Variation Analysis in Premenopausal and Postmenopausal Breast Cancer Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Patients
2.2. Immunohistochemical Staining
2.3. FISH Analysis
2.4. Molecular Classification
2.5. Targeting NGS Panel Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Evaluation of Tumors by Histological Subtypes
3.3. Molecular Classification Analysis Results
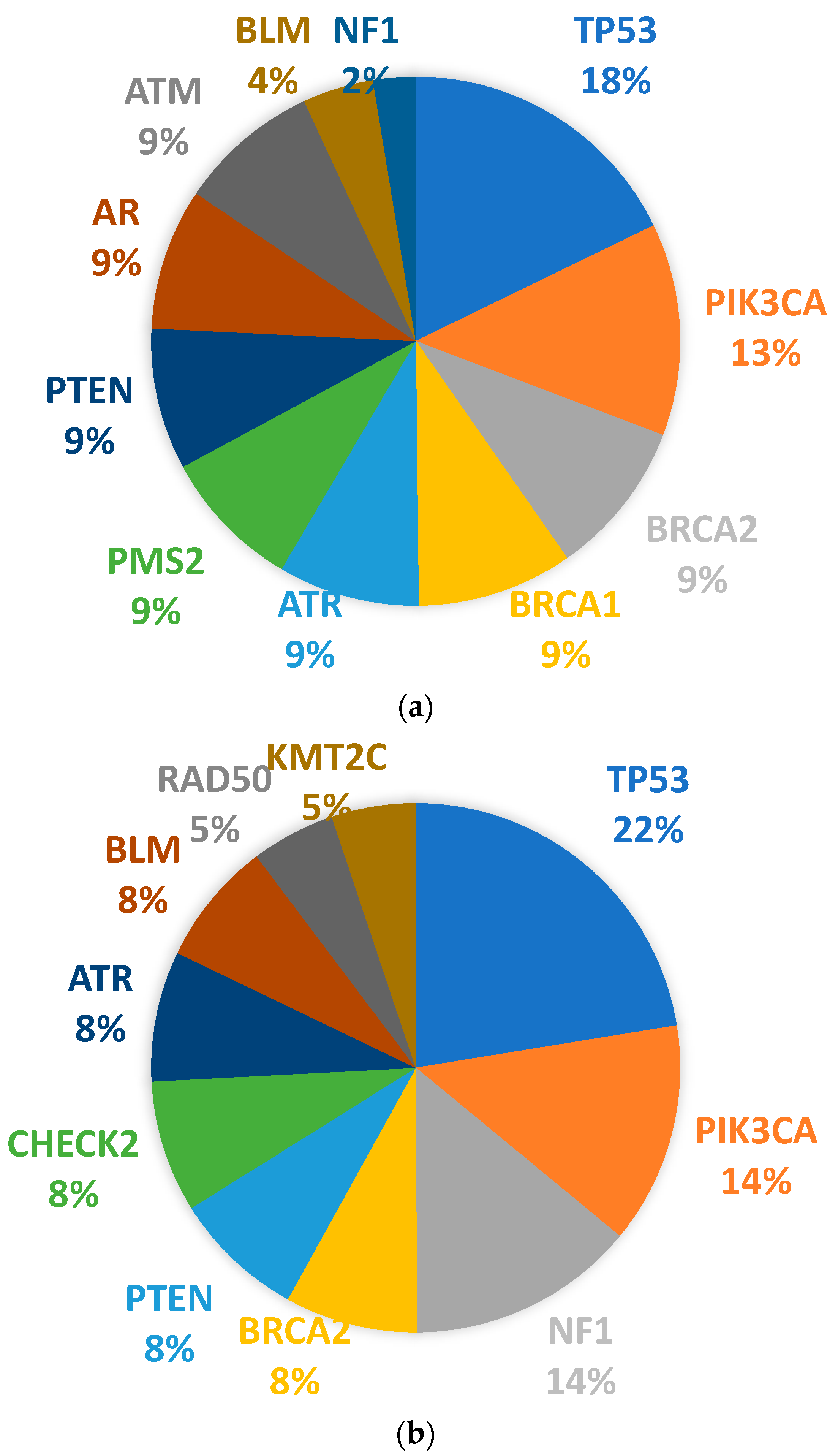
3.4. Somatic Mutation Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Tin Tin, S.; Reeves, G.K.; Key, T.J. Endogenous hormones and risk of invasive breast cancer in pre-and post-menopausal women: Findings from the UK Biobank. Br. J. Cancer 2021, 125, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Mahlow, J.; Goold, E.A.; Jedrzkiewicz, J.; Gulbahce, H.E. What to expect from the new ASCO/CAP guideline recommendations for hormone receptor testing in breast cancer: A national reference laboratory experience. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kunheri, B.; Raj, R.V.; Vijaykumar, D.K.; Pavithran, K. Impact of St. Gallen surrogate classification for intrinsic breast cancer sub-types on disease features, recurrence, and survival in South Indian patients. Indian J. Cancer 2020, 57, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J.; et al. ACMG SF v3. 0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Tenea-Cojan, T.S.; Georgescu, C.V.; Corici, O.M.; Voinea, B.; Georgescu, D.M.; Vidrighin, C.; Firulescu, S.; Ilie, D.; Paun, I. Histopathological study on con-servatively operated breast carcinomas. Curr. Health Sci. J. 2016, 42, 269–282. [Google Scholar] [PubMed]
- Chu, K.C.; Anderson, W.F.; Fritz, A.; Ries, L.A.; Brawley, O.W. Frequency distributions of breast cancer characteristics classified by estrogen receptor and progesterone receptor status for eight racial/ethnic groups. Cancer 2001, 1, 37–45. [Google Scholar] [CrossRef]
- Amanat, A.; Waris, S.; Wali, N.; Alam, S.; Masood, A.; Aslam, M. Determination of Estrogen Receptor, Progesterone Receptor & Human Epidermal Growth Factor Receptor 2 Status in Breast Cancer: A Single-Center Experience. J. Sharif Med. Dent. Coll. Lahore Pak. 2022, 8, 47–51. [Google Scholar]
- Turner, K.M.; Yeo, S.K.; Holm, T.M.; Shaughnessy, E.; Guan, J.L. Heterogeneity within molecular subtypes of breast cancer. Am. J. Physiol.-Cell Physiol. 2021, 321, C343–C354. [Google Scholar] [CrossRef]
- Yu, R.; Peng, M.; Zhao, S.; Wang, Z.; Ma, Y.; Zhang, X.; Lv, X.; Wang, S.; Ju, S.; Zhao, R.; et al. Comprehensive Characterization of the Function of Metabolic Genes and Establishment of a Prediction Model in Breast Cancer. Dis. Markers 2022, 2022, 3846010. [Google Scholar] [CrossRef]
- do Nascimento, R.G.; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know? Mastology 2020, 30, e20200024. [Google Scholar] [CrossRef]
- Pandit, P.; Patil, R.; Palwe, V.; Gandhe, S.; Patil, R.; Nagarkar, R. Prevalence of Molecular Subtypes of Breast Cancer: A Single Institutional Experience of 2062 Patients. Eur. J. Breast Health 2020, 16, 39–43. [Google Scholar] [CrossRef]
- Ozmen, V. Breast Cancer in Turkey: Clinical and Histopathological Characteristics (Analysis of 13.240 Patients). J. Breast Health 2014, 10, 98–105. [Google Scholar] [CrossRef]
- Murase, K.; Yanai, A.; Saito, M.; Imamura, M.; Miyagawa, Y.; Takatsuka, Y.; Inoue, N.; Ito, T.; Hirota, S.; Sasa, M.; et al. Biological Characteristics of Luminal Subtypes in Pre- and Postmenopausal Estrogen Receptor-Positive and HER2-Negative Breast Cancers. Breast Cancer 2012, 21, 52–57. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. J. Am. Med. Assoc. 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Liao, S.; Hartmaier, R.J.; McGuire, K.P.; Puhalla, S.L.; Luthra, S.; Chandran, U.R.; Ma, T.; Bhargava, R.; Modugno, F.; Davidson, N.E.; et al. The molecular landscape of premenopausal breast cancer. Breast Cancer Res. 2015, 17, 104. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F. Comprehensive molecular portraits of human breast tumors. Nature 2012, 490, 61. [Google Scholar]
- Olivier, M.; Bouaoun, L.; Villar, S.; Robitaille, A.; Cahais, V.; Heguy, A.; Byrnes, G.; Le Calvez-Kelm, F.; Torres-Mejía, G.; Alvarado-Cabrero, I.; et al. Molecular features of premenopausal breast cancers in Latin American women: Pilot results from the PRECAMA study. PLoS ONE 2019, 14, e0210372. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.G.; Masciari, S.; Gelman, R.; Miron, A.; Miron, P.; Foley, K.; Richardson, A.L.; Krop, I.E.; Verselis, S.J.; Dillon, D.A.; et al. Prevalence of germline TP53 mutations in HER2-positive Breast Cancer Patients. Breast Cancer Res. Treat. 2013, 139, 193. [Google Scholar] [CrossRef] [PubMed]
- Darb-Esfahani, S.; Denkert, C.; Stenzinger, A.; Salat, C.; Sinn, B.; Schem, C.; Endris, V.; Klare, P.; Schmitt, W.; Blohmer, J.-U.; et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget 2016, 7, 67686. [Google Scholar] [CrossRef] [PubMed]
- Román-Rosales, A.A.; García-Villa, E.; Herrera, L.A.; Gariglio, P.; Díaz-Chávez, J. Mutant p53 gain of function induces HER2 over-expression in cancer cells. BMC Cancer 2018, 18, 709. [Google Scholar] [CrossRef] [PubMed]
- Nagy, T.R.; Maistro, S.; Encinas, G.; Katayama, M.L.H.; de Lima Pereira, G.F.; Gaburo-Júnior, N.; Franco, L.A.M.; de Gouvêa, A.C.R.C.; Diz, M.d.P.E.; Leite, L.A.S.; et al. Germline and Somatic mutations in postmenopausal breast cancer patients. Clinics 2021, 76, e2837. [Google Scholar] [CrossRef] [PubMed]
- Hagio, K.; Amano, T.; Hayashi, H.; Takeshita, T.; Oshino, T.; Kikuchi, J.; Ohhara, Y.; Yabe, I.; Kinoshita, I.; Nishihara, H.; et al. Impact of clinical targeted sequencing on endocrine responsiveness in estrogen receptor-positive, HER2-negative metastatic breast cancer. Sci. Rep. 2021, 11, 8109. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Y.; Ma, W.L.; Lu, Y.S. Role of alpelisib in the treatment of PIK3CA-mutated breast cancer: Patient selection and clinical perspectives. Ther. Clin. Risk Manag. 2021, 17, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Gymnopoulos, M.; Elsliger, M.A.; Vogt, P.K. Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. USA 2007, 104, 5569–5574. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Razavi, P.; Johnson, J.L.; Shao, H.; Shah, H.; Antoine, A.; Ladewig, E.; Gorelick, A.; Lin, T.-Y.; Toska, E.; et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science 2019, 366, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lau, Q.C.; Salto-Tellez, M.; Putti, T.C.; Loh, M.; Sukumar, S. Mutational hotspot in exon 20 of PIK3CA in breast cancer among Singapore Chinese. Cancer Biol. Ther. 2006, 5, 544–548. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Lopez-Ilasaca, M.; Pinto, J.A.; Chirinos-Arias, M.; Doimi, F.; Neciosup, S.P.; Rojas, K.I.; Vidaurre, T.; Balko, J.M.; Arteaga, C.L.; et al. PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastatic breast cancers. Hematol. Oncol. Stem Cell Ther. 2014, 7, 142–148. [Google Scholar] [CrossRef]
- Desriani; Al-Ahwani, F. The sensitivity and efficacy method of PIK3CA exon 9 E545A as a high diagnostic accuracy in breast cancer. J. Genet. Eng. Biotechnol. 2018, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mangone, F.R.; Bobrovnitchaia, I.G.; Salaorni, S.; Manuli, E.; Nagai, M.A. PIK3CA exon 20 mutations are associated with poor prognosis in breast cancer patients. Clinics 2012, 67, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.S.; Deffenbaugh, A.M.; Reid, J.E.; Hulick, M.; Ward, B.E.; Lingenfelter, B.; Gumpper, K.L.; Scholl, T.; Tavtigian, S.V.; Pruss, D.R.; et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10,000 individuals. J. Clin. Oncol. 2002, 20, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Barakeh, D.H.; Aljelaify, R.; Bashawri, Y.; Almutairi, A.; Alqubaishi, F.; Alnamnakani, M.; Almubarak, L.; Al Naeem, A.; Almushawah, F.; Alrashed, M.; et al. Landscape of somatic mutations in breast cancer: New opportunities for targeted therapies in Saudi Arabian patients. Oncotarget 2021, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Guindalini, R.S.C.; Viana, D.; Kitajima, J.P.; Valim, A.; Schlesinger, D.; Kok, F.; Folgueira, M.A.A.K. Detection of inherited mutations in Brazilian breast cancer patients using multi-gene panel testing. In Proceedings of the 2018 American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, 1–5 June 2018; Volume 36. [Google Scholar]
- Encinas, G.; Sabelnykova, V.Y.; de Lyra, E.C.; Hirata Katayama, M.L.; Maistro, S.; de Vasconcellos Valle, P.W.M.; de Lima Pereira, G.F.; Rodrigues, L.M.; de Menezes Pacheco Serio, P.A.; de Gouvêa, A.C.R.C.; et al. Somatic mutations in early onset luminal breast cancer. Oncotarget 2018, 9, 22460–22479. [Google Scholar] [CrossRef] [PubMed]
- Guindalini, R.S.C.; Viana, D.V.; Kitajima, J.P.F.W.; Rocha, V.M.; López, R.V.M.; Zheng, Y.; Freitas, É.; Monteiro, F.P.M.; Valim, A.; Schlesinger, D.; et al. Detection of germline variants in Brazilian breast cancer patients using multigene panel testing. Sci. Rep. 2022, 12, 4190. [Google Scholar] [CrossRef]
- Abulkhair, O.; Al Balwi, M.; Makram, O.; Alsubaie, L.; Faris, M.; Shehata, H.; Hashim, A.; Arun, B.; Saadeddin, A.; Ibrahim, E. Prevalence of BRCA1 and BRCA2 Mutations Among High-Risk Saudi Patients with Breast Cancer. J. Glob. Oncol. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Nassar, A.; Abouelhoda, M.; Mansour, O.; Loutfy, S.A.; Hafez, M.M.; Gomaa, M.; Bahnassy, A.; Youssef, A.S.E.-D.; Lotfy, M.M.; Ismail, H.; et al. Targeted next generation sequencing identifies somatic mutations in a cohort of Egyptian breast cancer patients. J. Adv. Res. 2020, 24, 149–157. [Google Scholar] [CrossRef]
- Zografos, E.; Andrikopoulou, A.; Papatheodoridi, A.M.; Kaparelou, M.; Bletsa, G.; Liontos, M.; Dimopoulos, M.-A.; Zagouri, F. Multi-Gene Mutation Profiling by Targeted Next-Generation Sequencing in Premenopausal Breast Cancer. Genes 2022, 13, 1362. [Google Scholar] [CrossRef]
- Ross, D.S.; Pareja, F. Molecular Pathology of Breast Tumors: Diagnostic and Actionable Genetic Alterations. Surg. Pathol. Clin. 2021, 14, 455–471. [Google Scholar] [CrossRef]
- Wallace, M.D.; Pfefferle, A.D.; Shen, L.; McNairn, A.J.; Cerami, E.G.; Fallon, B.L.; Rinaldi, V.D.; Southard, T.L.; Perou, C.M.; Schimenti, J.C. Comparative oncogenomics implicates the neurofibromin 1 gene (NF1) as a breast cancer driver. Genetics 2012, 92, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.S.; McPherson, J.R.; Ong, C.K.; Rozen, S.G.; The, B.T.; Lee, A.S.; Callen, D.F. The NF1 gene revisited—From bench to bedside. Oncotarget 2014, 15, 5873–5892. [Google Scholar] [CrossRef] [PubMed]
- Kallionpää, R.A.; Huovinen, R.; Peltonen, S.; Peltonen, J. 486P The influence of NF1 germline and somatic mutations on breast cancer patient survival. Ann. Oncol. 2023, 34, S386. [Google Scholar] [CrossRef]
- Chaudhry, U.S.; Yang, L.; Askeland, R.W.; Fajardo, L.L. Metaplastic breast cancer in a patient with Neurofibromatosis. J. Clin. Imaging Sci. 2015, 5, 17. [Google Scholar] [CrossRef] [PubMed]


| ACVR1B | BMPR1A | CDKN2A | ERCC4 | GATA3 | MDM2 | NBN | PMS1 | SEPT9 | XRRC2 |
| AKT1 | BRCA1 | CHEK2 | ESR1 | GEN1 | MED12 | NCOR1 | PMS2 | SMAD4 | XRRC3 |
| APC | BRCA2 | CSMD1 | EXT2 | HERC1 | MEN1 | NEK2 | PPM1L | SMARCA4 | ZBED4 |
| AR | BRIP1 | CTNNB1 | EXOC2 | HOXB13 | MLH1 | NF1 | PTEN | STK11 | - |
| ATM | CASP8 | DIRAS3 | FAM175A | IRAK4 | MRE11A | PALB2 | PTGFR | SYNE1 | - |
| ATR | CBFB | EGFR | FBXO32 | ITCH | MSH2 | PALLD | RAD50 | TGFB1 | - |
| AXIN2 | CCND1 | EP300 | FANCC | KMT2C | MSH6 | PBRM1 | RAD51C | TP53 | - |
| BAP1 | CDH1 | EPCAM | FBXO32 | KRAS | MUC16 | PCGF2 | RAD51D | TRAF5 | - |
| BARD1 | CDK4 | ERBB2 | FGFR1 | MAP2K4 | MUTYH | PIK3CA | RB1 | VHL | - |
| BLM | CDK6 | ERBB3 | FGFR2 | MAP3K1 | MYC | PIK3R1 | RET | WEE1 | - |
| Features | Postmenopausal | Premenopausal | p Value |
|---|---|---|---|
| n = 105 | n = 149 | ||
| Age at diagnosis, median (range), years | 59.54 ± 9.01 | 42.11 ± 5.51 | 0.000 * |
| Special Histopathology Subtypes n (%) | |||
| Invasive ductal carcinoma | 87 (82.86) | 129 (86.58) | 0.186 |
| Invasive lobular carcinoma | 10 (9.52) | 16 (10.74) | |
| Other special types of carcinomas | 8 (7.62) | 4 (2.68) | |
| Hormone receptor status | |||
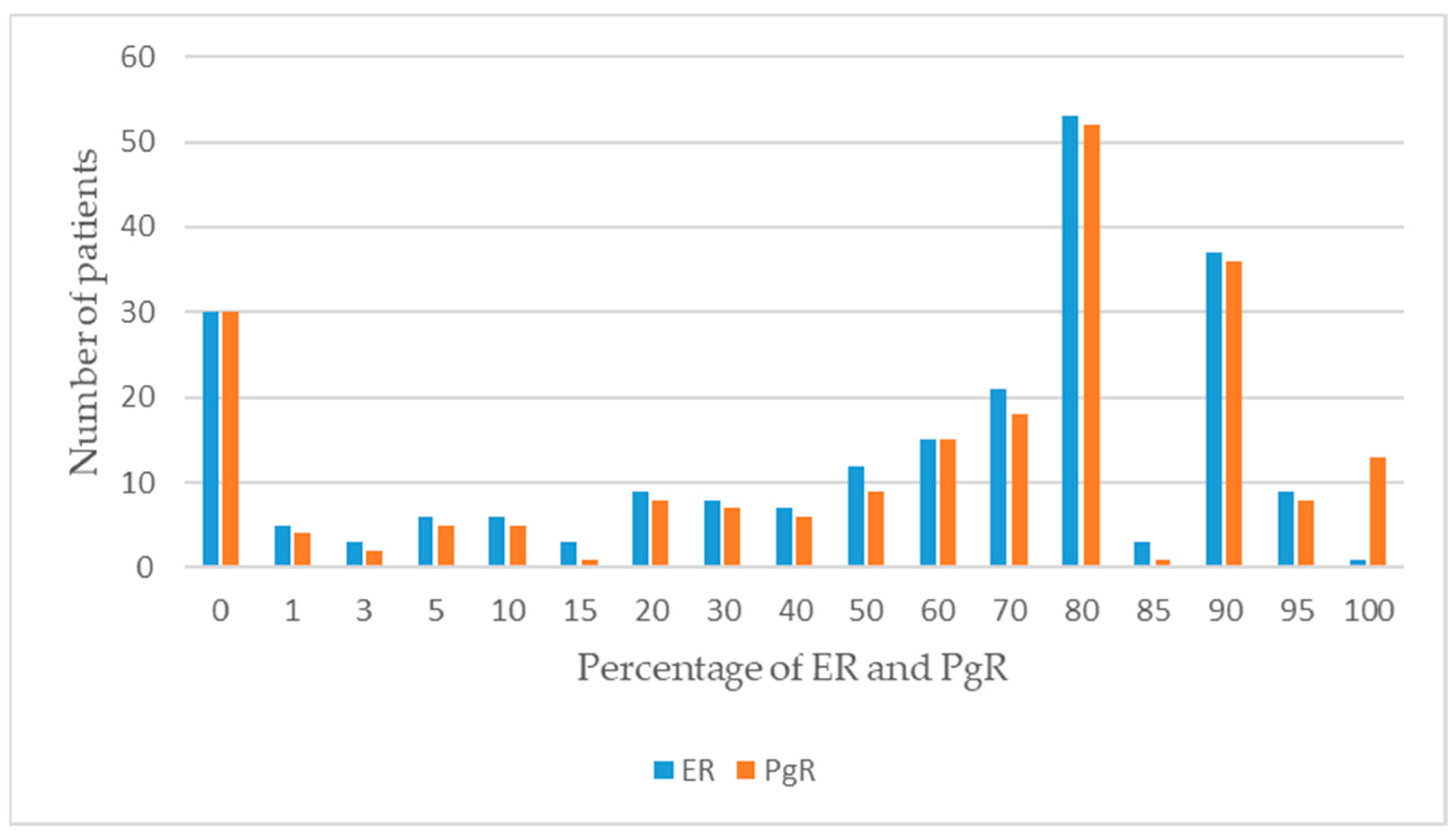
| ER(+)/PgR(+) | 78 (74.29) | 95 (63.76) | 0.008 * |
| ER(−)/PgR(−) | 25 (23.81) | 35 (23.49) | |
| ER(+)/PgR(−) | 2 (1.90) | 16 (10.74) | |
| ER(−)/PgR(+) | 0 (0.00) | 3 (2.01) | |
| Tumor Subtype n (%) | |||
| Luminal A | 41 (39.05) | 52 (34.90) | 0.154 |
| Luminal B-HER2 (+) | 26 (24.76) | 39 (26.17) | |
| Luminal B-HER2 (−) | 17 (16.19) | 12 (8.05) | |
| HER2 positive | 7 (6.67) | 16 (10.74) | |
| Triple Negative | 14 (13.33) | 30 (20.13) |
| Genes | Mutations |
|---|---|
| TP53 | |
| Frameshift variants | Exon 4 c.267delC Exon 5 c.389delT Exon 5 c.390_426delCAACAAGATGTTTT Exon 5 p.481delG Exon 7 c.737_740delTGAA Exon 7 c.754delC Exon 7 c.774dupA Exon 7 c.780delC Exon 8 c.803-805delACA Exon 10 c.1024delC Exon 13 c.323_329dupGTTTCCG Exon 13 c.576dupG Exon 4 c.158G>A Exon 4 c.372C>A Exon 5 c.497C>G Exon 5 c.499C>T Exon 8 c.916C>T Exon 8 c.1024C>T Exon 20 c.1024C>T Exon 5 c.469G>T Exon 5 c.524G>A Exon 5 c.730G>T Exon 6 c.584T>C Exon 6 c.659A>G Exon 7 c.524G>A Exon 7 c.742C>T Exon 7 c.743G>A Exon 8 c.818G>A Exon 8 c.853G>A Exon 10 c.329G>C Exon 11 c.818G>A Exon 13 c.856G>A Exon 6 c.920-1G>T Exon 9 c.920-2A>T Exon 11 c.994-2A>G |
| Nonsense variants | |
| Missense variants | |
| Splice acceptor variants | |
| PIK3CA | |
| Nonsense variants | Exon 2 c.277C>T |
| Exon 3 c.353G>A | |
| Exon 5 c.1035T>A | |
| Exon 7 c.3127A>G | |
| Exon 9 c.1624G>A | |
| Exon 9 c.1633G>A | |
| Exon 9 c.1634A>C | |
| Exon 10 c.3127A>G | |
| Exon 14 c.2176G>A | |
| Exon 18 c.1637A>G | |
| Exon 19 c.2702G>T | |
| Exon 21 c.23145G>C | |
| Missense variants | Exon 20 c.3140A>G |
| Exon 20 c.3140 A>T | |
| BRCA2 | Exon 7 c.3847_3848delGT Exon 10 c.1813delA Exon 11 c.3539delA Exon 11 c.5073delA Exon 18 c.8331+1delG Exon 23 c.9097delA Exon 11 c.4440T>G Exon 18 c.1103C>G Exon 20 c.8504C>G Exon 25 c.9382C>T |
| Frameshift variants | |
| Nonsense variants | |
| NF1 | |
| Nonsense variant | Exon 13 c.1400C>T |
| Intron variant | Exon 19 c.2325+3A>G |
| PTEN | |
| Frameshift variants | Exon 8 c.802-2delA |
| Exon 15 c.692_708delCCACACGACGGGAAGAC | |
| Nonsense variants | Exon 8 c.697C>T |
| Exon 8 c.1003C>T | |
| Missense variants | Exon 5 c.407G>A |
| Exon 10 c.397G>A | |
| Exon 18 c.389G>A | |
| Splice donor variant | Exon 4 c.253+1G>C |
| ATR | |
| Frameshift variants | Exon 10 c.2320delA |
| Exon 10 c.2319_2320delAA | |
| Exon 10 c.2320duplA | |
| Nonsense variant | Exon 6 c.3547C>T |
| CHEK2 | Exon 8 c.1450_1451delCCinsT Exon 12 c.1361G>A Exon 6 c.737A>G Exon 10 c.1427C>T Exon 10 c.1556C>T Exon 12 c.1312G>T Exon 14 c.1556C>T |
| Frameshift variant | |
| Nonsense variant | |
| Missense variants | |
| BLM | |
| Frameshift variants | Exon 7 c.1544delA |
| Exon 7 c.2320delA | |
| Nonsense variant | Exon 8 c.1642C>T |
| BRCA1 | |
| Frameshift variants | Exon 3 c.3794delA |
| Exon 10 c.1961delA | |
| Exon 10 c.3333delA | |
| Exon 10 c.3770_3771delAG | |
| Exon 16 c.5030_5033delCTAA | |
| Exon 2 c.66dupA | |
| Exon 19 c.5266dupC | |
| Splice donor variant | Exon 3 c.134+2T>C |
| Splice acceptor variant | Exon 4 c.135-2A >G |
| PMS2 | |
| Frameshift variants | Exon 11 c.1239delA |
| Exon 11 c.2165delA | |
| ATM | |
| Frameshift variant | Exon 6 c.640delT |
| Nonsense variant | Exon 7 c.742C>T |
| Missense variants | Exon 8 c.1009C>T |
| Exon 17 c.2572T>C | |
| Exon 22 c.3161C>G | |
| Exon 50 c.7463G>A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdogdu, I.H.; Orenay-Boyacioglu, S.; Boyacioglu, O.; Gurel, D.; Akdeniz, N.; Meteoglu, I. Variation Analysis in Premenopausal and Postmenopausal Breast Cancer Cases. J. Pers. Med. 2024, 14, 434. https://doi.org/10.3390/jpm14040434
Erdogdu IH, Orenay-Boyacioglu S, Boyacioglu O, Gurel D, Akdeniz N, Meteoglu I. Variation Analysis in Premenopausal and Postmenopausal Breast Cancer Cases. Journal of Personalized Medicine. 2024; 14(4):434. https://doi.org/10.3390/jpm14040434
Chicago/Turabian StyleErdogdu, Ibrahim Halil, Seda Orenay-Boyacioglu, Olcay Boyacioglu, Duygu Gurel, Nurten Akdeniz, and Ibrahim Meteoglu. 2024. "Variation Analysis in Premenopausal and Postmenopausal Breast Cancer Cases" Journal of Personalized Medicine 14, no. 4: 434. https://doi.org/10.3390/jpm14040434





