Significant Increase in Oxidative Stress Indices in Erythrocyte Membranes of Obese Patients with Metabolically-Associated Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Eating Habits
2.3. Biochemical Measurements
2.4. Red Blood Cell Membrane Fatty Acid Profile
2.5. Fibroblast Growth Factor 21 (FGF21) and Oxidized Low-Density Lipoprotein (oxLDL) Assay
2.6. Small- and Dense-LDL (sdLDL) Score Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaya, E.; Yilmaz, Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J. Clin. Transl. Hepatol. 2022, 10, 329–338. [Google Scholar] [CrossRef]
- Kunduraci, Y.E.; Ozbek, H. Does the Energy Restriction Intermittent Fasting Diet Alleviate Metabolic Syndrome Biomarkers? A Randomized Controlled Trial. Nutrients 2020, 12, 3213. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Capo, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Olusi, S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1159–1164. [Google Scholar] [CrossRef]
- Cazzola, R.; Rondanelli, M.; Russo-Volpe, S.; Ferrari, E.; Cestaro, B. Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J. Lipid. Res. 2004, 45, 1846–1851. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Bonfiglio, C.; Franco, I.; Mirizzi, A.; De Leonardis, G.; Cozzolongo, R.; Giannuzzi, V.; Giannelli, G.; et al. Aerobic Physical Activity and a Low Glycemic Diet Reduce the AA/EPA Ratio in Red Blood Cell Membranes of Patients with NAFLD. Nutrients 2018, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Osella, A.R.; Caruso, M.G.; Pesole, P.L.; Lippolis, A.; Tutino, V.; Bonfiglio, C.; De Nunzio, V.; Scavo, M.P.; Mirizzi, A.; et al. Nonalcoholic Fatty Liver Disease: Focus on New Biomarkers and Lifestyle Interventions. Int. J. Mol. Sci. 2021, 22, 3899. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Negro, R.; Arre, V.; Depalo, N.; Carrieri, L.; Rizzi, F.; Mastrogiacomo, R.; Serino, G.; Notarnicola, M.; De Nunzio, V.; et al. The oleic/palmitic acid imbalance in exosomes isolated from NAFLD patients induces necroptosis of liver cells via the elongase-6/RIP-1 pathway. Cell Death Dis. 2023, 14, 635. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; Bonfiglio, C.; Cozzolongo, R.; Giannuzzi, V.; De Nunzio, V.; De Leonardis, G.; Abbrescia, D.I.; Franco, I.; et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2017, 16, 160. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Tentes, I.; Anagnostopoulos, K. Red Blood Cell Dysfunction in Non-Alcoholic Fatty Liver Disease: Marker and Mediator of Molecular Mechanisms. Maedica 2020, 15, 513–516. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Tentes, I.; Anagnostopoulos, K. Erythrocytes contribute to the immunometabolic cross-talk. Immunometabolism 2021, 3, e210015. [Google Scholar] [CrossRef]
- Ayats-Vidal, R.; Bosque-Garcia, M.; Cordobilla, B.; Asensio-De la Cruz, O.; Garcia-Gonzalez, M.; Castro-Marrero, J.; Lopez-Rico, I.; Domingo, J.C. Changes of Erythrocyte Fatty Acids after Supplementation with Highly Concentrated Docosahexaenoic Acid (DHA) in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial. J. Clin. Med. 2023, 12, 3704. [Google Scholar] [CrossRef]
- Hatherill, J.R.; Till, G.O.; Ward, P.A. Mechanisms of oxidant-induced changes in erythrocytes. Agents Actions 1991, 32, 351–358. [Google Scholar] [CrossRef]
- Notarnicola, M.; De Nunzio, V.; Lippolis, T.; Tutino, V.; Cisternino, A.M.; Iacovazzi, P.A.; Milella, R.A.; Gasparro, M.; Negro, R.; Polignano, M.; et al. Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial. Biomedicines 2022, 10, 2310. [Google Scholar] [CrossRef]
- El-Eshmawy, M.M. Impact of obesity on liver function tests: Is nonalcoholic fatty liver disease the only player? A review article. Porto Biomed. J. 2023, 8, e228. [Google Scholar] [CrossRef]
- Reyes, C.; Nova-Lamperti, E.; Duran-Sandoval, D.; Rojas, D.; Gajardo, J.; Guzman-Gutierrez, E.; Bustos-Ruiz, C.; Ormazabal, V.; Zuniga, F.A.; Escudero, C.; et al. Loxin Reduced the Inflammatory Response in the Liver and the Aortic Fatty Streak Formation in Mice Fed with a High-Fat Diet. Int. J. Mol. Sci. 2022, 23, 7329. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryc, E.; Krauze, K.; Szczesniak, A.; Goryniak-Mikolajczyk, A.; Serwin, N.; Sleboda-Taront, D.; Jacek, R.; Heryc, R.; Michalczyk, A.; Dolegowska, B. Activity of erythrocyte antioxidant enzymes in healthy women depends on age, BMI, physical activity, and diet. J. Health Popul. Nutr. 2022, 41, 35. [Google Scholar] [CrossRef] [PubMed]
- Krychtiuk, K.A.; Kastl, S.P.; Pfaffenberger, S.; Lenz, M.; Hofbauer, S.L.; Wonnerth, A.; Koller, L.; Katsaros, K.M.; Pongratz, T.; Goliasch, G.; et al. Association of small dense LDL serum levels and circulating monocyte subsets in stable coronary artery disease. PLoS ONE 2015, 10, e0123367. [Google Scholar] [CrossRef]
- Gentile, M.; Iannuzzo, G.; Mattiello, A.; Rubba, F.; Panico, S.; Rubba, P. Association between body shape index and small dense LDL particles in a cohort of mediterranean women: Findings from Progetto ATENA. J. Clin. Biochem. Nutr. 2017, 61, 130–134. [Google Scholar] [CrossRef]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; De Nunzio, V.; Gigante, I.; De Leonardis, G.; Veronese, N.; Rotolo, O.; Reddavide, R.; Stasi, E.; et al. Nutrition and lipidomic profile in colorectal cancers. Acta Biomed. 2018, 89, 87–96. [Google Scholar] [CrossRef]
- Stevanovic, M.; Vekic, J.; Bogavac-Stanojevic, N.; Janac, J.; Stjepanovic, Z.; Zeljkovic, D.; Trifunovic, B.; Spasojevic-Kalimanovska, V.; Zeljkovic, A. Significance of LDL and HDL subclasses characterization in the assessment of risk for colorectal cancer development. Biochem. Med. 2018, 28, 030703. [Google Scholar] [CrossRef]
- Bjornheden, T.; Babyi, A.; Bondjers, G.; Wiklund, O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis 1996, 123, 43–56. [Google Scholar] [CrossRef]
- Tribble, D.L.; Rizzo, M.; Chait, A.; Lewis, D.M.; Blanche, P.J.; Krauss, R.M. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am. J. Med. 2001, 110, 103–110. [Google Scholar] [CrossRef]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. "Bligh and Dyer" and Folch Methods for Solid-Liquid-Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Amezaga, J.; Ugartemendia, G.; Larraioz, A.; Bretana, N.; Iruretagoyena, A.; Camba, J.; Urruticoechea, A.; Ferreri, C.; Tueros, I. Omega 6 polyunsaturated fatty acids in red blood cell membrane are associated with xerostomia and taste loss in patients with breast cancer. Prostaglandins Leukot Essent Fat. Acids 2021, 173, 102336. [Google Scholar] [CrossRef] [PubMed]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Sztolsztener, K.; Chabowski, A.; Harasim-Symbor, E.; Bielawiec, P.; Konstantynowicz-Nowicka, K. Arachidonic Acid as an Early Indicator of Inflammation during Non-Alcoholic Fatty Liver Disease Development. Biomolecules 2020, 10, 1133. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 2008, 34, 171–180. [Google Scholar] [CrossRef]
- Sarajlic, P.; Vigor, C.; Avignon, A.; Zhou, B.; Oger, C.; Galano, J.M.; Durand, T.; Sultan, A.; Back, M. Omega-3 to omega-6 fatty acid oxidation ratio as a novel inflammation resolution marker for metabolic complications in obesity. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Reynes, C.; Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Bouzas, C.; Garcia, S.; Mateos, D.; Casares, M.; Gomez, C.; Ugarriza, L.; et al. Polyunsaturated and Saturated Oxylipin Plasma Levels Allow Monitoring the Non-Alcoholic Fatty Liver Disease Progression to Severe Stages. Antioxidants 2023, 12, 711. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; D’Alessandro, A.; Ahmed, M.H.; Zhang, Y.; Song, A.; Ko, T.P.; Nemkov, T.; Reisz, J.A.; Wu, H.; Adebiyi, M.; et al. Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci. Rep. 2017, 7, 15281. [Google Scholar] [CrossRef]
- Benson, T.W.; Weintraub, N.L.; Kim, H.W.; Seigler, N.; Kumar, S.; Pye, J.; Horimatsu, T.; Pellenberg, R.; Stepp, D.W.; Lucas, R.; et al. A single high-fat meal provokes pathological erythrocyte remodeling and increases myeloperoxidase levels: Implications for acute coronary syndrome. Lab. Investig. 2018, 98, 1300–1310. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Fibroblast growth factor 21 in metabolic syndrome. Front. Endocrinol. 2023, 14, 1220426. [Google Scholar] [CrossRef]
- Karamfilova, V.; Assyov, Y.; Nedeva, I.; Gateva, A.; Ivanova, I.; Cherkezov, I.; Mateva, L.; Kamenov, Z. Fibroblast Growth Factor 21 as a Marker of Prediabetes in Patients with Non-alcoholic Fatty Liver Disease. Turk. J. Gastroenterol. 2022, 33, 233–239. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Ferreri, R.; Amezaga, J.; Tueros, I. Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Rajaram, S.; Damasceno, N.R.T.; Braga, R.A.M.; Martinez, R.; Kris-Etherton, P.; Sala-Vila, A. Effect of Nuts on Markers of Inflammation and Oxidative Stress: A Narrative Review. Nutrients 2023, 15, 1099. [Google Scholar] [CrossRef]
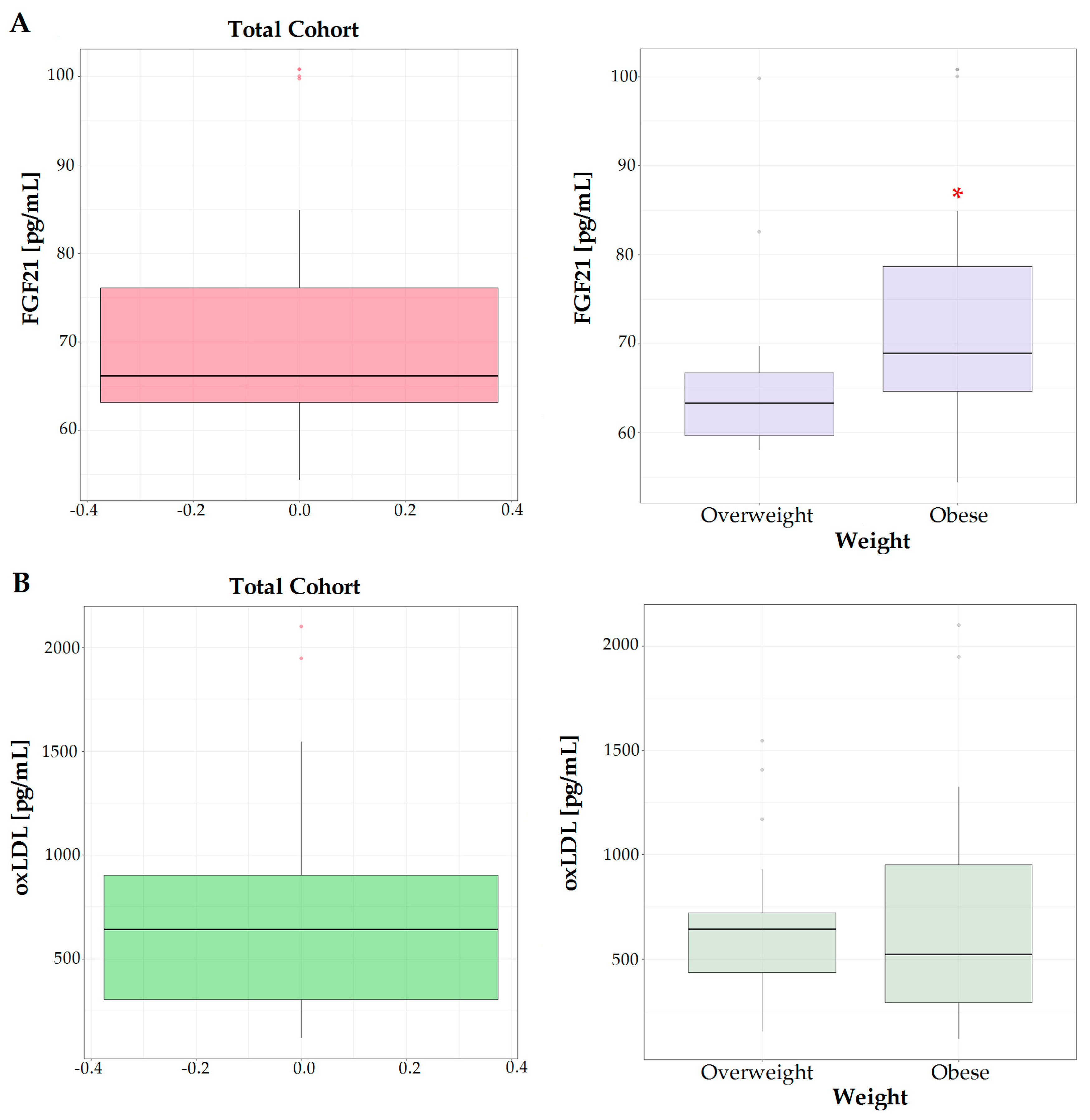
| Parameters * | Total Cohort (n = 42) | Overweight (n = 19) | Obese (n = 23) | p ^ |
|---|---|---|---|---|
| Gender (M) (%) | 26 (61.90) | 9 (47.37) | 17 (73.91) | 0.08 ѱ |
| Age (yrs) | 47.4 ± 8.46 | 45.5 ± 9.44 | 49.0 ± 7.38 | 0.14 |
| BMI (Kg/m2) | 29.2 ± 5.52 | 25.3 ± 2.41 | 32.4 ± 5.31 | <0.0001 |
| RBC (106/µL) | 4.93 ± 0.51 | 4.77 ± 0.58 | 5.06 ± 0.40 | 0.04 |
| Hb (g/dL) | 14.7 ± 1.69 | 13.9 ± 1.82 | 15.3 ± 1.29 | 0.007 |
| Hematocrit (%) | 43.7 ± 4.37 | 42.1 ± 5.09 | 45.0 ± 3.25 | 0.04 |
| MCV (FL) | 88.8 ± 3.64 | 88.0 ± 2.68 | 89.4 ± 4.23 | 0.09 |
| Platelets (103/µL) | 247 ± 72.29 | 228 ± 47.37 | 263 ± 85.57 | 0.16 |
| WBCs (103/µL) | 6.00 ± 1.79 | 5.38 ± 1.37 | 6.51 ± 1.95 | 0.04 |
| Glucose (mg/dL) | 90.5 ± 13.62 | 86.7 ± 12.54 | 93.6 ± 13.94 | 0.09 |
| Insulin (µUI/mL) | 13.5 ± 5.96 | 11.9 ± 3.80 | 14.8 ± 7.10 | 0.15 |
| HOMA test | 3.08 ± 1.67 | 2.53 ± 0.88 | 3.54 ± 2.02 | 0.14 |
| AST (U/L) | 20.5 ± 6.04 | 19.8 ± 4.06 | 21.1 ± 7.33 | 0.84 |
| ALT (U/L) | 25.9 ± 13.24 | 20.9 ± 11.32 | 30.0 ± 13.53 | 0.002 |
| AST/ALT Ratio | 0.91 ± 0.35 | 1.09 ± 0.38 | 0.76 ± 0.23 | 0.002 |
| GGT (U/L) | 30.8 ± 23.46 | 21.8 ± 20.34 | 37.9 ± 23.70 | 0.0005 |
| Cholesterol (mg/dL) | 202 ± 41.24 | 190 ± 34.18 | 212 ± 44.69 | 0.08 |
| HDL | 49.7 ± 12.16 | 52.5 ± 11.60 | 47.4 ± 12.38 | 0.11 |
| LDL (mg/dL) | 129 ± 38.11 | 115 ± 30.52 | 140 ± 40.48 | 0.03 |
| Triglycerides (mg/dL) | 126 ± 72.83 | 104 ± 82.19 | 144 ± 60.01 | 0.02 |
| sdLDL score (%) | 1.96 ± 3.14 | 1.50 ± 2.69 | 2.32 ± 3.47 | 0.39 |
| PCR (mg/dL) | 0.23 ± 0.32 | 0.16 ± 0.21 | 0.28 ± 0.38 | 0.14 |
| ALP (U/L) | 67.8 ± 22.69 | 68.0 ± 27.88 | 67.7 ± 21.39 | 0.61 |
| Ferritin (ng/mL) | 299 ± 252.77 | 281 ± 181.27 | 307 ± 285.52 | 0.94 |
| RBC Membrane FA% * | Total Cohort (n = 42) | Overweight (n = 19) | Obese (n = 23) | p ^ |
|---|---|---|---|---|
| Saturated fatty acids | ||||
| Palmitic acid | 22.0 ± 1.73 | 22.4 ± 1.70 | 21.6 ± 1.69 | 0.16 |
| Stearic acid | 18.0 ± 1.75 | 17.7 ± 2.06 | 18.2 ± 1.45 | 0.35 |
| Monounsaturated fatty acids | ||||
| Palmitoleic acid | 0.16 ± 0.15 | 0.18 ± 0.16 | 0.14 ± 0.14 | 0.36 |
| Oleic acid | 15.5 ± 1.37 | 15.6 ± 1.58 | 15.3 ± 1.19 | 0.73 |
| cis-Vaccenic acid | 0.74 ± 0.14 | 0.74 ± 0.13 | 0.73 ± 0.15 | 0.74 |
| Polyunsaturated fatty acids | ||||
| LA | 10.4 ± 1.41 | 10.8 ± 1.60 | 10.1 ± 1.17 | 0.04 |
| DGLA | 1.75 ± 0.38 | 1.71 ± 0.45 | 1.79 ± 0.32 | 0.14 |
| AA | 14.7 ± 1.65 | 13.9 ± 1.35 | 15.4 ± 1.58 | 0.003 |
| EPA | 0.58 ± 0.28 | 0.55 ± 0.22 | 0.61 ± 0.32 | 0.83 |
| DPA | 1.85 ± 0.34 | 1.79 ± 0.29 | 1.90 ± 0.38 | 0.34 |
| DHA | 3.74 ± 0.74 | 3.78 ± 0.64 | 3.71 ± 0.83 | 0.71 |
| Total fatty acids | ||||
| Total SFAs | 41.5 ± 2.42 | 41.8 ± 2.13 | 41.3 ± 2.65 | 0.37 |
| Total MUFAs | 18.0 ± 1.80 | 18.1 ± 2.20 | 17.9 ± 1.43 | 0.85 |
| Total PUFAs | 36.4 ± 2.23 | 35.8 ± 2.17 | 36.9 ± 2.21 | 0.19 |
| Total n-9 PUFAs | 15.8 ± 1.45 | 15.9 ± 1.71 | 15.8 ± 1.23 | 0.91 |
| Total n-6 PUFAs | 30.1 ± 2.44 | 29.6 ± 2.41 | 30.6 ± 2.41 | 0.16 |
| Total n-3 PUFAs | 6.23 ± 1.06 | 6.20 ± 0.97 | 6.25 ± 1.14 | 0.99 |
| Fatty acid index | ||||
| SI | 1.17 ± 0.15 | 1.15 ± 0.18 | 1.19 ± 0.12 | 0.43 |
| SFAs/MUFAs | 1.15 ± 0.09 | 1.17 ± 0.09 | 1.13 ± 0.09 | 0.18 |
| AA/DHA | 4.09 ± 0.98 | 3.78 ± 0.78 | 4.35 ± 1.07 | 0.11 |
| AA/EPA | 30.6 ± 13.62 | 30.0 ± 13.50 | 31.2 ± 13.99 | 0.66 |
| Omega3 index | 4.33 ± 0.90 | 4.34 ± 0.75 | 4.31 ± 1.03 | 0.79 |
| n-6/n-3 PUFAs | 5.00 ± 1.04 | 4.91 ± 0.99 | 5.08 ± 1.10 | 0.68 |
| Free RSI | 30.2 ± 1.84 | 29.5 ± 1.92 | 30.7 ± 1.61 | 0.04 |
| UI | 128 ± 7.13 | 125 ± 6.21 | 130 ± 7.23 | 0.02 |
| PI | 106 ± 8.18 | 103 ± 5.91 | 109 ± 9.08 | 0.05 |
| Parameters * | Total Cohort (n = 42) | Overweight (n = 19) | Obese (n = 23) | p ^ |
|---|---|---|---|---|
| Macronutrients | ||||
| Energy intake (Kcal/day) | 2057 ± 308.04 | 1871 ± 248.34 | 2210 ± 268.59 | <0.0001 |
| Proteins (g) | 99.8 ± 23.12 | 88.4 ± 16.20 | 109 ± 24.02 | 0.005 |
| Proteins (%) | 19.4 ± 3.21 | 19.0 ± 2.90 | 20.0 ± 3.47 | 0.35 |
| Lipids (g) | 95.7 ± 22.21 | 79.4 ± 14.56 | 109 ± 18.04 | <0.0001 |
| Lipids (%) | 41.6 ± 5.40 | 38.3 ± 5.00 | 44.4 ± 3.98 | 0.0001 |
| Carbohydrates (g) | 210 ± 39.56 | 212 ± 42.76 | 208 ± 37.62 | 0.99 |
| Carbohydrates (%) | 38.6 ± 6.35 | 42.4 ± 5.36 | 35.5 ± 5.39 | 0.0003 |
| Starch (g) | 84.3 ± 24.35 | 83.3 ± 25.44 | 85.1 ± 23.95 | 0.50 |
| Sugars (g) | 52.2 ± 21.34 | 59.9 ± 25.99 | 45.8 ± 14.21 | 0.04 |
| Total Fiber (g) | 10.4 ± 2.55 | 10.6 ± 2.50 | 10.3 ± 2.64 | 0.67 |
| Micronutrients | ||||
| Sodium (mg) | 886 ± 828.15 | 654 ± 149.61 | 1078 ± 1083.90 | 0.09 |
| Potassium (mg) | 1168 ± 620.39 | 1055 ± 331.16 | 1262 ± 779.33 | 0.65 |
| Iron (mg) | 7.16 ± 4.54 | 5.79 ± 1.50 | 8.29 ± 5.79 | 0.16 |
| Calcium (mg) | 443 ± 129.51 | 461 ± 107.77 | 429 ± 145.84 | 0.44 |
| Phosphorus (mg) | 837 ± 218.69 | 730 ± 179.49 | 924 ± 322.03 | 0.09 |
| Thiamine (mg) | 0.53 ± 0.35 | 0.45 ± 0.14 | 0.60 ± 0.44 | 0.42 |
| Riboflavina (mg) | 0.63 ± 0.20 | 0.60 ± 0.20 | 0.66 ± 0.20 | 0.33 |
| Niacin (mg) | 10.4 ± 5.03 | 9.04 ± 2.59 | 11.6 ± 6.22 | 0.25 |
| Vitamin A (mcg) | 389 ± 160.24 | 358 ± 126.70 | 415 ± 182.28 | 0.45 |
| Vitamin C (mg) | 44.7 ± 50.16 | 55.6 ± 64.35 | 35.7 ± 33.38 | 0.53 |
| Vitamin E (mg) | 8.36 ± 3.73 | 6.90 ± 3.63 | 9.57 ± 3.43 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutino, V.; De Nunzio, V.; Donghia, R.; Aloisio Caruso, E.; Cisternino, A.M.; Iacovazzi, P.A.; Mastrosimini, A.M.; Fernandez, E.A.; Giannuzzi, V.; Notarnicola, M. Significant Increase in Oxidative Stress Indices in Erythrocyte Membranes of Obese Patients with Metabolically-Associated Fatty Liver Disease. J. Pers. Med. 2024, 14, 315. https://doi.org/10.3390/jpm14030315
Tutino V, De Nunzio V, Donghia R, Aloisio Caruso E, Cisternino AM, Iacovazzi PA, Mastrosimini AM, Fernandez EA, Giannuzzi V, Notarnicola M. Significant Increase in Oxidative Stress Indices in Erythrocyte Membranes of Obese Patients with Metabolically-Associated Fatty Liver Disease. Journal of Personalized Medicine. 2024; 14(3):315. https://doi.org/10.3390/jpm14030315
Chicago/Turabian StyleTutino, Valeria, Valentina De Nunzio, Rossella Donghia, Emanuela Aloisio Caruso, Anna Maria Cisternino, Palma Aurelia Iacovazzi, Anna Margherita Mastrosimini, Elizabeth Alicia Fernandez, Vito Giannuzzi, and Maria Notarnicola. 2024. "Significant Increase in Oxidative Stress Indices in Erythrocyte Membranes of Obese Patients with Metabolically-Associated Fatty Liver Disease" Journal of Personalized Medicine 14, no. 3: 315. https://doi.org/10.3390/jpm14030315





