A Fast Scoring of Human Primary Respiratory Epithelia Grown at Air–Liquid Interface (ALI) to Assess Epithelial Morphology in Research and Personalized Medicine Settings
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Morphology of Epithelia Grown at the Air–Liquid Interface
3.2. Difference in Morphology of SAEC and HBEC Epithelia and the Effect of Cortisol Can Be Distinguished via the ALI Histology Score
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fulcher, M.; Gabriel, S.E.; Burns, K.; Yankaskas, J.R.; Randell, S.H. Well-Differentiated Human Airway Epithelial Cell Cultures. In Human Cell Culture Protocols; Picot, J., Ed.; Methods in Molecular Medicine; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Legebeke, J.; Horton, K.L.; Jackson, C.L.; Coles, J.; Harris, A.; Wai, H.A.; Holloway, J.W.; Wheway, G.; Baralle, D.; Lucas, J.S. Temporal Whole-Transcriptomic Analysis of Characterized In Vitro and Ex Vivo Primary Nasal Epithelia. Front. Cell Dev. Biol. 2022, 10, 907511. [Google Scholar] [CrossRef]
- Noel, S.; Servel, N.; Hatton, A.; Golec, A.; Rodrat, M.; Ng, D.R.S.; Li, H.; Pranke, I.; Hinzpeter, A.; Edelman, A.; et al. Correlating genotype with phenotype using CFTR-mediated whole-cell Cl-currents in human nasal epithelial cells. J. Physiol. 2022, 600, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Park, B.; Bhowmik, D.; Nishida, K.; Lauver, M.; Putcha, N.; Gao, P.; Ramanathan, M.; Hansel, N.; Biswal, S.; et al. Strong correlation between air-liquid interface cultures and in vivo transcriptomics of nasal brush biopsy. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1056–L1062. [Google Scholar] [CrossRef] [PubMed]
- Brewington, J.J.; Filbrandt, E.T.; LaRosa, F.J.; Moncivaiz, J.D.; Ostmann, A.J.; Strecker, L.M.; Clancy, J.P. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight 2018, 3, e99385. [Google Scholar] [CrossRef]
- Müller, L.; Brighton, L.E.; Carson, J.L.; Fischer, W.A., II; Jaspers, I. Culturing of Human Nasal Epithelial Cells at the Air Liquid Interface. JoVE J. Vis. Exp. 2013, 80, e50646. [Google Scholar]
- Gras, D.; Bourdin, A.; Vachier, I.; de Senneville, L.; Bonnans, C.; Chanez, P. An ex vivo model of severe asthma using reconstituted human bronchial epithelium. J. Allergy Clin. Immunol. 2012, 129, 1259–1266.e1. [Google Scholar] [CrossRef]
- Zarcone, M.C.; Duistermaat, E.; van Schadewijk, A.; Jedynska, A.; Hiemstra, P.S.; Kooter, I.M. Cellular response of mucociliary differentiated primary bronchial epithelial cells to diesel exhaust. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L111–L123. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Yue, X.; Goldmann, T.; Gülsen, A.; Kugler, C.; Yu, X.; Petersen, F. Air exposure and cell differentiation are essential for investigation of SARS-CoV-2 entry genes in human primary airway epithelial cells in vitro. Front. Med. 2022, 9, 897695. [Google Scholar] [CrossRef]
- Diabasana, Z.; Perotin, J.-M.; Belgacemi, R.; Ancel, J.; Mulette, P.; Launois, C.; Delepine, G.; Dubernard, X.; Mérol, J.-C.; Ruaux, C.; et al. Chr15q25 Genetic Variant rs16969968 Alters Cell Differentiation in Respiratory Epithelia. Int. J. Mol. Sci. 2021, 22, 6657. [Google Scholar] [CrossRef]
- Wiszniewski, L.; Jornot, L.; Dudez, T.; Pagano, A.; Rochat, T.; Lacroix, J.S.; Suter, S.; Chanson, M. Long-Term Cultures of Polarized Airway Epithelial Cells from Patients with Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2006, 34, 39–48. [Google Scholar] [CrossRef]
- Von Schledorn, L.; Puertollano Martín, D.; Cleve, N.; Zöllner, J.; Roth, D.; Staar, B.O.; Hegermann, J.; Ringshausen, F.C.; Nawroth, J.; Martin, U.; et al. Primary Ciliary Dyskinesia Patient-Specific hiPSC-Derived Airway Epithelium in Air-Liquid Interface Culture Recapitulates Disease Specific Phenotypes In Vitro. Cells 2023, 12, 1467. [Google Scholar] [CrossRef]
- Wong, A.P.; Chin, S.; Xia, S.; Garner, J.; Bear, C.E.; Rossant, J. Efficient generation of functional CFTR-expressing airway epithelial cells from human pluripotent stem cells. Nat. Protoc. 2015, 10, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Bear, C.E.; Chin, S.; Pasceri, P.; Thompson, T.O.; Huan, L.-J.; Ratjen, F.; Ellis, J.; Rossant, J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012, 30, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Grainge, C.L.; Davies, D.E. Epithelial injury and repair in airways diseases. Chest 2013, 144, 1906–1912. [Google Scholar] [CrossRef]
- Bhowmick, R.; Gappa-Fahlenkamp, H. Cells and Culture Systems Used to Model the Small Airway Epithelium. Lung 2016, 194, 419–428. [Google Scholar] [CrossRef]
- Chang, E.H.; Pouladi, N.; Guerra, S.; Jandova, J.; Kim, A.; Li, H.; Li, J.; Morgan, W.; Stern, D.A.; Willis, A.L.; et al. Epithelial cell responses to rhinovirus identify an early-life-onset asthma phenotype in adults. J. Allergy Clin. Immunol. 2022, 150, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Ben Brahim, C.; Courageux, C.; Jolly, A.; Ouine, B.; Cartier, A.; de la Grange, P.; de Koning, L.; Leroy, P. Proliferation Genes Repressed by TGF-β Are Downstream of Slug/Snail2 in Normal Bronchial Epithelial Progenitors and Are Deregulated in COPD. Stem Cell Rev. Rep. 2021, 17, 703–718. [Google Scholar] [CrossRef]
- Rodenburg, L.W.; van der Windt, I.S.; Dreyer, H.H.M.; Smits, S.M.A.; den Hertog-Oosterhoff, L.A.; Aarts, E.M.; Beekman, J.M.; Amatngalim, G.D. Protocol for generating airway organoids from 2D air liquid interface-differentiated nasal epithelia for use in a functional CFTR assay. STAR Protoc. 2023, 4, 102337. [Google Scholar] [CrossRef]
- Jonsdottir, H.R.; Siegrist, D.; Julien, T.; Padey, B.; Bouveret, M.; Terrier, O.; Pizzorno, A.; Huang, S.; Samby, K.; Wells, T.N.C.; et al. Molnupiravir combined with different repurposed drugs further inhibits SARS-CoV-2 infection in human nasal epithelium in vitro. Biomed. Pharmacother. 2022, 150, 113058. [Google Scholar] [CrossRef]
- Lee, R.E.; Reidel, B.; Nelson, M.R.; Macdonald, J.K.; Kesimer, M.; Randell, S.H. Air-Liquid interface cultures to model drug delivery through the mucociliary epithelial barrier. Adv. Drug Deliv. Rev. 2023, 198, 114866. [Google Scholar] [CrossRef] [PubMed]
- Leni, Z.; Ess, M.N.; Keller, A.; Allan, J.D.; Hellén, H.; Saarnio, K.; Williams, K.R.; Brown, A.S.; Salathe, M.; Baumlin, N.; et al. Role of Secondary Organic Matter on Soot Particle Toxicity in Reconstituted Human Bronchial Epithelia Exposed at the Air-Liquid Interface. Environ. Sci. Technol. 2022, 56, 17007–17017. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef]
- Muller, L.; Jaspers, I. Epithelial cells, the “switchboard” of respiratory immune defense responses: Effects of air pollutants. Swiss Med. Wkly. 2012, 142, w13653. [Google Scholar] [CrossRef] [PubMed]
- Künzi, L.; Ryter, S.; Cornelius, A.; Leni, Z.; Baumlin, N.; Salathe, M.; Walser, M.; Engler, O.; Geiser, M. Transport of Designed Ankyrin Repeat Proteins through reconstituted human bronchial epithelia and protection against SARS-CoV-2. Sci. Rep. 2023, 13, 5537. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Stanke, F.; Janciauskiene, S.; Tamm, S.; Wrenger, S.; Raddatz, E.L.; Jonigk, D.; Braubach, P. Effect of Alpha-1 Antitrypsin on CFTR Levels in Primary Human Airway Epithelial Cells Grown at the Air-Liquid-Interface. Molecules 2021, 26, 2639. [Google Scholar] [CrossRef]
- Sham, P.C.; Curtis, D. Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann. Hum. Genet. 1995, 59, 97–105. [Google Scholar] [CrossRef]
- Weng, C.-M.; Lee, M.-J.; Chao, W.; Lin, Y.-R.; Chou, C.-J.; Chen, M.-C.; Chou, C.-L.; Tsai, I.-L.; Lin, C.-H.; Fan Chung, K.; et al. Airway epithelium IgE-FcεRI cross-link induces epithelial barrier disruption in severe T2-high asthma. Mucosal Immunol. 2023, 16, 685–698. [Google Scholar] [CrossRef]
- Veerati, P.C.; Reid, A.T.; Nichol, K.S.; Wark, P.A.B.; Knight, D.A.; Bartlett, N.W.; Grainge, C.L. Mechanical forces suppress antiviral innate immune responses from asthmatic airway epithelial cells following rhinovirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L206–L214. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C.; Lai, Y.; Altman, M.C.; Barrow, K.A.; Dill-McFarland, K.A.; Liu, M.; Hamerman, J.A.; Lacy-Hulbert, A.; Piliponsky, A.M.; Ziegler, S.F.; et al. Rhinovirus infection of the airway epithelium enhances mast cell immune responses via epithelial-derived interferons. J. Allergy Clin. Immunol. 2023, 151, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Husami, A.; Arora, K.; Zhang, W.; Kadri, F.; Yarlagadda, S.; Moon, C.; Mun, K.S.; Zhang, K.; Huang, Y.; et al. A personalized medicine approach to optimize care for a pediatric cystic fibrosis patient with atypical clinical symptoms. Pediatr. Pulmonol. 2023, 59, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shrivastava, A.; Zhang, C.; Pollok, B.A.; Finkbeiner, W.E.; Gibb, E.R.; Ly, N.P.; Illek, B. Functional Profiling of CFTR-Directed Therapeutics Using Pediatric Patient-Derived Nasal Epithelial Cell Models. Front. Pediatr. 2020, 8, 536. [Google Scholar] [CrossRef]
- Lo Cicero, S.; Castelli, G.; Blaconà, G.; Bruno, S.M.; Sette, G.; Pigliucci, R.; Villella, V.R.; Esposito, S.; Zollo, I.; Spadaro, F.; et al. L1077P CFTR pathogenic variant function rescue by Elexacaftor-Tezacaftor-Ivacaftor in cystic fibrosis patient-derived air-liquid interface (ALI) cultures and organoids: In vitro guided personalized therapy of non-F508del patients. Respir. Res. 2023, 24, 217. [Google Scholar] [CrossRef] [PubMed]
- Sette, G.; Lo Cicero, S.; Blaconà, G.; Pierandrei, S.; Bruno, S.M.; Salvati, V.; Castelli, G.; Falchi, M.; Fabrizzi, B.; Cimino, G.; et al. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient-derived nasal epithelial conditionally reprogrammed stem cells. Eur. Respir. J. 2021, 58, 2100908. [Google Scholar] [CrossRef]
- Dy, A.B.C.; Girkin, J.; Marrocco, A.; Collison, A.; Mwase, C.; O’Sullivan, M.J.; Phung, T.-K.N.; Mattes, J.; Koziol-White, C.; Gern, J.E.; et al. Rhinovirus infection induces secretion of endothelin-1 from airway epithelial cells in both in vitro and in vivo models. Respir. Res. 2023, 24, 205. [Google Scholar] [CrossRef]
- Awatade, N.T.; Reid, A.T.; Nichol, K.S.; Budden, K.F.; Veerati, P.C.; Pathinayake, P.S.; Grainge, C.L.; Hansbro, P.M.; Wark, P.A.B. Comparison of commercially available differentiation media on cell morphology, function, and anti-viral responses in conditionally reprogrammed human bronchial epithelial cells. Sci. Rep. 2023, 13, 11200. [Google Scholar] [CrossRef]
- Van Barneveld, A.; Stanke, F.; Claass, A.; Ballmann, M.; Tümmler, B. CFTR protein analysis of splice site mutation 2789+5 G-A. J. Cyst. Fibros. 2008, 7, 165–167. [Google Scholar] [CrossRef]
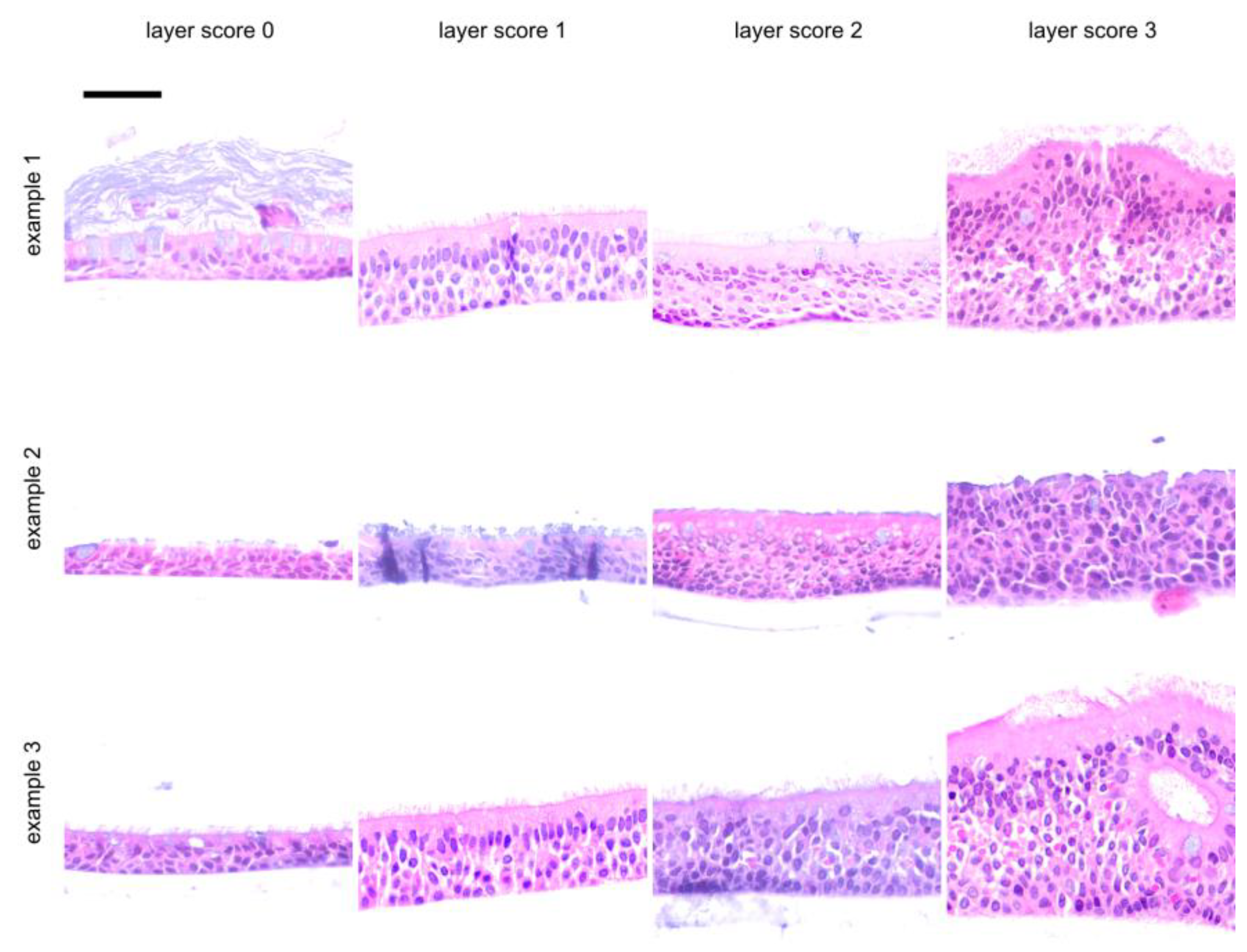
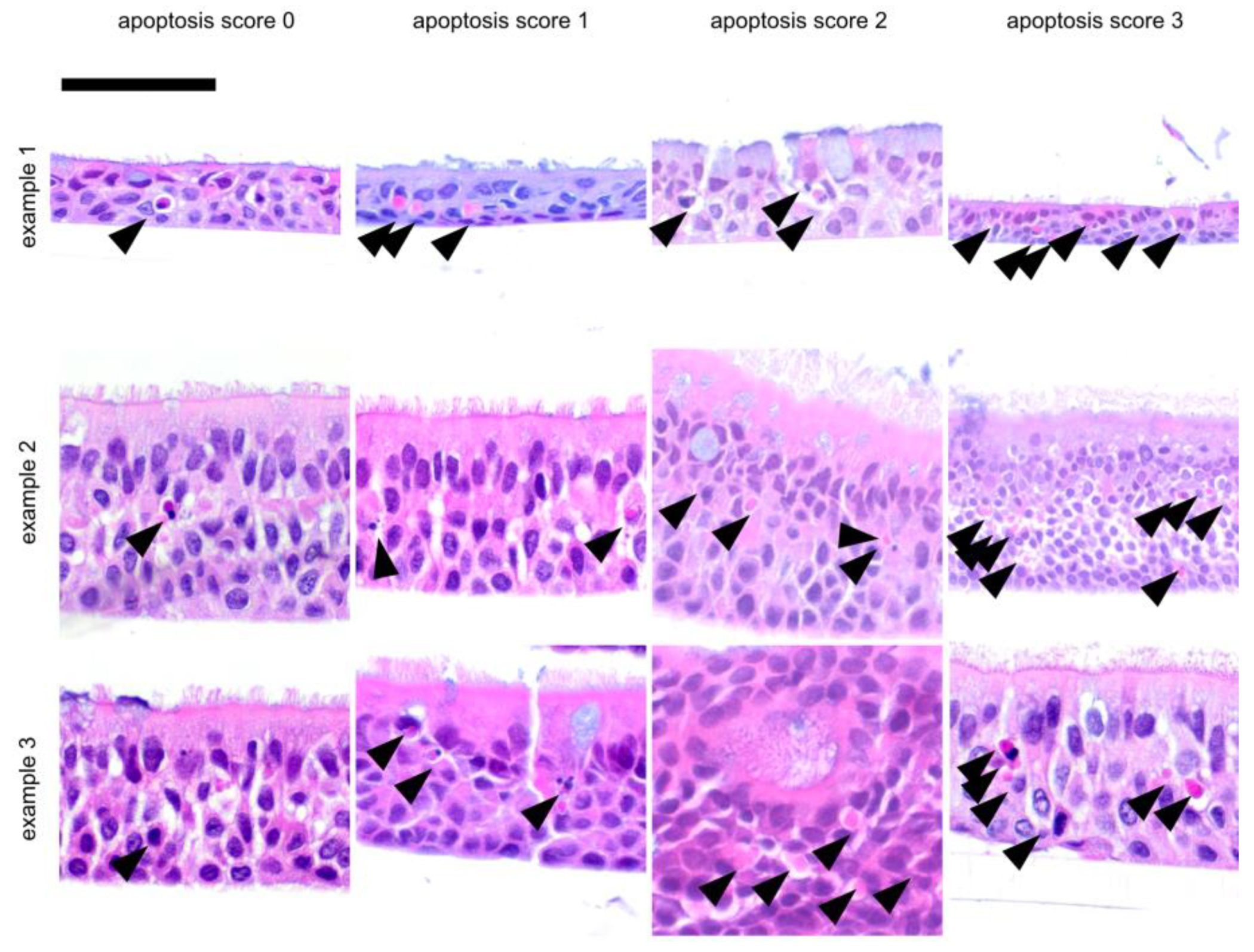
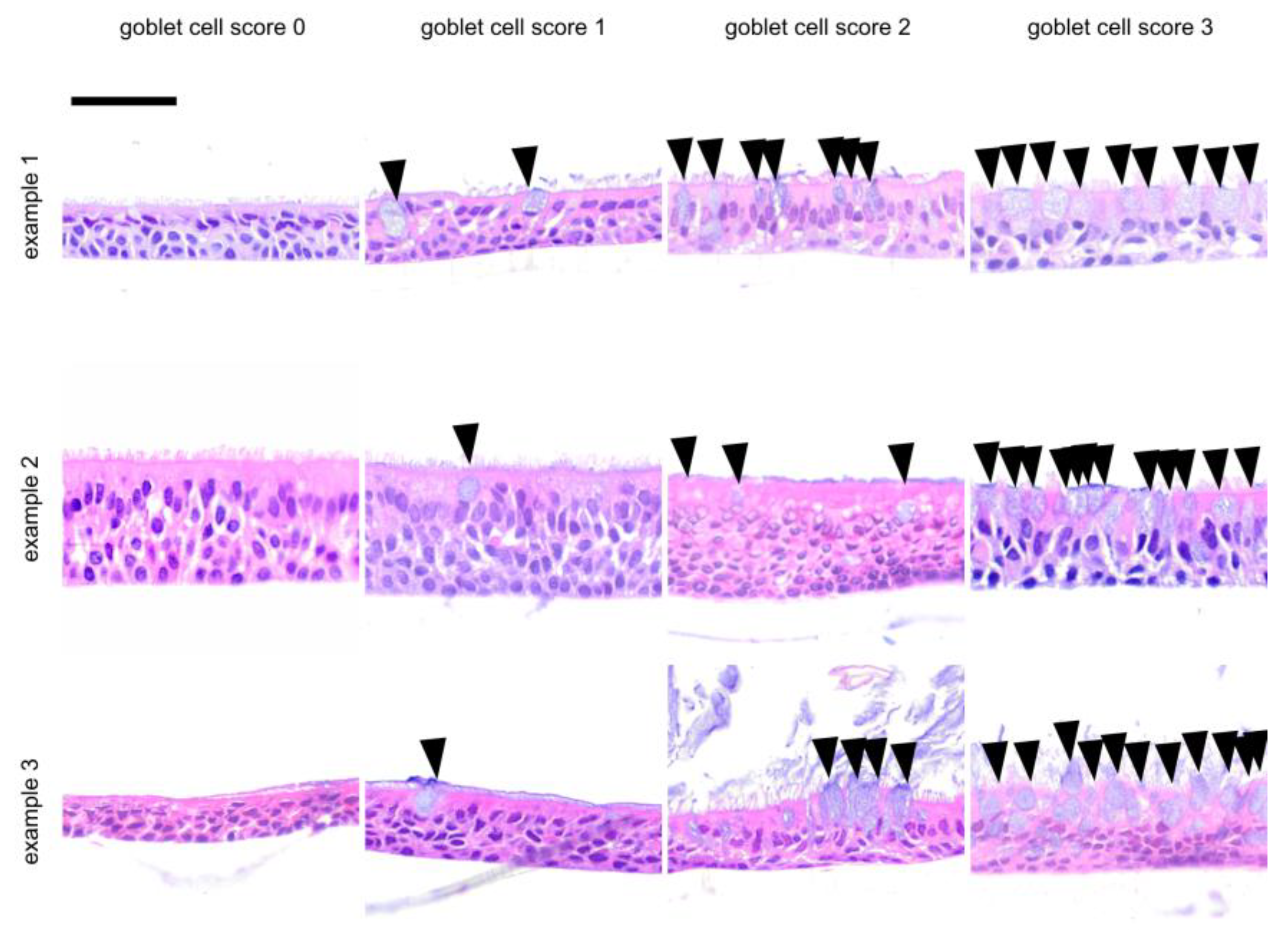

| Score | Layer | Apoptosis | Goblet Cell Score | Air Inclusion | ||||
|---|---|---|---|---|---|---|---|---|
| Data Set | Study 1 1 | Study 2 2 | Study 1 1 | Study 2 2 | Study 1 1 | Study 2 2 | Study 1 1 | Study 2 2 |
| 0 | 5 [10.9%] | 6 [21.4%] | 19 [42.2%] | 4 [14.3%] | 4 [9.1%] | 7 [25.9%] | 23 [52.3%] | 16 [59.3%] |
| 1 | 34 [73.9%] | 19 [67.9%] | 8 [17.8%] | 14 [50%] | 30 [68.2%] | 10 [37.0%] | 15 [34.1%] | 9 [33.3%] |
| 2 | 4 [8.7%] | 2 [7.1%] | 14 [31.1%] | 9 [32.1%] | 6 [13.6%] | 3 [11.1%] | 5 [11.3%] | 2 [7.4%] |
| 3 | 3 [6.5%] | 1 [3.6%] | 4 [8.9%] | 1 [3.6%] | 4 [9.1%] | 7 [25.9%] | 1 [2.3%] | 0 [0%] |
| p 3 | 1 | 0.03 | 1 | 0.9 | ||||
| Score | Layer | Apoptosis | Goblet Cell Score | Air Inclusion | ||||
|---|---|---|---|---|---|---|---|---|
| Data Set | SAEC | HBEC | SAEC | HBEC | SAEC | HBEC | SAEC | HBEC |
| 0 | 10 [33.4%] | 1 [2.3%] | 13 [43.3%] | 10 [23.2%] | 2 [6.9%] | 9 [21.4%] | 16 [55.2%] | 23 [54.8%] |
| 1 | 18 [60%] | 35 [79.5%] | 8 [26.7%] | 14 [32.6%] | 12 [41.4%] | 28 [66.7%] | 9 [31.0%] | 15 [35.7%] |
| 2 | 1 [3.3%] | 5 [11.4%] | 8 [26.7%] | 15 [34.9%] | 6 [20.7%] | 3 [7.1%] | 4 [13.8%] | 3 [7.1%] |
| 3 | 1 [3.3%] | 3 [6.8%] | 1 [3.3%] | 4 [9.3%] | 9 [31.0%] | 2 [4.8%] | 0 [0%] | 1 [2.4%] |
| p 1 | 0.001 | 0.07 | 0.002 | 0.67 | ||||
| Score | Layer | Apoptosis | Goblet Cell Score | Air Inclusion | ||||
|---|---|---|---|---|---|---|---|---|
| Cortisol | YES 1 | NO (2d) 2 | YES 1 | NO (2d) 2 | YES 1 | NO (2d) 2 | YES 1 | NO(2d) 2 |
| 0 | 6 [14.3%] | 5 [15.6%] | 13 [31.7%] | 10 [21.3%] | 2 [4.9%] | 9 [30%] | 23 [56.1%] | 16 [53.3%] |
| 1 | 34 [80.9%] | 19 [59.4%] | 9 [21.9%] | 13 [40.6%] | 22 [53.7%] | 18 [60%] | 15 [36.6%] | 9 [30%] |
| 2 | 0 [0%] | 6 [18.8%] | 17 [41.5%] | 6 [18.7%] | 8 [19.5%] | 1 [3.3%] | 2 [4.9%] | 5 [16.7%] |
| 3 | 2 [4.8%] | 2 [6.2%] | 2 [4.9%] | 3 [9.4%] | 9 [21.9%] | 2 [6.7%] | 1 [2.4%] | 0 [0%] |
| p 3 | 0.01 | 0.04 | 0.0001 | 0.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutsch, C.T.; Feng, L.; Gómez Hohn, A.; Brandt, L.; Tamm, S.; Janciauskiene, S.; Stanke, F.; Jonigk, D.; Dittrich, A.-M.; Braubach, P. A Fast Scoring of Human Primary Respiratory Epithelia Grown at Air–Liquid Interface (ALI) to Assess Epithelial Morphology in Research and Personalized Medicine Settings. J. Pers. Med. 2024, 14, 109. https://doi.org/10.3390/jpm14010109
Lutsch CT, Feng L, Gómez Hohn A, Brandt L, Tamm S, Janciauskiene S, Stanke F, Jonigk D, Dittrich A-M, Braubach P. A Fast Scoring of Human Primary Respiratory Epithelia Grown at Air–Liquid Interface (ALI) to Assess Epithelial Morphology in Research and Personalized Medicine Settings. Journal of Personalized Medicine. 2024; 14(1):109. https://doi.org/10.3390/jpm14010109
Chicago/Turabian StyleLutsch, Christopher T., Longhua Feng, Ana Gómez Hohn, Lennart Brandt, Stephanie Tamm, Sabina Janciauskiene, Frauke Stanke, Danny Jonigk, Anna-Maria Dittrich, and Peter Braubach. 2024. "A Fast Scoring of Human Primary Respiratory Epithelia Grown at Air–Liquid Interface (ALI) to Assess Epithelial Morphology in Research and Personalized Medicine Settings" Journal of Personalized Medicine 14, no. 1: 109. https://doi.org/10.3390/jpm14010109





