Personalizing Nutrition Strategies: Bridging Research and Public Health
Abstract
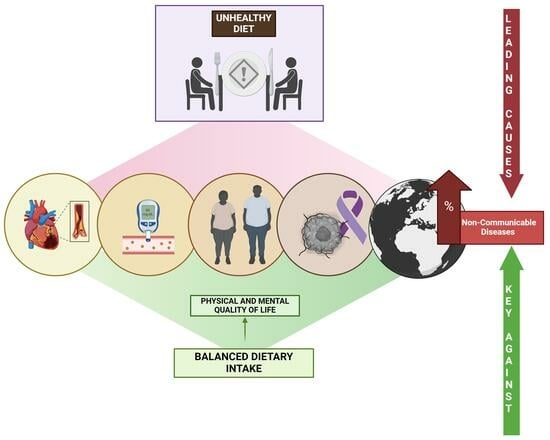
:1. Introduction
2. Materials and Methods
3. Diet and Microbiota
3.1. Unveiling the Crucial Link: Nutrition, Gut Microbiota, and Health
3.2. The Quality of Diet: Straight to Modulating the Composition of Microbiota
3.3. Physical Activity: The Other Factor of Modulation
4. Diet and Hormonal Disruption
4.1. Deciphering the Interplay: Nutrition, Adipokines, and Hormone Sensitivity
4.2. Appetite Regulation and Hormonal Dynamics
4.3. Impact on Pancreatic Function and Insulin Sensitivity
4.4. Sex Hormones and Metabolic Health
5. Diet and Inflammation
5.1. Metabolic Inflammation: Diet, Oxidative Stress, and Health Implications
5.2. Harnessing Nutritional Anti-Inflammatory Power: Insights from the Mediterranean Diet and Plant-Based Diets
6. Diet Pattern and Cardiovascular Health
6.1. Cardiovascular Health Overview
6.2. Toward Cardiovascular Wellness: Navigating Dietary Pathways for Health
7. Role of Dietary Pattern on Metabolic Health
7.1. Metabolic Pathways
7.2. Metabolomic Profile and Diet Patterns
7.3. Molecular Biomakers
7.4. Food and “Omics”
8. Dietary Patterns on Mental Well-Being
8.1. Mental Health Overview
8.2. Toward Mental Wellness: The Nutritional Paradigm Shift
9. The Impact of Dietary Patterns on Cancer Development
9.1. Exploring the Link between Dietary Habits and Cancer: Insights into Macronutrient Quality
9.2. Lipid Quality and Cancer Progression
9.3. Protective Role of Fiber Consumption in Cancer
10. Dietary Patterns on Oral Health
10.1. Role of Oral Microbiota in Health
10.2. Characteristics of the Diet
10.3. Dietary Patterns
11. The Importance of Dietary Patterns on Visual Health
11.1. Diet and Eye Health
11.2. Natural Molecules in Food/Supplements That Impact Visual Health
11.2.1. Omega-3 Fatty Acids
11.2.2. Antioxidants
11.2.3. Carotenoids: Lutein/Zeaxanthin
11.2.4. B-Complex Vitamin
11.2.5. Calcium
12. Practical Applications
12.1. Future Research Directions
- -
- Enhancing the sensitivity of NMR spectroscopy to detect molecular components at lower concentrations, expanding its utility in characterizing complex mixtures in foods.
- -
- Designing training and education programs targeting food scientists to increase the understanding and proficiency in NMR spectroscopy, reducing the entry barrier and fostering its use in nutrition and food science research.
- -
- Investigating and developing new analytical techniques and approaches harnessing the advantages of NMR spectroscopy in dietary pattern analysis, such as identifying specific biomarkers of certain diets or assessing food quality and authenticity.
- -
- Promoting interdisciplinary collaboration among food scientists, chemists, physicists, and NMR experts to address technical and scientific challenges associated with the application of NMR spectroscopy in dietary and food research.
- -
- Exploring the integration of NMR spectroscopy with other analytical techniques, such as mass spectrometry, to gain a more comprehensive understanding of food composition and molecular structure and their relationship to human health.
- -
- Establishing collaborations with industry to develop practical applications of NMR spectroscopy in assessing food quality and safety, potentially leading to the creation of innovative tools and technologies for the food industry.
- -
- Investigating the intricate interplay between dietary constituents and the host metabolome to delineate the underlying mechanisms governing disease pathogenesis.
- -
- Examining the longitudinal effects of dietary interventions on metabolic profiles and subsequent health outcomes to inform personalized dietary recommendations.
- -
- Exploring the potential of metabolomics to identify novel biomarkers predictive of dietary adherence and susceptibility to chronic diseases.
12.2. Practical Applications
- -
- Encouraging compliance with anti-inflammatory dietary patterns:
- -
- Executing Specific Dietary Interventions According to Metabolomic Profiling:
- -
- Incorporating Metabolomics-Based Biomarkers into Clinical Practice:
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thapa, D.K.; Acharya, K.; Karki, A.; Cleary, M. Health facility readiness to provide antenatal care (ANC) and non-communicable disease (NCD) services in Nepal and Bangladesh: Analysis of facility-based surveys. PLoS ONE 2023, 18, e0281357. [Google Scholar] [CrossRef]
- Santos, A.C.; Willumsen, J.; Meheus, F.; Ilbawi, A.; Bull, F.C. The cost of inaction on physical inactivity to public health-care systems: A population-attributable fraction analysis. Lancet Glob. Health 2023, 11, e32–e39. [Google Scholar] [CrossRef]
- David, M.; James, C. Ready for the Next Crisis? Investing in Health System Resilience; OECD: Paris, France, 2023. [Google Scholar] [CrossRef]
- Bertolina, C.; Farotto, M.; Crivellari, S.; Giacchero, F.; Grasso, C.; Bertolotti, M.; Maconi, A. Summary of the 2016 World Health Organization Report and 2021 Compendium on environmental diseases. Work. Pap. Public Health 2023, 11. [Google Scholar] [CrossRef]
- McGuire, S.U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.K.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary Patterns: Challenges and Opportunities in Dietary Patterns Research. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef]
- Joy, E.J.; Green, R.; Agrawal, S.; Aleksandrowicz, L.; Bowen, L.; Kinra, S.; I Macdiarmid, J.; Haines, A.; Dangour, A.D. Dietary patterns and non-communicable disease risk in Indian adults: Secondary analysis of Indian Migration Study data. Public Health Nutr. 2017, 20, 1963–1972. [Google Scholar] [CrossRef]
- Johnston, J.D. Physiological Responses to Food Intake throughout the Day. Nutr. Res. Rev. 2014, 27, 107–118. [Google Scholar] [CrossRef]
- Bressan, J.; Hermsdorff, H.H.M.; Zulet, M.Á.; Martínez, J.A. Impacto hormonal e inflamatório de diferentes composições dietéticas: Ênfase em padrões alimentares e fatores dietéticos específicos. Arq. Bras. Endocrinol. Metabol. 2009, 53, 572–581. [Google Scholar] [CrossRef]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res. 2022, 211, 113049. [Google Scholar] [CrossRef]
- Bonaccio, M.; Iacoviello, L.; de Gaetano, G.; on behalf of the Moli-sani Investigators. The Mediterranean diet: The reasons for a success. Thromb. Res. 2012, 129, 401–404. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M. Mediterranean Diet: From a Healthy Diet to a Sustainable Dietary Pattern. Front. Nutr. 2015, 2, 15. [Google Scholar] [CrossRef]
- Milte, C.M.; McNaughton, S.A. Dietary patterns and successful ageing: A systematic review. Eur. J. Nutr. 2016, 55, 423–450. [Google Scholar] [CrossRef]
- Stein, C.; Cunha-Cruz, J.; Hugo, F.N. Is dietary pattern a mediator of the relationship between socioeconomic status and dental caries? Clin. Oral. Investig. 2021, 25, 5441–5447. [Google Scholar] [CrossRef]
- Boadi-kusi, S.B.; Asiamah, E.; Ocansey, S.; Abu, S.L. Nutrition knowledge and dietary patterns in ophthalmic patients. Clin. Exp. Optom. 2021, 104, 78–84. [Google Scholar] [CrossRef]
- Francisco, S.G.; Smith, K.M.; Aragonès, G.; Whitcomb, E.A.; Weinberg, J.; Wang, X.; Bejarano, E.; Taylor, A.; Rowan, S. Dietary Patterns, Carbohydrates, and Age-Related Eye Diseases. Nutrients 2020, 12, 2862. [Google Scholar] [CrossRef]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Salonen, A.; Virta, L.J.; Kekkonen, R.A.; de Vos, W.M. Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding. JAMA Pediatr. 2016, 170, 750. [Google Scholar] [CrossRef]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices with Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Song, M.; Wang, D.D.; Franzosa, E.A.; Cao, Y.; Joshi, A.; Drew, D.A.; Mehta, R.; Ivey, K.L.; et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Weiss, E.; Eklund, M.; Aumiller, T.; Louis, S.; Rings, A.; Messner, S.; Camarinha-Silva, A.; Seifert, J.; Bischoff, S.C.; et al. Intestinal Microbiota and Microbial Metabolites Are Changed in a Pig Model Fed a High-Fat/Low-Fiber or a Low-Fat/High-Fiber Diet. PLoS ONE 2016, 11, e0154329. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Wieser, V.; Tilg, H. Dietary Factors: Major Regulators of the Gut’s Microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Belinchón-deMiguel, P.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F.; Martínez-Guardado, I.; Villanueva-Tobaldo, C.V.; Clemente-Suárez, V.J. Advances in Understanding the Interplay between Dietary Practices, Body Composition, and Sports Performance in Athletes. Nutrients 2024, 16, 571. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.R.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals with Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef]
- Morrison, K.E.; Jašarević, E.; Howard, C.D.; Bale, T.L. It’s the fiber, not the fat: Significant effects of dietary challenge on the gut microbiome. Microbiome 2020, 8, 15. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Cai, C.W.; Ran, Z.H. Therapeutic modulation of gut microbiota in inflammatory bowel disease: More questions to be answered. J. Dig. Dis. 2016, 17, 800–810. [Google Scholar] [CrossRef]
- Li, X.; Su, C.; Jiang, Z.; Yang, Y.; Zhang, Y.; Yang, M.; Zhang, X.; Du, Y.; Zhang, J.; Wang, L.; et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes 2021, 7, 36. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Ringel, Y.; Heyman, M.B.; Foster, J.A.; Bercik, P.; Shulman, R.J.; Versalovic, J.; Verdu, E.F.; Dinan, T.G.; Hecht, G.; et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 2013, 4, 17–27. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Probert, H.M.; Smejkal, C.W.; Gibson, G.R. Using probiotics and prebiotics to improve gut health. Drug Discov. Today 2003, 8, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- O’sullivan, O.; Cronin, O.; Clarke, S.F.; Murphy, E.F.; Molloy, M.G.; Shanahan, F.; Cotter, P.D. Exercise and the microbiota. Gut Microbes 2015, 6, 131–136. [Google Scholar] [CrossRef]
- Rodriguez, J.; Neyrinck, A.M.; Van Kerckhoven, M.; Gianfrancesco, M.A.; Renguet, E.; Bertrand, L.; Cani, P.D.; Lanthier, N.; Cnop, M.; Paquot, N.; et al. Physical activity enhances the improvement of body mass index and metabolism by inulin: A multicenter randomized placebo-controlled trial performed in obese individuals. BMC Med. 2022, 20, 110. [Google Scholar] [CrossRef]
- Daniel, H. Diet and the gut microbiome: From hype to hypothesis. Br. J. Nutr. 2020, 124, 521–530. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef]
- Shehzad, A.; Iqbal, W.; Shehzad, O.; Lee, Y.S. Adiponectin: Regulation of its production and its role in human diseases. Hormones 2012, 11, 8–20. [Google Scholar] [CrossRef]
- Yu, Y.; Fernandez, I.D.; Meng, Y.; Zhao, W.; Groth, S.W. Gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers in loss of control eating: A scoping review. Appetite 2021, 166, 105442. [Google Scholar] [CrossRef]
- Bouassida, A.; Zalleg, D.; Bouassida, S.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Leptin, its implication in physical exercise and training: A short review. J. Sports Sci. Med. 2006, 5, 172–181. [Google Scholar]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Caruso, C.; Candore, G. The Role of Adipose Tissue and Adipokines in Obesity-Related Inflammatory Diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]
- Sarmento-Cabral, A.; Peinado, J.R.; Halliday, L.C.; Malagon, M.; Castaño, J.; Kineman, R.D.; Luque, R. Adipokines (Leptin, Adiponectin, Resistin) Differentially Regulate All Hormonal Cell Types in Primary Anterior Pituitary Cell Cultures from Two Primate Species. Sci. Rep. 2017, 7, 43537. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Kyrou, I.; Mattu, H.S.; Chatha, K.; Randeva, H.S. Fat Hormones, Adipokines. In Endocrinology of the Heart in Health and Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 167–205. [Google Scholar] [CrossRef]
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58. [Google Scholar] [CrossRef]
- Engin, A. Diet-Induced Obesity and the Mechanism of Leptin Resistance. Adv. Exp. Med. Biol. 2017, 960, 381–397. [Google Scholar] [CrossRef]
- Andreoli, M.F.; Donato, J.; Cakir, I.; Perello, M. Leptin resensitisation: A reversion of leptin-resistant states. J. Endocrinol. 2019, 241, R81–R96. [Google Scholar] [CrossRef]
- Dinu, M.; Colombini, B.; Pagliai, G.; Cesari, F.; Gori, A.; Giusti, B.; Marcucci, R.; Sofi, F. Effects of a dietary intervention with Mediterranean and vegetarian diets on hormones that influence energy balance: Results from the CARDIVEG study. Int. J. Food Sci. Nutr. 2020, 71, 362–369. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Ma, W.; Huang, T.; Zheng, Y.; Wang, M.; Bray, G.A.; Sacks, F.M.; Qi, L. Weight-Loss Diets, Adiponectin, and Changes in Cardiometabolic Risk in the 2-Year POUNDS Lost Trial. J. Clin. Endocrinol. Metab. 2016, 101, 2415–2422. [Google Scholar] [CrossRef]
- Monzillo, L.U.; Hamdy, O.; Horton, E.S.; Ledbury, S.; Mullooly, C.; Jarema, C.; Porter, S.; Ovalle, K.; Moussa, A.; Mantzoros, C.S. Effect of Lifestyle Modification on Adipokine Levels in Obese Subjects with Insulin Resistance. Obes. Res. 2003, 11, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of Weight Loss and Lifestyle Changes on Vascular Inflammatory Markers in Obese Women. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef]
- Mahbouli, S.; Der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.-P.; Rossary, A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 2017, 38, 3254–3264. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Kucharska, A.; Rumińska, M.; Pyrżak, B. Thyroid Dysfunction in Obese and Overweight Children. Endokrynol. Pol. 2017, 68, 54–60. [Google Scholar] [CrossRef]
- Trayhurn, P.; Bing, C. Appetite and energy balance signals from adipocytes. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1237–1249. [Google Scholar] [CrossRef]
- Arch, J.; Stock, M.; Trayhurn, P. Leptin resistance in obese humans: Does it exist and what does it mean? Int. J. Obes. 1998, 22, 1159–1163. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tomé, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef]
- Feinle-Bisset, C. Modulation of hunger and satiety. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Sam, A.H.; Troke, R.C.; Tan, T.M.; Bewick, G.A. The role of the gut/brain axis in modulating food intake. Neuropharmacology 2012, 63, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Coccurello, R.; Maccarrone, M. Hedonic Eating and the ‘Delicious Circle’: From Lipid-Derived Mediators to Brain Dopamine and Back. Front. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef]
- Brereton, M.F.; Vergari, E.; Zhang, Q.; Clark, A. Alpha-, Delta- and PP-cells. J. Histochem. Cytochem. 2015, 63, 575–591. [Google Scholar] [CrossRef]
- Reinehr, T.; Enriori, P.J.; Harz, K.; Cowley, M.A.; Roth, C.L. Pancreatic polypeptide in obese children before and after weight loss. Int. J. Obes. 2006, 30, 1476–1481. [Google Scholar] [CrossRef]
- Rose, A.J. Role of Peptide Hormones in the Adaptation to Altered Dietary Protein Intake. Nutrients 2019, 11, 1990. [Google Scholar] [CrossRef]
- Karra, E.; Chandarana, K.; Batterham, R.L. The role of peptide YY in appetite regulation and obesity. J. Physiol. 2009, 587, 19–25. [Google Scholar] [CrossRef]
- Neary, M.T.; Batterham, R.L. Peptide YY: Food for thought. Physiol. Behav. 2009, 97, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Le Roux, C.W.; Cohen, M.A.; Park, A.J.; Ellis, S.M.; Patterson, M.; Frost, G.S.; Ghatei, M.A.; Bloom, S.R. Pancreatic Polypeptide Reduces Appetite and Food Intake in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Hoover, S.E.; Gower, B.A.; Cedillo, Y.E.; Chandler-Laney, P.C.; Deemer, S.E.; Goss, A.M. Changes in Ghrelin and Glucagon following a Low Glycemic Load Diet in Women with PCOS. J. Clin. Endocrinol. Metab. 2021, 106, e2151–e2161. [Google Scholar] [CrossRef] [PubMed]
- Zipf, W.B.; O’dorisio, T.M.; Cataland, S.; Dixon, K. Pancreatic Polypeptide Responses to Protein Meal Challenges in Obese but Otherwise Normal Children and Obese Children with Prader-Willi Syndrome*. J. Clin. Endocrinol. Metab. 1983, 57, 1074–1080. [Google Scholar] [CrossRef]
- Makris, M.C.; Alexandrou, A.; Papatsoutsos, E.G.; Malietzis, G.; Tsilimigras, D.I.; Guerron, A.D.; Moris, D. Ghrelin and Obesity: Identifying Gaps and Dispelling Myths. A Reappraisal. In Vivo 2017, 31, 1047–1050. [Google Scholar] [CrossRef]
- Klementova, M.; Thieme, L.; Haluzik, M.; Pavlovicova, R.; Hill, M.; Pelikanova, T.; Kahleova, H. A Plant-Based Meal Increases Gastrointestinal Hormones and Satiety More Than an Energy- and Macronutrient-Matched Processed-Meat Meal in T2D, Obese, and Healthy Men: A Three-Group Randomized Crossover Study. Nutrients 2019, 11, 157. [Google Scholar] [CrossRef]
- Di Mauro, A.; Tuccinardi, D.; Watanabe, M.; Del Toro, R.; Monte, L.; Giorgino, R.; Rampa, L.; Rossini, G.; Kyanvash, S.; Soare, A.; et al. The Mediterranean diet increases glucagon-like peptide 1 and oxyntomodulin compared with a vegetarian diet in patients with type 2 diabetes: A randomized controlled cross-over trial. Diabetes Metab. Res. Rev. 2021, 37, e3406. [Google Scholar] [CrossRef]
- Wynne, K.; Bloom, S.R. The role of oxyntomodulin and peptide tyrosine–tyrosine (PYY) in appetite control. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 612–620. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef]
- Chawla, S.; Beretoulis, S.; Deere, A.; Radenkovic, D. The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients 2021, 13, 2525. [Google Scholar] [CrossRef]
- Stachowicz, M.; Lebiedzińska, A. The effect of diet components on the level of cortisol. Eur. Food Res. Technol. 2016, 242, 2001–2009. [Google Scholar] [CrossRef]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Associations between Sleep, Cortisol Regulation, and Diet: Possible Implications for the Risk of Alzheimer Disease. Adv. Nutr. 2016, 7, 679–689. [Google Scholar] [CrossRef]
- Polito, R.; Messina, G.; Valenzano, A.; Scarinci, A.; Villano, I.; Monda, M.; Cibelli, G.; Porro, C.; Pisanelli, D.; Monda, V.; et al. The Role of Very Low Calorie Ketogenic Diet in Sympathetic Activation through Cortisol Secretion in Male Obese Population. J. Clin. Med. 2021, 10, 4230. [Google Scholar] [CrossRef]
- Marketon, J.I.W.; Glaser, R. Stress hormones and immune function. Cell Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef]
- Montalvo, C.P.; Díaz, N.H.; Galdames, L.A.; Andrés, M.E.; Larraín, R.E. Short communication: Effect of vitamins E and C on cortisol production by bovine adrenocortical cells in vitro. J. Dairy. Sci. 2011, 94, 3495–3497. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.; Preut, R.; Schommer, K.; Schürmeyer, T.H. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 2002, 159, 319–324. [Google Scholar] [CrossRef]
- Diaz, E.; Ruiz, F.; Hoyos, I.; Zubero, J.; Gravina, L.; Gil, J.; Irazusta, J.; Gil, S.M. Cell damage, antioxidant status, and cortisol levels related to nutrition in ski mountaineering during a two-day race. J. Sports Sci. Med. 2010; 9, 338–346. [Google Scholar]
- Aguiló, A.; Monjo, M.; Moreno, C.; Martinez, P.; Martínez, S.; Tauler, P. Vitamin C supplementation does not influence plasma and blood mononuclear cell IL-6 and IL-10 levels after exercise. J. Sports Sci. 2014, 32, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effects of Low-Carbohydrate versus Mediterranean Diets on Weight Loss, Glucose Metabolism, Insulin Kinetics and β-Cell Function in Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Foley, P.J. Effect of low carbohydrate diets on insulin resistance and the metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Richter, E. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans. Am. J. Clin. Nutr. 1996, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef]
- Whittaker, J.; Harris, M. Low-carbohydrate diets and men’s cortisol and testosterone: Systematic review and meta-analysis. Nutr. Health 2022, 28, 543–554. [Google Scholar] [CrossRef]
- Whittaker, J.; Wu, K. Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. J. Steroid Biochem. Mol. Biol. 2021, 210, 105878. [Google Scholar] [CrossRef]
- Bennett, F.; Ingram, D. Diet and female sex hormone concentrations: An intervention study for the type of fat consumed. Am. J. Clin. Nutr. 1990, 52, 808–812. [Google Scholar] [CrossRef]
- Thomas, H.V.; Davey, G.K.; Key, T.J. Oestradiol and sex hormone-binding globulin in premenopausal and post-menopausal meat-eaters, vegetarians and vegans. Br. J. Cancer 1999, 80, 1470–1475. [Google Scholar] [CrossRef]
- Karelis, A.D.; Fex, A.; Filion, M.-E.; Adlercreutz, H.; Aubertin-Leheudre, M. Comparison of sex hormonal and metabolic profiles between omnivores and vegetarians in pre- and post-menopausal women. Br. J. Nutr. 2010, 104, 222–226. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Corapi, S.; Gable, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Reproductive Hormone Levels in Females and Males: A Review of Human Trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Mousavi, Y.; Höckerstedt, K. Diet and Breast Cancer. Acta Oncol. 1992, 31, 175–181. [Google Scholar] [CrossRef]
- Neary, M.T.; Batterham, R.L. Gut hormones: Implications for the treatment of obesity. Pharmacol. Ther. 2009, 124, 44–56. [Google Scholar] [CrossRef]
- Adlercreutz, H. Western diet and Western diseases: Some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990, 50, 3–23. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of ‘Western Diet’ in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, G.K.; Mrakovcic-Sutic, I.; Žeželj, S.P.; Šuša, B.; Rahelić, D.; Majanović, S.K. The Efficacy of an Energy-Restricted Anti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; Britto, J.S.J.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef]
- Osorio-Conles, O.; Olbeyra, R.; Moizé, V.; Ibarzabal, A.; Giró, O.; Viaplana, J.; Jiménez, A.; Vidal, J.; de Hollanda, A. Positive Effects of a Mediterranean Diet Supplemented with Almonds on Female Adipose Tissue Biology in Severe Obesity. Nutrients 2022, 14, 2617. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and Inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Masana, M.F.; Tyrovolas, S.; Kollia, N.; Chrysohoou, C.; Skoumas, J.; Haro, J.M.; Tousoulis, D.; Papageorgiou, C.; Pitsavos, C.; Panagiotakos, D.B. Dietary Patterns and Their Association with Anxiety Symptoms among Older Adults: The ATTICA Study. Nutrients 2019, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Dietary patterns and the risk of CVD and all-cause mortality in older British men. Br. J. Nutr. 2016, 116, 1246–1255. [Google Scholar] [CrossRef]
- Whalen, K.A.; McCullough, M.L.; Flanders, W.D.; Hartman, T.J.; Judd, S.; Bostick, R.M. Paleolithic and Mediterranean Diet Pattern Scores Are Inversely Associated with Biomarkers of Inflammation and Oxidative Balance in Adults. J. Nutr. 2016, 146, 1217–1226. [Google Scholar] [CrossRef]
- Zade, M.R.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Nelson, W.G.; DeWeese, T.L.; DeMarzo, A.M. The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Rev. 2002, 21, 3–16. [Google Scholar] [CrossRef]
- Anand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.M.; et al. Food Consumption and its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and Inflammation: Possible Effects on Immunity, Chronic Diseases, and Life Span. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Rao, L. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetusa, L.R.; Sacdaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Leona, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.G.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.; Pontefract, B.; Mishcon, H.; Black, C.; Sutton, S.; Theberge, C. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients with Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.G. Cardiovascular Disease. N. Engl. J. Med. 2003, 349, 60–72. [Google Scholar] [CrossRef]
- Mbewu, A.; Mbanya, J.-C. Cardiovascular Disease; WHO: Geneva, Switzerland, 2006; ISBN 9789241547178. [Google Scholar]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Altesha, M.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Dietary Restrictions and Nutrition in the Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2019, 124, 952–965. [Google Scholar] [CrossRef]
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, Genetic Factors, and Their Interaction in Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8761. [Google Scholar] [CrossRef] [PubMed]
- Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [CrossRef]
- Ori, Z.; Ori, I. Principles of Continuous Risk Monitoring of Body Composition, Insulin Resistance, Endothelial Dysfunction and Nutrition to Improve General Health and Prevent Cardiovascular Disease and Cancer. J. Health Pol. Manag. 2021, 4, 1–4. [Google Scholar]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef]
- Li, J.; Guasch-Ferré, M.; Chung, W.; Ruiz-Canela, M.; Toledo, E.; Corella, D.; Bhupathiraju, S.N.; Tobias, D.K.; Tabung, F.K.; Hu, J.; et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020, 41, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet and cardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Lari, A.; Sohouli, M.H.; Fatahi, S.; Cerqueira, H.S.; Santos, H.O.; Pourrajab, B.; Rezaei, M.; Saneie, S.; Rahideh, S.T. The effects of the Dietary Approaches to Stop Hypertension (DASH) diet on metabolic risk factors in patients with chronic disease: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Said, M.S.; El Sayed, I.T.; Ibrahim, E.E.; Khafagy, G.M. Effect of DASH Diet Versus Healthy Dietary Advice on the Estimated Atherosclerotic Cardiovascular Disease Risk. J. Prim. Care Community Health 2021, 12, 215013272098095. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, N.; Yang, R.; Liao, X.-Y.; Zhang, Y.; Zhu, B.-F.; Zhao, Q.; Chen, L.; Zhang, Y.-G.; Lei, Y. Effects of the Modified DASH Diet on Adults with Elevated Blood Pressure or Hypertension: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 725020. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; van Daalen, K.R.; Thayyil, A.; de Cocco, M.T.A.R.R.; Caputo, D.; Oliver-Williams, C. A Systematic Review of the Association between Vegan Diets and Risk of Cardiovascular Disease. J. Nutr. 2021, 151, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and vegan diets and the risk of cardiovascular disease, ischemic heart disease and stroke: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Nutr. 2023, 62, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; Vialichka, V.; Biesiekierska, M.; Balcerczyk, A.; Pirola, L. Effects of ketogenic diet and ketone bodies on the cardiovascular system: Concentration matters. World J. Diabetes 2020, 11, 584–595. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.P.S.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension—A Narrative Review. Nutrients 2021, 13, 2567. [Google Scholar] [CrossRef]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; Petersen, K.S.; Skulas-Ray, A.C.; Richter, C.K.; Proctor, D.N.; Kris-Etherton, P.M. Replacing Saturated Fat with Walnuts or Vegetable Oils Improves Central Blood Pressure and Serum Lipids in Adults at Risk for Cardiovascular Disease: A Randomized Controlled-Feeding Trial. J. Am. Heart Assoc. 2019, 8, e011512. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Oxidative stress and beta-cell dysfunction. Pflug. Arch. 2010, 460, 703–718. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. [Google Scholar] [CrossRef]
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New Aspects of Amino Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.-W.; Ren, Z.; Mehrkanoon, S.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J.; Eussen, S.J.P.M.; Zeegers, M.P.; Wesselius, A. Plasma Metabolomic Profiling of Dietary Patterns Associated with Glucose Metabolism Status: The Maastricht Study. BMC Med. 2022, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Brennan, L.; McNulty, B.A.; Bloomfield, J.F.; Duff, D.J.; Devlin, N.F.C.; Gibney, M.J.; Flynn, A.; Walton, J.; Nugent, A.P. Plasma Fatty Acid Patterns Reflect Dietary Habits and Metabolic Health: A Cross-Sectional Study. Mol. Nutr. Food Res. 2016, 60, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.-C. The Metabolic Signature Associated with the Western Dietary Pattern: A Cross-Sectional Study. Nutr. J. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Giardina, S.; Cañueto, D.; Salas-Salvadó, J.; Cañellas, N.; Bulló, M. Changes in Plasma Metabolite Concentrations after a Low-Glycemic Index Diet Intervention. Mol. Nutr. Food Res. 2019, 63, e1700975. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of Mediterranean Diet on Plasma Metabolites and Their Relationship with Insulin Resistance and Gut Microbiota Composition in a Crossover Randomized Clinical Trial. Clin. Nutr. 2021, 40, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Baden, M.Y.; Guasch-Ferré, M.; Wittenbecher, C.; Li, J.; Li, Y.; Wan, Y.; Bhupathiraju, S.N.; Tobias, D.K.; Clish, C.B.; et al. Plasma Metabolite Profiles Related to Plant-Based Diets and the Risk of Type 2 Diabetes. Diabetologia 2022, 65, 1119–1132. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med.—Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Ojo, O.; Adebowale, F.; Wang, X.-H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Wang, Z.; Ni, X.; Zhang, L.; Sun, L.; Zhu, X.; Zhou, Q.; Yang, Z.; Yuan, H. Toll-Like Receptor 4 and Inflammatory Micro-Environment of Pancreatic Islets in Type-2 Diabetes Mellitus: A Therapeutic Perspective. Diabetes Metab. Syndr. Obes. 2020, 13, 4261–4272. [Google Scholar] [CrossRef]
- Garibotto, G.; Carta, A.; Picciotto, D.; Viazzi, F.; Verzola, D. Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J. Nephrol. 2017, 30, 719–727. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; De Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk. Circulation 2014, 129 (Suppl. S2), S76–S99. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Lovegrove, J.A.; Mensink, R.P.; Schwab, U. Dietary Fatty Acids: Is it Time to Change the Recommendations? Ann. Nutr. Metab. 2016, 68, 249–257. [Google Scholar] [CrossRef]
- Chiu, S.; Williams, P.T.; Krauss, R.M. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: A randomized controlled trial. PLoS ONE 2017, 12, e0170664. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Kotnik, P.; Fischer-Posovszky, P.; Wabitsch, M. RBP4: A controversial adipokine. Eur. J. Endocrinol. 2011, 165, 703–711. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Margioris, A.N. Fatty acids and postprandial inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Soeters, M.R.; Soeters, P.B.; Schooneman, M.G.; Houten, S.M.; Romijn, J.A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1397–E1407. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Ragucci, M.; Di Donato, O.; Della Pepa, G.; Della Corte, G.; Griffo, E.; Anniballi, G.; Giacco, A.; Mancini, M.; et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.W.; Koopman, F.A.; Visscher, J.P.M.; de Hair, M.J.; Gerlag, D.M.; Tak, P.P. Hormone, metabolic peptide, and nutrient levels in the earliest phases of rheumatoid arthritis—Contribution of free fatty acids to an increased cardiovascular risk during very early disease. Clin. Rheumatol. 2017, 36, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef]
- Luu, N.-T.; Madden, J.; Calder, P.C.; Grimble, R.F.; Shearman, C.P.; Chan, T.; Dastur, N.; Howell, W.M.; Rainger, G.E.; Nash, G.B. Dietary Supplementation with Fish Oil Modifies the Ability of Human Monocytes to Induce an Inflammatory Response. J. Nutr. 2007, 137, 2769–2774. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef]
- Pacheco, Y.M.; López, S.; Bermúdez, B.; Abia, R.; Villar, J.; Muriana, F.J.G. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J. Nutr. Biochem. 2008, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Deckelbaum, R.J. Omega-3 fatty acids: Time to get the messages right! Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 91–93. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef]
- Cicerale, S.; Breslin, P.A.S.; Beauchamp, G.K.; Keast, R.S.J. Sensory Characterization of the Irritant Properties of Oleocanthal, a Natural Anti-Inflammatory Agent in Extra Virgin Olive Oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Role of resveratrol-induced CD11b+ Gr-1+ myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3+ T cells and amelioration of chronic colitis in IL-10−/− mice. Brain Behav. Immun. 2012, 26, 72–82. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3: The good oil. Nutr. Bull. 2017, 42, 132–140. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n -3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, C.R.; Rounova, O.; Hahn, S.E.; Diamandis, E.P.; Goldberg, D.M. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin. Chim. Acta 1996, 246, 163–182. [Google Scholar] [CrossRef]
- Fauconneau, B.; Waffo-Teguo, P.; Huguet, F.; Barrier, L.; Decendit, A.; Merillon, J.-M. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997, 61, 2103–2110. [Google Scholar] [CrossRef]
- Jang, D.-S.; Kang, B.-S.; Ryu, S.Y.; Chang, I.-M.; Min, K.R.; Kim, Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem. Pharmacol. 1999, 57, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis Jr, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut Microbiota and Type 1 Diabetes Mellitus: The Effect of Mediterranean Diet. Front. Nutr. 2021, 7, 612773. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018, 118, 74–100.e11. [Google Scholar] [CrossRef]
- Waterworth, D.M.; Ricketts, S.L.; Song, K.; Chen, L.; Zhao, J.H.; Ripatti, S.; Aulchenko, Y.S.; Zhang, W.; Yuan, X.; Lim, N.; et al. Genetic Variants Influencing Circulating Lipid Levels and Risk of Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2264–2276. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Song, R.J.; Xu, X.; Gerszten, R.E.; Ngo, D.; Clish, C.B.; Corlin, L.; Ma, J.; Xanthakis, V.; Jacques, P.F.; et al. Proteomic and Metabolomic Correlates of Healthy Dietary Patterns: The Framingham Heart Study. Nutrients 2020, 12, 1476. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takahashi, S.; Saito, K. Omics and Integrated Omics for the Promotion of Food and Nutrition Science. J. Tradit. Complement. Med. 2011, 1, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Lupianez-Barbero, A.; Estruch, R.; Corella, D.; Fitó, M.; Arós, F.; Ruiz-Canela, M.; Salas-Salvadó, J.; et al. Metabolomic Pattern Analysis after Mediterranean Diet Intervention in a Nondiabetic Population: A 1- and 3-Year Follow-up in the PREDIMED Study. J. Proteome Res. 2015, 14, 531–540. [Google Scholar] [CrossRef]
- World Health Organization. World Mental Health Report: Transforming Mental Health for All; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Buckley, N.; Glasson, E.J.; Chen, W.; Epstein, A.; Leonard, H.; Skoss, R.; Jacoby, P.; Blackmore, A.M.; Srinivasjois, R.; Bourke, J.; et al. Prevalence estimates of mental health problems in children and adolescents with intellectual disability: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2020, 54, 970–984. [Google Scholar] [CrossRef]
- Vasileva, M.; Graf, R.K.; Reinelt, T.; Petermann, U.; Petermann, F. Research review: A meta-analysis of the international prevalence and comorbidity of mental disorders in children between 1 and 7 years. J. Child. Psychol. Psychiatry 2021, 62, 372–381. [Google Scholar] [CrossRef]
- Lakhan, R.; Agrawal, A.; Sharma, M. Prevalence of Depression, Anxiety, and Stress during COVID-19 Pandemic. J. Neurosci. Rural. Pract. 2020, 11, 519. [Google Scholar] [CrossRef]
- Wu, T.; Jia, X.; Shi, H.; Niu, J.; Yin, X.; Xie, J.; Wang, X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 91–98. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Braz. J. Psychiatry 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Yazd, D.; Wheeler, S.; Zuo, S. Key Risk Factors Affecting Farmers’ Mental Health: A Systematic Review. Int. J. Environ. Res. Public. Health 2019, 16, 4849. [Google Scholar] [CrossRef]
- Lavallee, K.; Zhang, X.C.; Michalak, J.; Schneider, S.; Margraf, J. Vegetarian diet and mental health: Cross-sectional and longitudinal analyses in culturally diverse samples. J. Affect. Disord. 2019, 248, 147–154. [Google Scholar] [CrossRef]
- Bremner, J.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapapor, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Gehlich, K.H.; Beller, J.; Lange-Asschenfeldt, B.; Köcher, W.; Meinke, M.C.; Lademann, J. Consumption of fruits and vegetables: Improved physical health, mental health, physical functioning and cognitive health in older adults from 11 European countries. Aging Ment. Health 2020, 24, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Santos, L.E.; Ferreira, S.T. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front. Neurosci. 2019, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Hu, Y.F.; Li, C.F.; Yuang, S.; Song, Y.; Zheng, M.; Gongo, J.; He, Q.Q. Improving the Metabolic and Mental Health of Children with Obesity: A School-Based Nutrition Education and Physical Activity Intervention in Wuhan, China. Nutrients 2020, 12, 194. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R. Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef]
- Greetfeld, M.; Hessler-Kaufmann, J.B.; Brandl, B.; Skurk, T.; Holzapfel, C.; Quadflieg, N.; Schlegl, S.; Hauner, H.; Voderholzer, U. Orthorexic tendencies in the general population: Association with demographic data, psychiatric symptoms, and utilization of mental health services. Eat. Weight Disord.—Stud. Anorex. Bulim. Obes. 2021, 26, 1511–1519. [Google Scholar] [CrossRef]
- Rosli, H.; Shahar, S.; Din, N.C.; Haron, H.; Rajab, N.F. Prevalence of Poor Mental Health and Cognitive Status among Middle-Aged Adults and Its Predictors in Relation to Polyphenols Intake. Malays. J. Med. Sci. 2019, 26, 72–89. [Google Scholar] [CrossRef]
- Samodien, E.; Johnson, R.; Pheiffer, C.; Mabasa, L.; Erasmus, M.; Louw, J.; Chellan, N. Diet-induced hypothalamic dysfunction and metabolic disease, and the therapeutic potential of polyphenols. Mol. Metab. 2019, 27, 1–10. [Google Scholar] [CrossRef]
- Meléndez, G.P.P.; Valero-Jara, V.; Acevedo-Hernández, P.; Thomas-Valdés, S. Impact of polyphenols on stress and anxiety: A systematic review of molecular mechanisms and clinical evidence. Crit. Rev. Food Sci. Nutr. 2022, 64, 2340–2357. [Google Scholar] [CrossRef]
- Micek, A.; Jurek, J.; Owczarek, M.; Guerrera, I.; Alfio Torrisi, S.; Castellano, S.; Grosso, G.; Alshatwi, A.A.; Godos, J. Polyphenol-Rich Beverages and Mental Health Outcomes. Antioxidants 2023, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and ‘At-Risk’ Individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Hoffbrand, A.V. Mandatory UK folic acid fortification. Lancet 2021, 398, 1961–1962. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef]
- Kimmel, M.; Jin, W.; Xia, K.; Lun, K.; Azcarate-Peril, A.; Plantinga, A.; Wu, M.; Ataei, S.; Rackers, H.; Carroll, I.; et al. Metabolite trajectories across the perinatal period and mental health: A preliminary study of tryptophan-related metabolites, bile acids and microbial composition. Behav. Brain Res. 2022, 418, 113635. [Google Scholar] [CrossRef] [PubMed]
- Hosker, D.K.; Elkins, R.M.; Potter, M.P. Promoting Mental Health and Wellness in Youth Through Physical Activity, Nutrition, and Sleep. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Chattu, V.; Aeri, B. Nutritional aspects of depression in adolescents—A systematic review. Int. J. Prev. Med. 2019, 10, 42. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocr. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Knauf, C.; Abot, A.; Wemelle, E.; Cani, P.D. Targeting the Enteric Nervous System to Treat Metabolic Disorders? ‘Enterosynes’ as Therapeutic Gut Factors. Neuroendocrinology 2020, 110, 139–146. [Google Scholar] [CrossRef]
- Järbrink-Sehgal, E.; Andreasson, A. The gut microbiota and mental health in adults. Curr. Opin. Neurobiol. 2020, 62, 102–114. [Google Scholar] [CrossRef]
- Desai, V.; Kozyrskyj, A.L.; Lau, S.; Sanni, O.; Dennet, L.; Walter, J.; Ospina, M.B. Effectiveness of Probiotic, Prebiotic, and Synbiotic Supplementation to Improve Perinatal Mental Health in Mothers: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 622181. [Google Scholar] [CrossRef]
- Johnson, D.; Thurairajasingam, S.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Exploring the Role and Potential of Probiotics in the Field of Mental Health: Major Depressive Disorder. Nutrients 2021, 13, 1728. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Probiotics in human mental health and diseases—A minireview. Trop. J. Pharm. Res. 2021, 18, 889–895. [Google Scholar] [CrossRef]
- Freijy, T.M.; Cribb, L.; Oliver, G.; Metri, N.-J.; Opie, R.S.; Jacka, F.N.; Hawrelak, J.A.; Rucklidge, J.J.; Ng, C.H.; Sarris, J. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: The ‘Gut Feelings’ randomised controlled trial. Front. Neurosci. 2023, 16, 1097278. [Google Scholar] [CrossRef]
- Requejo, O.H.; Rodríguez, M.C.R. Nutrition and cancer. Nutr. Hosp. 2015, 32 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging: A role for prostate cancer? Biochim. Biophys. Acta (BBA)—Rev. Cancer 2009, 1795, 83–91. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. Elife 2019, 8, e43653. [Google Scholar] [CrossRef]
- Chao, T.; Furth, E.E.; Vonderheide, R.H. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982. [Google Scholar] [CrossRef]
- Silvera, S.A.N.; Jain, M.; Howe, G.R.; Miller, A.B.; Rohan, T.E. Dietary carbohydrates and breast cancer risk: A prospective study of the roles of overall glycemic index and glycemic load. Int. J. Cancer 2005, 114, 653–658. [Google Scholar] [CrossRef]
- Grasgruber, P.; Hrazdira, E.; Sebera, M.; Kalina, T. Cancer Incidence in Europe: An Ecological Analysis of Nutritional and Other Environmental Factors. Front. Oncol. 2018, 8, 151. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the BODY. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Supabphol, S.; Seubwai, W.; Wongkham, S.; Saengboonmee, C. High glucose: An emerging association between diabetes mellitus and cancer progression. J. Mol. Med. 2021, 99, 1175–1193. [Google Scholar] [CrossRef]
- Czekajło, A.; Różańska, D.; Mandecka, A.; Konikowska, K.; Madalińska, M.; Szuba, A.; Regulska-Ilow, B. Glycemic load and carbohydrates content in the diets of cancer patients. Rocz. Panstw. Zakl. Hig. 2017, 68, 261–268. [Google Scholar]
- Dang, C.V. Rethinking the Warburg Effect with Myc Micromanaging Glutamine Metabolism. Cancer Res. 2010, 70, 859–862. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between Hyperglycemia and Survival in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef]
- Wei, M.; Bradhorst, S.; Shelehchi, M.; Mirzaei, H.; Chen, C.W.; Budniak, J.; Groshen, S.; Mack, Q.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Brenna, J.T.; Shen, Y.; Ye, K. Higher ratio of plasma omega-6/omega-3 fatty acids is associated with greater risk of all-cause, cancer, and cardiovascular mortality: A population-based cohort study in UK Biobank. medRxiv 2023. Preprint. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.H.; Chen, Y.Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Li, H.; Yu, D.; Cai, H.; Gao, J.; Gao, Y.; Luu, H.N.; Tran, H.; Xiang, Y.-B.; Zheng, W.; et al. Dietary fatty acids and colorectal cancer risk in men: A report from the Shanghai Men’s Health Study and a meta-analysis. Int. J. Cancer 2021, 148, 77–89. [Google Scholar] [CrossRef]
- Han, S.N.; Leka, L.S.; Lichtenstein, A.H.; Ausman, L.M.; Schaefer, E.J.; Meydani, S.N. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J. Lipid Res. 2002, 43, 445–452. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Pischon, T.; Hankinson, S.E.; Rifai, N.; Joshipura, K.; Willet, W.C.; Rimm, E.B. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 2004, 79, 606–612. [Google Scholar] [CrossRef]
- Michels, N.; Specht, I.O.; Heitmann, B.L.; Chajès, V.; Huybrechts, I. Dietary trans-fatty acid intake in relation to cancer risk: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 758–776. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Chen, S.; Chien, Y.; Ma, S.; Zheng, R.; Zhao, P.; Zhang, L.; Liu, Y.; Yu, Q.; Deng, Q.; Zhang, K. Dietary fibre intake and risk of breast cancer: A systematic review and meta-analysis of epidemiological studies. Oncotarget 2016, 7, 80980–80989. [Google Scholar] [CrossRef]
- Vieytes, C.A.M.; Taha, H.M.; Burton-Obanla, A.A.; Douglas, K.G.; Arthur, A.E. Carbohydrate Nutrition and the Risk of Cancer. Curr. Nutr. Rep. 2019, 8, 230–239. [Google Scholar] [CrossRef]
- Bøhn, S.K.; Myhrstad, M.C.; Thoresen, M.; Holden, M.; Karlsen, A.; Tunheim, S.H.; Erlund, I.; Svendsen, M.; Seljeflot, I.; Moskaug, J.O.; et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med. 2010, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M. Nutraceuticals and Mediterranean Diet. Med. Aromat. Plants 2012, 1, e126. [Google Scholar] [CrossRef]
- Vasto, S.; Barera, A.; Rizzo, C.; Carlo, M.; Caruso, C.; Panotopoulos, G. Mediterranean Diet and Longevity: An Example of Nutraceuticals? Curr. Vasc. Pharmacol. 2013, 12, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Comba, A.; Maestri, D.M.; Berra, M.A.; Garcia, C.P.; Undurti, N.D.; Eynard, A.R.; Pasqualini, M.E. Effect of ω-3 and ω-9 fatty acid rich oils on lipoxygenases and cyclooxygenases enzymes and on the growth of a mammary adenocarcinoma model. Lipids Health Dis. 2010, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, L. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis 2005, 26, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Pandey, M.K.; Ahn, k.S.; Yi, T.; Chaturvedi, M.M.; Liu, M.; Aggarwal, B.B. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-κB–regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-κBα kinase, leading to potentiation of apoptosis. Blood 2008, 111, 4880–4891. [Google Scholar] [CrossRef]
- Carvalho, A.L.N.; Annoni, R.; Torres, L.H.; Durão, A.; Shimada, A.L.; Almeida, F.; Hebeda, C.; Lopes, F.; Dolhnikoff, M.; Martins, M.; et al. Anacardic Acids from Cashew Nuts Ameliorate Lung Damage Induced by Exposure to Diesel Exhaust Particles in Mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 549879. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Griessl, T.; Zechel-Gran, S.; Olejniczak, S.; Weigel, M.; Hain, T.; Domann, E. High-resolution taxonomic examination of the oral microbiome after oil pulling with standardized sunflower seed oil and healthy participants: A pilot study. Clin. Oral. Investig. 2021, 25, 2689–2703. [Google Scholar] [CrossRef]
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O‘brien, D. The Prevention of Infections in Older Adults: Oral Health. J. Am. Geriatr. Soc. 2020, 68, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontology 2000 2016, 70, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Mukherjee, A.; Jantsch, V.; Khan, R.; Hartung, W.; Fischer, R.; Jantsch, j.; Ehrenstein, B.; Konig, M.F.; Andrade, F. Rheumatoid Arthritis-Associated Autoimmunity Due to Aggregatibacter actinomycetemcomitans and Its Resolution with Antibiotic Therapy. Front. Immunol. 2018, 9, 2352. [Google Scholar] [CrossRef]
- Kriebel, K.; Hieke, C.; Müller-Hilke, B.; Nakata, M.; Kreikemeyer, B. Oral Biofilms from Symbiotic to Pathogenic Interactions and Associated Disease –Connection of Periodontitis and Rheumatic Arthritis by Peptidylarginine Deiminase. Front. Microbiol. 2018, 9, 53. [Google Scholar] [CrossRef]
- Dutzan, N.; Kajikawa, T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; et al. A dysbiotic microbiome triggers T H 17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, 463. [Google Scholar] [CrossRef]
- Kilian, M. The oral microbiome—Friend or foe? Eur. J. Oral. Sci. 2018, 126, 5–12. [Google Scholar] [CrossRef]
- Silva, L.M.; Brenchley, L.; Moutsopoulos, N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol. Rev. 2019, 287, 226–235. [Google Scholar] [CrossRef]
- Feldens, C.A.; Pinheiro, L.L.; Cury, J.A.; Mendonça, F.; Groisman, M.; Costa, R.A.H.; Pereira, H.C.; Vieira, A.R. Added Sugar and Oral Health: A Position Paper of the Brazilian Academy of Dentistry. Front. Oral Health 2022, 3, 869112. [Google Scholar] [CrossRef] [PubMed]
- OMS. Guideline: Sugars Intake for Adults and Children; OMS: Geneva, Switzerland, 2015. [Google Scholar]
- Brun, A.; Nuzzo, A.; Prouvost, B.; Diallo, D.; Hamdan, S.; Meseguer, E.; Guidoux, C.; Lavallée, P.; Amarenco, P.; Lesèche, G.; et al. Oral microbiota and atherothrombotic carotid plaque vulnerability in periodontitis patients. A cross-sectional study. J. Periodontal Res. 2021, 56, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Wide, U.; Arvidsson, L.; Eiben, G.; Hakeberg, M. Dietary intake and meal patterns among young adults with high caries activity: A cross-sectional study. BMC Oral Health 2022, 22, 190. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Tennert, C.; Reinmuth, A.C.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. Microbiologyopen 2020, 9, e1056. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Ndokaj, A.; Jedlinski, M.; Ardan, R.; Bietolini, S.; Ottolenghi, L. Impact of Green Tea (Camellia sinensis) on periodontitis and caries. Systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2021, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Walter, C.; Borof, K.; Petersen, E.; Lieske, B.; Kasapoudis, D.; Jalilvand, N.; Beikler, T.; Jagemann, B.; Zyriax, B.-C.; et al. Association between dietary pattern and periodontitis—A cross-sectional study. Nutrients 2021, 13, 4167. [Google Scholar] [CrossRef] [PubMed]
- Blostein, F.A.; Jansen, E.C.; Jones, A.D.; Marshall, T.A.; Foxman, B. Dietary patterns associated with dental caries in adults in the United States. Community Dent. Oral Epidemiol. 2020, 48, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Almoraie, N.M.; Saqaan, R.; Alharthi, R.; Alamoudi, A.; Badh, L.; Shatwan, I.M. Snacking patterns throughout the life span: Potential implications on health. Nutr. Res. 2021, 91, 81–94. [Google Scholar] [CrossRef] [PubMed]
- García, C.B.; Miralles, E.G.; Martínez, L.M. Association between eating behavior pattern and caries in a population of children aged 3 to 9 years in the province of Alicante. Nutr. Hosp. 2022, 39, 33–38. [Google Scholar] [CrossRef]
- Davis, E.; Martinez, G.; Blostein, F.; Marschall, T.; Jones, A.d.; Jansen, E.; McNeil, D.W.; Neiswanger, K.; Marazita, M.L.; Foxman, B. Dietary Patterns and Risk of a New Carious Lesion Postpartum: A Cohort Study. J. Dent. Res. 2022, 101, 295–303. [Google Scholar] [CrossRef]
- Sherawat, J.; Rana, M.; Thakur, S. Associaton between dental health status and changing dietary and lifestyle patterns among selected population of Shimla (Himachal Pradesh, India). Indian. J. Dent. Sci. 2022, 14, 109–115. [Google Scholar]
- Tilton, E.; Keels, M.; Simancas-Pallares, M. Child nutrition patterns are associated with primary dentition dental caries. Pediatr. Dent. 2021, 15, 205–210. [Google Scholar]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Yousaf, M.; Aslam, T.; Saeed, S.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Individual, Family, and Socioeconomic Contributors to Dental Caries in Children from Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2022, 19, 7114. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.; Garemo, M.; Mulder, A. Determinants of dental caries in children in the Middle East and North Africa region: A systematic review based on literature published from 2000 to 2019. BMC Oral Health 2021, 21, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Waterhouse, B.; Aggarwal, V.R.; Bloor, K.; Doran, T. Effect of sugar-sweetened beverages on oral health: A systematic review and meta-analysis. Eur. J. Public Health 2021, 31, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.; Muthukumaraswamy, N.S.; Dharamarajan, L.; Jing, K.; Vaithilingam, R. Alcohol consumption is associated with periodontitis A systematic review and meta-analysis of observational studies. Commun. Dent. Health 2020, 37, 12–21. [Google Scholar]
- Zupo, R.; Castellana, F.; De Nucci, S.; Dibello, V.; Lozupone, M.; Giannelli, G.; De Pergola, G.; Panza, F.; Sardone, R.; Boeing, H. Beverages Consumption and Oral Health in the Aging Population: A Systematic Review. Front. Nutr. 2021, 8, 762383. [Google Scholar] [CrossRef]
- Chien, J.; Hwang, J.H.; Nilaad, S.; Masso-Silva, J.A.; Ahn, S.J.; McEachern, E.K.; Moshensky, A.; Byun, M.-K.; Crotty Alexander, L.E. Cigarette Smoke Exposure Promotes Virulence of Pseudomonas aeruginosa and Induces Resistance to Neutrophil Killing. Infect. Immun. 2020, 88, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, T.; Morita, M.; Yamamoto, T.; Inagaki, K.; Wang, P.L.; Ito, H.; Morozumi, T.; Takeshita, T.; Suzuki, N.; Shigeishi, H.; et al. Smoking and periodontal microorganisms. Jpn. Dent. Sci. Rev. 2019, 55, 88–94. [Google Scholar] [CrossRef]
- Shapiro, H.; Goldenberg, K.; Ratiner, K.; Elinav, E. Smoking-induced microbial dysbiosis in health and disease. Clin. Sci. 2022, 136, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Vandana, M.; Patwardhan, S.; Kaushik, K.S. Looking beyond the smokescreen: Can the oral microbiome be a tool or target in the management of tobacco-associated oral cancer? Ecancermedicalscience 2021, 15, 1179. [Google Scholar] [CrossRef]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia—Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Yu, N.; Van Dyke, T. Periodontitis: A host mediated disruption of microbial homeostasis. Curr. Oral Health Rep. 2020, 7, 3–11. [Google Scholar] [CrossRef]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 19, 152–169. [Google Scholar] [CrossRef]
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hay, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109884. [Google Scholar] [CrossRef]
- Lukiw, W.J. Gastrointestinal (GI) Tract Microbiome-Derived Neurotoxins—Potent Neuro-Inflammatory Signals from the GI Tract via the Systemic Circulation Into the Brain. Front. Cell Infect. Microbiol. 2020, 10, 22. [Google Scholar] [CrossRef]
- Jungbauer, G.; Stähli, A.; Zhu, X.; Alberi, L.A.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease—A causative relationship? Periodontology 2000 2022, 89, 59–82. [Google Scholar] [CrossRef]
- Fieldhouse, J.L.P.; Doorduijn, A.S.; de Leeuw, F.A.; Verhaar, B.J.H.; Koene, T.; Wesselman, L.M.P.; Schueren, M.V.; Visser, M.; Rest, O.V.; Scheltens, P.; et al. A suboptimal diet is associated with poorer cognition: The NUDAD project. Nutrients 2020, 12, 703. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Gong, C.; Cuccioloni, M.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. FEBS J. 2021, 288, 2836–2855. [Google Scholar] [CrossRef]
- Laiola, M.; De Filippis, F.; Vitaglione, P.; Ercolini, D. A Mediterranean diet intervention reduces the levels of salivary periodontopathogenic bacteria in overweight and obese subjects. Appl. Env. Microbiol. 2020, 86, e00777-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tnag, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Placidi, G.; De Sienam, E.; Savastano, M.C.; Minnella, A.M.; Maceroni, M.; Midena, G.; Ziccardi, L.; Parisi, V.; Bertelli, M.; et al. USH2A-Related Retinitis Pigmentosa: Staging of Disease Severity and Morpho-Functional Studies. Diagnostics 2021, 11, 213. [Google Scholar] [CrossRef]
- Marino, V.; Dal Cortivo, G.; Maltese, P.E.; Placidi, G.; De Sienda, E.; Falsini, B.; Bertelli, M.; Dell’Orco, D. Impaired Ca2+ Sensitivity of a Novel GCAP1 Variant Causes Cone Dystrophy and Leads to Abnormal Synaptic Transmission between Photoreceptors and Bipolar Cells. Int. J. Mol. Sci. 2021, 22, 4030. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Placidi, G.; De Siena, E.; Chiurazzi, P.; Minnella, A.M.; Savastano, M.C.; Ziccardi, L.; Parisi, V.; Iarossi, G.; Percio, M.; et al. Genetic characteristics of 234 Italian patients with macular and cone/cone-rod dystrophy. Sci. Rep. 2022, 12, 3774. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.; Maltese, P.E.; Castori, M.; El Shamied, S.; Zeitz, C.; Audo, I.; Zulian, A.; Marinelli, C.; Benedetti, S.; Constantini, A.; et al. Molecular Epidemiology in 591 Italian Probands with Nonsyndromic Retinitis Pigmentosa and Usher Syndrome. Investig. Opthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Valero-Vello, M.; Peris-Martínez, C.; García-Medina, J.J.; Sanz-González, S.M.; Ramírez, A.I.; Fernández-Albarral, J.A.; Galarreta-Mira, D.; Zanón-Moreno, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the Antioxidant, Anti-Inflammatory, and Neuroprotective Potential of Natural Food and Nutritional Supplements for Ocular Health in the Mediterranean Population. Foods 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Brunes, A.; Hansen, M.B.; Heir, T. Loneliness among adults with visual impairment: Prevalence, associated factors, and relationship to life satisfaction. Health Qual. Life Outcomes 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, E.; Littlejohns, T.J.; Khawaja, A.P.; Llewellyn, D.J.; Ukoumunne, O.C.; Thiem, U. Visual Impairment, Eye Diseases, and Dementia Risk: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2021, 83, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Vision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Harb, E.N.; Wildsoet, C.F. Nutritional Factors and Myopia: An Analysis of National Health and Nutrition Examination Survey Data. Optom. Vis. Sci. 2021, 98, 458–468. [Google Scholar] [CrossRef]
- Zhang, J.; Tuo, J.; Wang, Z.; Zhu, A.; Machalińska, A.; Long, Q. Pathogenesis of Common Ocular Diseases. J. Ophthalmol. 2015, 2015, 1–2. [Google Scholar] [CrossRef]
- Seddon, J.M.; Rosner, B. Validated Prediction Models for Macular Degeneration Progression and Predictors of Visual Acuity Loss Identify High-Risk Individuals. Am. J. Ophthalmol. 2019, 198, 223–261. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Walchuk, C.; Suh, M. Nutrition and the aging retina: A comprehensive review of the relationship between nutrients and their role in age-related macular degeneration and retina disease prevention. Adv. Food Nutr. Res. 2020, 93, 293–332. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Gkerdi, A.; Reilly, J.; Tan, Z.; Shu, X. Association of Nutrients, Specific Dietary Patterns, and Probiotics with Age-related Macular Degeneration. Curr. Med. Chem. 2022, 29, 6141–6158. [Google Scholar] [CrossRef]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D. The relation between dietary intake and glaucoma: A systematic review. Acta Ophthalmol. 2018, 96, 550–556. [Google Scholar] [CrossRef]
- Ramdas, W.; Schouten, J.; Webers, C. The Effect of Vitamins on Glaucoma: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 359. [Google Scholar] [CrossRef]
- Jiang, H.; Yin, Y.; Wu, C.-R.; Liiu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef]
- Cecilia, O.-M.; Castellanos-González, J.A.; Navarro-Partida, J.; Cardona-Muñóz, E.G.; López-Contreras, A.K.; Roman-PIntos, L.M.; Robles-Rivera, R.R.; Rodríguez-Carrizalez, A.D. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed]
- Lanzetta, P.; Sarao, V.; Scanlon, P.H.; Barratt, J.; Porta, M.; Bandello, F.; Loewenstein, A.; Vision Academy. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020, 57, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, C.T.; Cagnone, G.; Heckel, E.; Sun, Y.; Cakir, B.; Tomita, Y.; Huang, S.; Lli, Q.; Britton, W.; et al. Dyslipidemia in retinal metabolic disorders. EMBO Mol. Med. 2019, 11, e10473. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science (1979) 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Maasen, K.; van Greevenbroek, M.M.; Scheijen, J.L.J.; van der Kallen, C.J.; Stehouwer, C.D.A.; Schalkwijk, C.G. High dietary glycemic load is associated with higher concentrations of urinary advanced glycation endproducts: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) Study. Am. J. Clin. Nutr. 2019, 110, 358–366. [Google Scholar] [CrossRef]
- Robles-Rivera, R.R.; Castellanos-González, J.A.; Olvera-Montaño, C.; Flores-Martin, R.A.; López-Contreras, A.K.; Arevalo-Simental, D.E.; Cardona-Muñoz, E.G.; Roman-Pintos, L.M.; Rodríguez-Carrizalez, A.D. Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2020, 2020, 3096470. [Google Scholar] [CrossRef] [PubMed]
- Molina-Leyva, I.; Molina-Leyva, A.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; García-Molina, L.; Bueno-Cavanillas, A. Effectiveness of Mediterranean Diet Implementation in Dry Eye Parameters: A Study of PREDIMED-PLUS Trial. Nutrients 2020, 12, 1289. [Google Scholar] [CrossRef]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Grégoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Féart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Si Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Zhao, Q.; Gao, F.; Jin, N.; Wang, D.; Wang, B.; Du, B.; Wei, R. Association between whole-grain intake and myopia in chinese children: A cross-sectional epidemiological study. BMC Ophthalmol. 2023, 23, 1. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Mares, J.; Clemons, T.E.; Van Asten, F.; Swaroop, A.; Chew, E.Y. Age-Related eye disease studies (AREDS) 1 and 2 reserach groups. Adherence to the Mediterranean Diet and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. In Ophthalmology; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1515–1528. [Google Scholar] [CrossRef]
- Raimundo, M.; Mira, F.; Cachulo, M.D.L.; Barreto, P.; Ribeiro, L.; Farinha, C.; Laíns, I.; Nunes, S.; Alves, D.; Figueira, J.; et al. Adherence to a Mediterranean diet, lifestyle and age-related macular degeneration: The Coimbra Eye Study—Report 3. Acta Ophthalmol. 2018, 96, e926–e932. [Google Scholar] [CrossRef]
- Napolitano, P.; Filippelli, M.; Davinelli, S.; Bartollino, S.; dell’Omo, R.; Costagliola, C. Influence of gut microbiota on eye diseases: An overview. Ann. Med. 2021, 53, 750–761. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021, 17, 818–833. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Cavuoto, K.M.; Banerjee, S.; Galor, A. Relationship between the microbiome and ocular health. Ocul. Surf. 2019, 17, 384–392. [Google Scholar] [CrossRef]
- Jayedi, A.; Mirzaei, K.; Rashidy-Pour, A.; Yekaninejad, M.S.; Zargar, M.-S.; Eidgahi, M.R.A. Dietary approaches to stop hypertension, mediterranean dietary pattern, and diabetic nephropathy in women with type 2 diabetes: A case-control study. Clin. Nutr. ESPEN 2019, 33, 164–170. [Google Scholar] [CrossRef]
- de Carvalho, G.B.; Dias-Vasconcelos, N.L.; Santos, R.K.F.; Brandão-Lima, P.N.; da Silva, D.G.; Pires, L.V. Effect of different dietary patterns on glycemic control in individuals with type 2 diabetes mellitus: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1999–2010. [Google Scholar] [CrossRef]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2019, 21, 454–466. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Melecchi, A.; Amato, R.; Lapi, D.; Dal monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Increased efficacy of dietary supplement containing wax ester-rich marine oil and xanthophylls in a mouse model of dry macular degeneration. Front. Pharmacol. 2022, 13, 1038730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.C.; Singh, S.; Craig, J.P.; Downie, L.E. Omega-3 fatty acids and eye health: Opinions and self-reported practice behaviors of optometrists in Australia and New Zealand. Nutrients 2020, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Drouin, G.; Rioux, V.; Legrand, P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 2019, 159, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.V.; Agnolazza, D.L.; German, O.L.; Garelli, A.; Politi, L.E.; Agbaba, M.-P.; Anderson, R.E.; Rotstein, N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J. Neurochem. 2016, 136, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.A.; Jacobs, R.J.; Braakhuis, A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019, 47, 106–127. [Google Scholar] [CrossRef]
- Choo, P.P.; Woi, P.J.; Bastion, M.L.C.; Omar, R.; Mustapha, M.; Din, N.M. Review of Evidence for the Usage of Antioxidants for Eye Aging. In BioMed Research International 2022; Hindawi Limited: London, UK, 2022. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Cammalleri, M.; Monte, M.D.; Amato, R.; Bagnoli, P.; Rusciano, D. A Dietary Combination of Forskolin with Homotaurine, Spearmint and B Vitamins Protects Injured Retinal Ganglion Cells in a Rodent Model of Hypertensive Glaucoma. Nutrients 2020, 12, 1189. [Google Scholar] [CrossRef]
- Castelli, V.; Paladini, A.; DÀngelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-pérez, M.P. Taurine Depletion Causes ipRGC Loss and Increases Light-Induced Photoreceptor Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, 1396. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Veetil, V.O.; Lanca, C.; Lee, B.L.; Godfrey, K.M.; Gluckman, P.D.; Shek, L.P.; Yap, F.; Tan, K.H.; Chong, Y.S.; et al. Maternal Lutein and Zeaxanthin Concentrations in Relation to Offspring Visual Acuity at 3 Years of Age: The GUSTO Study. Nutrients 2020, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Picone, S.; Gaiero, A.; Bellettato, M.; Montrone, G.; Riccobene, F.; Lista, G.; Pellegrini, G. Early pediatric benefit of lutein for maturing eyes and brain—An overview. Nutrients 2021, 13, 3239. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2021, 79, 544–573. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Rodríguez-Rodríguez, E.; Beltrán-De-miguel, B.; Sánchez-Prieto, M.; Estévez-Santiago, R. Changes in lutein status markers (Serum and faecal concentrations, macular pigment) in response to a lutein-rich fruit or vegetable (three pieces/day) dietary intervention in normolipemic subjects. Nutrients 2021, 13, 3614. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Medori, M.C.; Naureen, Z.; Dhuli, K.; Placidi, G.; Falsini, B.; Bertelli, M. Dietary supplements in retinal diseases, glaucoma, and other ocular conditions. J. Prev. Med. Hyg. 2022, 63, E189–E199. [Google Scholar] [CrossRef]
- Christen, W.G.; Glynn, R.J.; Manson, J.E.; MacFadyen, J.; Bubes, V.; Schvartz, M.; Buring, J.E.; Sesso, H.D.; Gaziano, J.M. Effects of Multivitamin Supplement on Cataract and Age-Related Macular Degeneration in a Randomized Trial of Male Physicians. Ophthalmology 2014, 121, 525–534. [Google Scholar] [CrossRef]
- Glaser, T.S.; Doss, L.E.; Shih, G.; Nigam, D.; Sperduto, R.D.; Ferris, F.L., 3rd; Agrón, E.; Clemons, T.E.; Chew, E.Y.; Age-Related Eye Disease Study Research Group. The Association of Dietary Lutein plus Zeaxanthin and B Vitamins with Cataracts in the Age-Related Eye Disease Study. Ophthalmology 2015, 122, 1471–1479. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Toulouie, S.; Chang, S.; Pan, J.; Snyder, K.; Yiu, G. Relationship of Retinal Vessel Caliber with Age-Related Macular Degeneration. J. Ophthalmol. 2022, 2022, 8210599. [Google Scholar] [CrossRef]
- Trinh, M.; Kalloniatis, M.; Nivison-Smith, L. Vascular Changes in Intermediate Age-Related Macular Degeneration Quantified Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2019, 8, 20. [Google Scholar] [CrossRef]
- Irnaten, M.; Duff, A.; Clark, A.; O’Brien, C. Intra-Cellular Calcium Signaling Pathways (PKC, RAS/RAF/MAPK, PI3K) in Lamina Cribrosa Cells in Glaucoma. J. Clin. Med. 2020, 10, 62. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, Y.J. The Relationship between Dietary Calcium and Age-Related Macular Degeneration. Nutrients 2023, 15, 671. [Google Scholar] [CrossRef]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Myoung, S.; Ahn, S. Recent Applications of Benchtop Nuclear Magnetic Resonance Spectroscopy. Magnetochemistry 2021, 7, 121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Peris-Ramos, H.C.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; David-Fernandez, S.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Personalizing Nutrition Strategies: Bridging Research and Public Health. J. Pers. Med. 2024, 14, 305. https://doi.org/10.3390/jpm14030305
Clemente-Suárez VJ, Peris-Ramos HC, Redondo-Flórez L, Beltrán-Velasco AI, Martín-Rodríguez A, David-Fernandez S, Yáñez-Sepúlveda R, Tornero-Aguilera JF. Personalizing Nutrition Strategies: Bridging Research and Public Health. Journal of Personalized Medicine. 2024; 14(3):305. https://doi.org/10.3390/jpm14030305
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Helia Carmen Peris-Ramos, Laura Redondo-Flórez, Ana Isabel Beltrán-Velasco, Alexandra Martín-Rodríguez, Susana David-Fernandez, Rodrigo Yáñez-Sepúlveda, and José Francisco Tornero-Aguilera. 2024. "Personalizing Nutrition Strategies: Bridging Research and Public Health" Journal of Personalized Medicine 14, no. 3: 305. https://doi.org/10.3390/jpm14030305