial Assessing the Efficacy and Safety of Pembrolizumab and Radiotherapy in Patients with Metastatic Triple-negative Breast Cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.C.; Pritchard, D.J.; Ritts, R.E.; Ivins, J.C.; Pierre, R.V. Effect of Surgery on the Quantity of Lymphocyte Subpopulations. J. Surg. Res. 1976, 21, 155–158. [Google Scholar] [CrossRef]





| Index | Reference Values |
|---|---|
| Lymphocytes (absolute count), ×109/L | 1.2–3.0 |
| T-lymphocytes (CD3+), ×106/mL | 950–1800 |
| T-lymphocytes (CD3+), % | 55–80 |
| T-helper cells (CD4+ CD3+), ×106/L | 570–1100 |
| T-helper cells (CD4+ CD3+), % | 31–51 |
| Cytotoxic T cells (CD8+ CD3+), ×106/L | 450–850 |
| Cytotoxic T cells (CD8+ CD3+), % | 12–30 |
| Immunoregulatory index (CD4+/CD8+), % | 1.0–2.5 |
| Natural killer cells (CD16+ CD56+ CD3−), ×106/L | 180–420 |
| Natural killer cells (CD16+ CD56+ CD3−), % | 4.0–18.0 |
| B-lymphocytes (CD19+), ×106/L | 150–400 |
| B-lymphocytes (CD19+), % | 5.0–19.0 |
| Level of Lymphocytes in Peripheral Blood | Without PCT (n = 33) | With PCT (n = 63) | ||
|---|---|---|---|---|
| Before EBRT (%) | After EBRT (%) | Before EBRT (%) | After EBRT (%) | |
| <1.2 × 109/L | 22.2 | 75.0 | 14.0 | 77.0 |
| 1.2–3.0 × 109/L | 75.0 | 25.0 | 82.0 | 23.0 |
| >3.0 × 109/L | 2.8 | 0 | 4.0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobzeva, I.; Astrelina, T.; Suchkova, Y.; Malivanova, T.; Usupzhanova, D.; Brunchukov, V.; Rastorgueva, A.; Nikitina, V.; Lubaeva, E.; Sukhova, M.; et al. Effect of Radiation Therapy on Composition of Lymphocyte Populations in Patients with Primary Breast Cancer. J. Pers. Med. 2023, 13, 1399. https://doi.org/10.3390/jpm13091399
Kobzeva I, Astrelina T, Suchkova Y, Malivanova T, Usupzhanova D, Brunchukov V, Rastorgueva A, Nikitina V, Lubaeva E, Sukhova M, et al. Effect of Radiation Therapy on Composition of Lymphocyte Populations in Patients with Primary Breast Cancer. Journal of Personalized Medicine. 2023; 13(9):1399. https://doi.org/10.3390/jpm13091399
Chicago/Turabian StyleKobzeva, Irina, Tatiana Astrelina, Yuliya Suchkova, Tatyana Malivanova, Daria Usupzhanova, Vitaliy Brunchukov, Anna Rastorgueva, Victoria Nikitina, Ekaterina Lubaeva, Marina Sukhova, and et al. 2023. "Effect of Radiation Therapy on Composition of Lymphocyte Populations in Patients with Primary Breast Cancer" Journal of Personalized Medicine 13, no. 9: 1399. https://doi.org/10.3390/jpm13091399