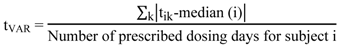Pattern of Timing Adherence Could Guide Recommendations for Personalized Intake Schedules
Abstract
:1. Introduction
2. Methods
- (a) Time variability of drug intake (tVAR) according to Equation (1) [10].
- (b) Dose-to-dose intervals as the time difference between two consecutive removals.
- (c) Weekend effects as the differences between objective adherence parameters on working days (Monday to Friday) and weekend days (Saturday and Sunday).
Statistical Analysis
3. Results
3.1. Patient Characteristics
| Dosing frequency | Number of drugs | Treatment schedule | N | % | ||||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Morning | Midday | Evening | At night | |||
| 1 × daily | 3.5 | 1–7 | X | 30 | 38.5 | |||
| X | 1 | 1.3 | ||||||
| X | 1 | 1.3 | ||||||
| 2 × daily | 5.0 | 2–11 | X | X | 30 | 38.5 | ||
| X | X | 2 | 2.6 | |||||
| X | X | 2 | 2.6 | |||||
| X | X | 1 | 1.3 | |||||
| 3 × daily | 7.0 | 3–10 | X | X | X | 5 | 6.4 | |
| X | X | X | 3 | 3.8 | ||||
| 4 × daily | 11.0 | 6–13 | X | X | X | X | 3 | 3.8 |
3.2. Objective Measures of Adherence
| Morning | Midday | Evening | ||||
|---|---|---|---|---|---|---|
| Median [h:min] | tVAR [min:s] | Median [h:min] | tVAR [min:s] | Median [h:min] | tVAR [min:s] | |
| N | 73 | 72 | 10 | 10 | 39 | 37 |
| Mean | 7:33 | 34:16 | 12:00 | 27:24 | 19:01 | 49:31 |
| SD | 1:00 | 28:50 | 00:33 | 29:37 | 1:35 | 50:43 |
| Median | 7:41 | 30:00 | 12:09 | 13:45 | 18:36 | 37:17 |
| IQR | 7:01–8:14 | 18:17–40:22 | 11:56–12:11 | 11:00–27:34 | 18:05–19:27 | 19:43–52:51 |
| Range | 4:00–9:23 | 00:43–228:45 | 10:28–12:35 | 6:51–103:26 | 16:02–23:26 | 02:43–250:34 |
| Treatment schedule | N | Mean interval [h:min] |
|---|---|---|
| Morning-Evening | 35 | 11:38 ± 1:49 |
| X-0-X-0 | 25 | 11:48 ± 1:53 |
| X-X-X-0 | 5 | 11:33 ± 1:01 |
| X-0-X-X | 2 | 10:10 ± 0:52 |
| X-X-X-X | 3 | 11:23 ± 3:07 |
| Morning-Midday | 8 | 4:32 ± 1:04 |
| Midday-Evening | 7 | 6:33 ± 0:31 |
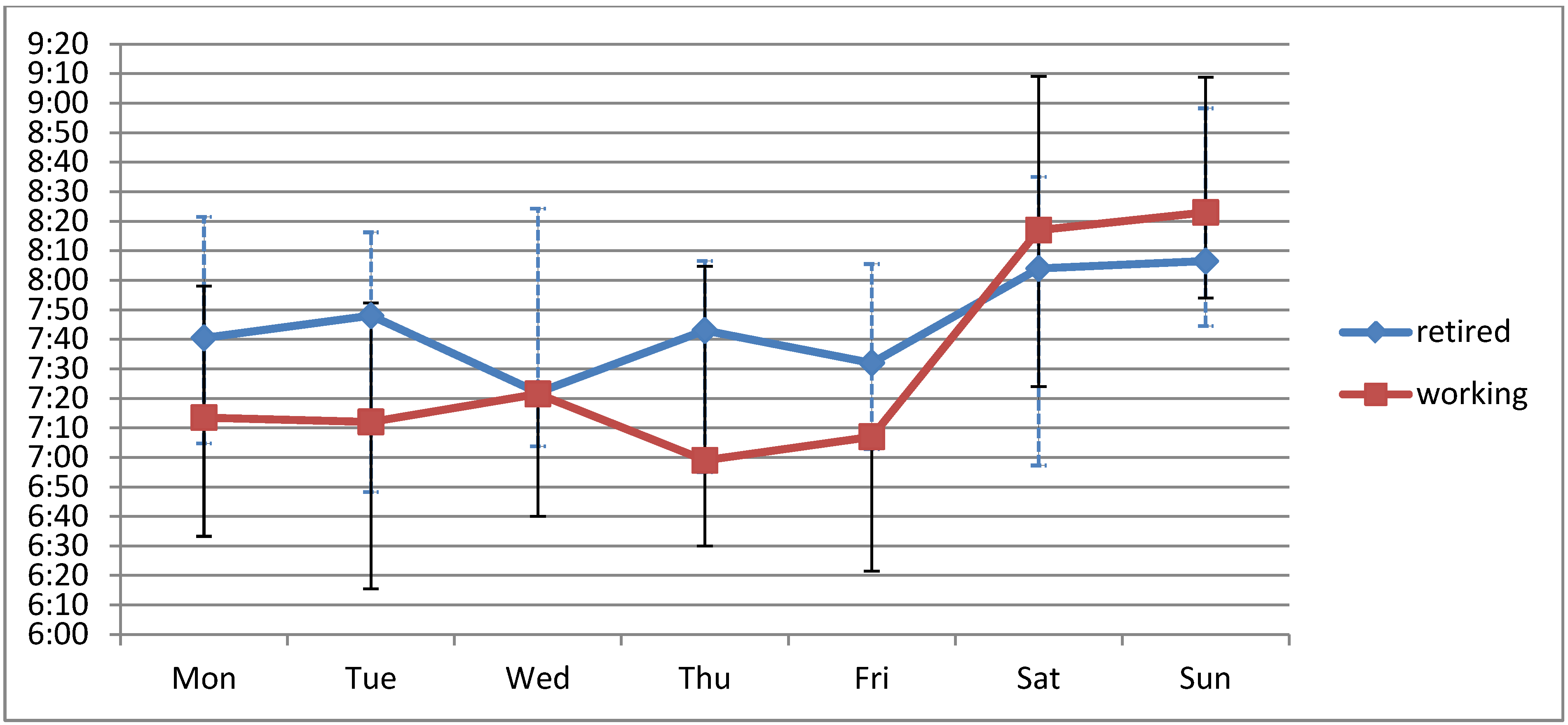
3.3. Weekend-Effect
3.4. Socio-Demographic Factors
3.5. Treatment Scheme and Subjective Adherence
3.6. Biomarker Response
4. Discussion
4.1. Main Findings
4.2. Objectively Measured Adherence and Biomarkers
4.3. Strengths and Limitations
5. Conclusion
Acknowledgments
Conflict of Interest
References and Notes
- DiMatteo, M. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med. Care 2004, 42, 200–209. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to long-term therapies—Evidence for action; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2003; pp. 1–194. [Google Scholar]
- Osterberg, L.; Blaschke, T. Adherence to Medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Cramer, J.A.; Mattson, R.H.; Prevey, M.; Scheyer, R.D.; Ouellette, V.L. How often is medication taken as prescribed? A novel assessment technique. JAMA 1989, 261, 3272–3277. [Google Scholar]
- Vrijens, B.; Vincze, G.; Kristanto, P.; Urquhart, J. Burnier, M. Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. Br. Med J. 2008, 336, 1114–1117. [Google Scholar]
- Bangsberg, D.; Hecht, F.; Charlebois, E.; Zolopa, A.; Holodniy, M.; Sheiner, L.; Bamberger, J.; Chesney, M.; Moss, A. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 2000, 14, 357–366. [Google Scholar]
- Milgrom, H.; Bender, B.; Ackerson, L.; Bowrya, P.; Smith, B.; Rand, C. Noncompliance and treatment failure in children with asthma. J. Allergy Clin. Immun. 1996, 98, 1051–1057. [Google Scholar] [CrossRef]
- Arnet, I.; Hersberger, K. Polymedication Electronic Monitoring System (POEMS)—Introducing a new technology as gold standard for compliance measurement. J. Patient Compliance 2012, 2, 48–49. [Google Scholar]
- Urquhart, J.; de Klerk, E. Contending paradigms for the interpretation of data on patient compliance with therapeutic drug regimens. Stat. Med. 1998, 17, 251–267. [Google Scholar] [CrossRef]
- Vrijens, B.; Goetghebeur, E. Comparing compliance patterns between randomized treatments. Control. Clin. Trials. 1997, 18, 187–203. [Google Scholar] [CrossRef]
- Goue Gohore, D.; Fenneteau, F.; Barrière, O.; Li, J.; Nekka, F. Rational drug delineation: A global sensitivity approach based on therapeutic tolerability to deviations in execution. Pharmacol. Pharm. 2010, 1, 42–52. [Google Scholar]
- Pearson, T.; Laurora, I.; Chu, H.; Kafonek, S. The lipid treatment assessment project (l-tap): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch. Intern. Med. 2000, 160, 459–467. [Google Scholar]
- Walter, P.; Arnet, I.; Hersberger, K. Fundamental progress in investigating drug resistance with Electronic Multidrug Compliance Monitoring (e-MCM). J. Patient Compliance 2011, 1, 42–47. [Google Scholar]
- Horne, R.; Weinman, J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. (Greenwich) 2008, 10, 348–354. [Google Scholar]
- Karow, T.; Lang-Roth, R. Allgemeine und Spezielle Pharmakologie; Elsevier: Pulheim, Germany, 2012. [Google Scholar]
- Burnier, M.; Schneider, M.P.; Chiolero, A.; Stubi, C.L.F.; Brunner, H.R. Electronic compliance monitoring in resistant hypertension: The basis for rational therapeutic decisions. J. Hypertens. 2001, 19, 335–341. [Google Scholar] [CrossRef]
- Plakogiannis, R.; Cohen, H. Optimal low-density lipoprotein cholesterol lowering—Morning versus evening statin administration. Ann. Pharmacother. 2007, 41, 106–110. [Google Scholar]
- Chapman, R.; Benner, J.; Petrilla, A.; Tierce, J.; Collins, S.; Battleman, D.; Schwartz, J. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch. Intern. Med. 2005, 165, 1147–1152. [Google Scholar]
- Osterberg, L.G.; Urquhart, J.; Blaschke, T.F. Understanding forgiveness: Minding and mining the gaps between pharmacokinetics and therapeutics. Clin. Pharmacol. Ther. 2010, 88, 457–459. [Google Scholar] [CrossRef]
- Delgado-Rodriguez, M.; Llorca, J. Bias. J. Epidemiol. Community Health 2004, 58, 635–641. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Walter, P.; Arnet, I.; Romanens, M.; Tsakiris, D.A.; Hersberger, K.E. Pattern of Timing Adherence Could Guide Recommendations for Personalized Intake Schedules. J. Pers. Med. 2012, 2, 267-276. https://doi.org/10.3390/jpm2040267
Walter P, Arnet I, Romanens M, Tsakiris DA, Hersberger KE. Pattern of Timing Adherence Could Guide Recommendations for Personalized Intake Schedules. Journal of Personalized Medicine. 2012; 2(4):267-276. https://doi.org/10.3390/jpm2040267
Chicago/Turabian StyleWalter, Philipp, Isabelle Arnet, Michel Romanens, Dimitrios A. Tsakiris, and Kurt E. Hersberger. 2012. "Pattern of Timing Adherence Could Guide Recommendations for Personalized Intake Schedules" Journal of Personalized Medicine 2, no. 4: 267-276. https://doi.org/10.3390/jpm2040267
APA StyleWalter, P., Arnet, I., Romanens, M., Tsakiris, D. A., & Hersberger, K. E. (2012). Pattern of Timing Adherence Could Guide Recommendations for Personalized Intake Schedules. Journal of Personalized Medicine, 2(4), 267-276. https://doi.org/10.3390/jpm2040267