Nanotribological Behavior of Carbon Based Thin Films: Friction and Lubricity Mechanisms at the Nanoscale
Abstract
:1. Introduction
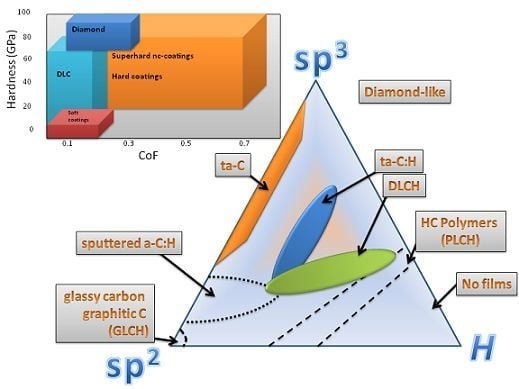
1.1. Definition
- (1)
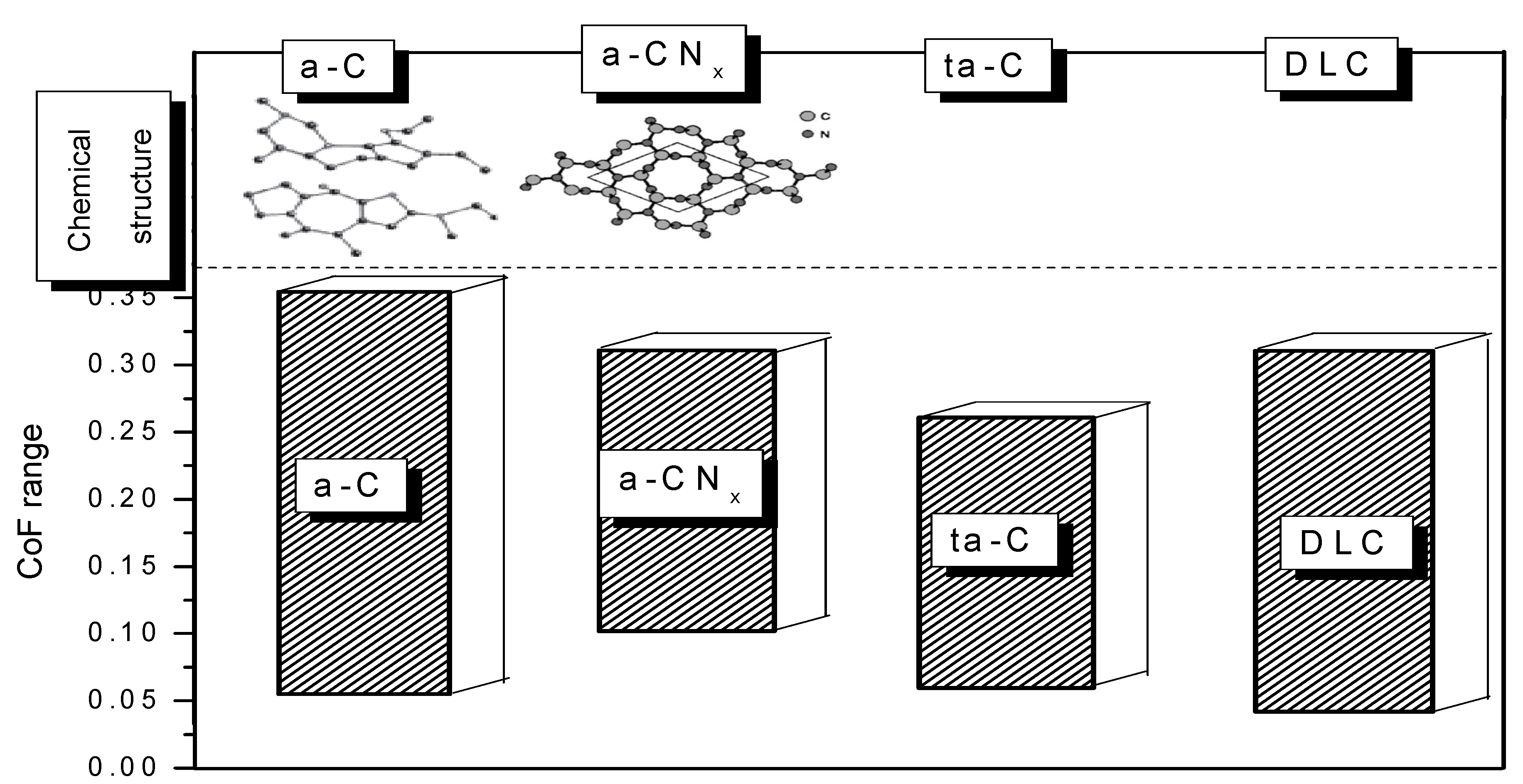
- a-C and hydrogenated amorphous carbon (a-C:H) films with a mixture of sp2 and sp3 bonding, highly sp3-boned material (ta-C) and sp2-bonded carbon
- (2)
- Carbon nitride (a-CNx)
- (3)
- Metal/Amorphous carbon (a-C:M) composite films
1.2. Scientific Fundamentals
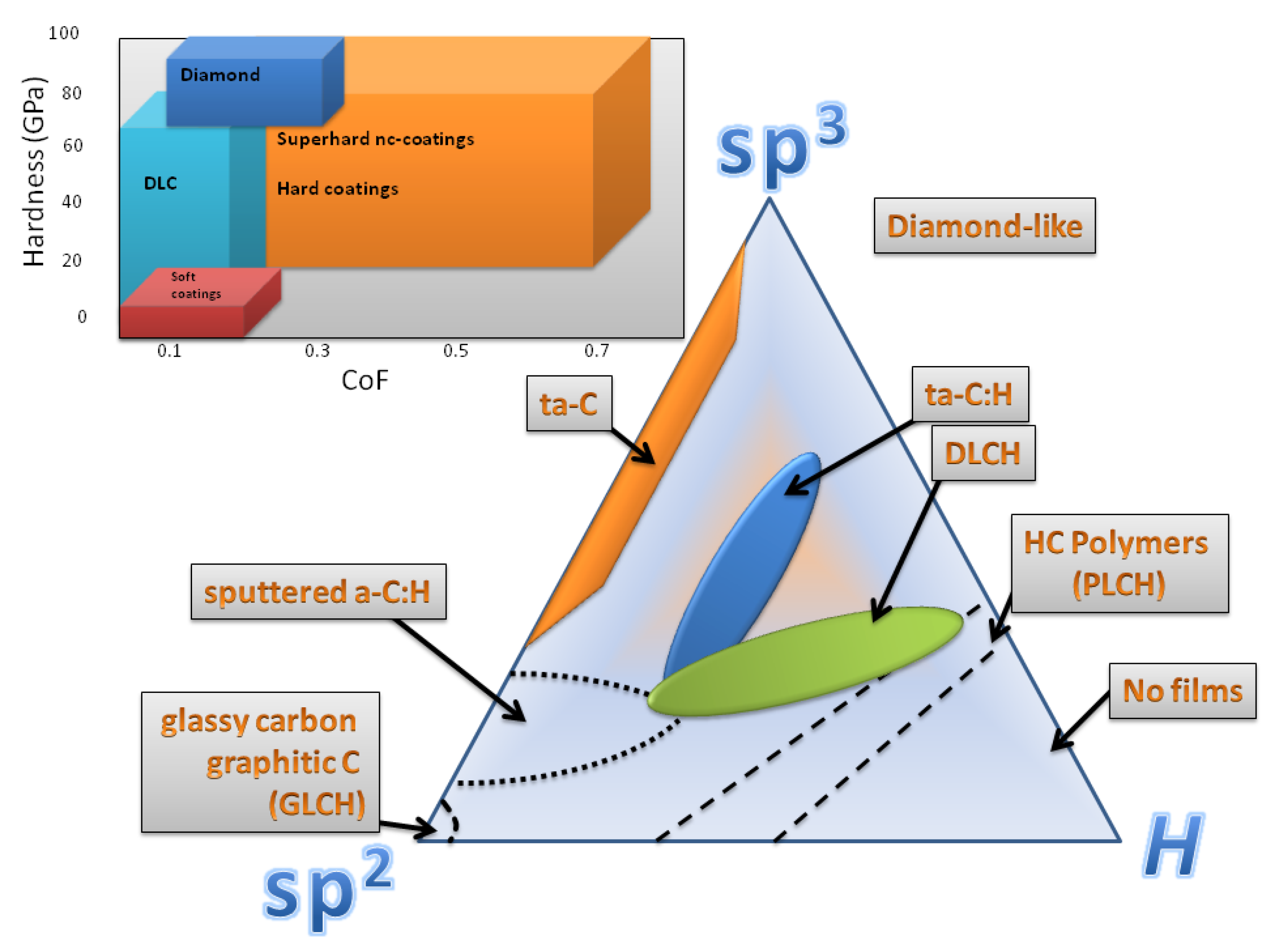
2. Discussion
2.1. Surface Chemistry
2.2. Bulk Chemistry—Additives
| Carbon Thin Film Type | Deposition method | Thickness (nm) | H3/E2 (GPa) | H/E | CoF at Load (mN) | |
|---|---|---|---|---|---|---|
| a-C sp3 rich multilayer [37] | MS | 90 | 0.235 | 0.093 | 0.25 | |
| 274 | 0.44 | 0.1231 | 0.2 | |||
| a-C multilayer [38] | MS | 115 | 0.383 | 0.1263 | ||
| a-C sp3 rich [37] | MS | 30 | 0.1704 | 0.0973 | ||
| a-C sp2 rich [37] | MS | 30 | 0.0303 | 0.0615 | 0.35 | 0.35 |
| a-C sp2 rich [37] | EB | 30 | 0.01 | 0.05 | ||
| a-C [37] | UBMS | 0.4087 | 0.1148 | 0.25 | 0.25 | |
| a-C:H [39] | PECVD | 1140 | 0.2244 | 0.125 | ||
| 550 | 0.3347 | 0.1343 | ||||
| ta-C [1] | S-bend | 0.649 | 0.123 | 0.05 | ||
| ta-C [1] | FCVA | 70 | 0.3704 | 0.111 | 0.1 | |
| ta-C:H [40] | EC | 70 | 1.389 | 1.167 | 0.01–0.12 | 1–20 |
| a-CNx [38] | MS | 222 | 0.2142 | 0.109 | 0.1–0.3 | 1–20 |
| ta-C [41] | FCVA | 76 | 0.8935 | 0.1057 | 0.1–0.15 | 1–20 |
| ta-C [42] | PLD | 120 | 0.07 | |||
| 130 | 0.07 | |||||
| 210 | 0.11 | |||||
| DLC:Ti [41] | CFUMS | 1000 | 0.2944 | 0.1056 | 0.1–0.4 | 1–80 |
| DLC:Mo [43] | MS | 650 | 0.2203 | 0.1192 | ||
| DLC:Silver [44] | PECVD | 500 | 0.17–0.24 | 1–20 | ||
| ta-C [45] | FCVA | 50 | 0.2518 | 0.07 | 0.08–0.14 | 1–5 |
| ta-C [46] | OPBD-FCVA | 0.861 | 0.1071 | |||
| a-CNx [45] | MS | 280 | 0.0274 | 0.07 | ||
| 0.1292 | 0.085 | |||||
| ta-C multilayer [46] | OPBD-FCVA | 0.8137 | 0.144 | 0.12 | ||
| a-C [47] | CFUBMS | 200 | 0.3456 | 0.12 | 0.1 | |
| a-C:H [48] | PIII | 70 | 0–0.6 | 0–14 | ||
| a-C:Ti [49] | PCVD | 500 | 0.05–0.1 | 1–200 | ||
| ta-C:Si [46] | FCVA | 5 | 0.23 | |||
| 20 | 0.12–0.24 | 1–18 | ||||
| 60 | 0.12–0.3 | 1–18 | ||||
| 80 | 0.1–0.25 | 1–7 | ||||
| a-CNx [50] | FCVA | 100 | 3.502 | 0.232 | ||
| 1.6 | 0.2 | |||||
| a-C [51] | Sputtering | 29 | 2.185 | 0.236 | 0.15–0.25 | 25–300 |
| 46 | 2.687 | 0.251 | 0.22–0.28 | 25–200 | ||
| 85 | 1.643 | 0.208 | 0.2–0.3 | 25–400 | ||
| DLC:9Cr18 | Vacuum Magnetic-Filtering Arc Plasma Deposition | 500 | 0.121 | 0.059 | 0.15–20 | 0–180 |
| DLC:40CrNiMo [52] | 500 | 0.185 | 0.064 | 0.15–40 | 0–84 | |
| a-C | PVD | 0.15–0.5 | 0.1 | |||
| a-C:H | PACVD | 0.48–0.37 | 0.14–0.11 | |||
| a-CNx [53] | PACVD | 0.1–0.18 | 0.1 | |||
| a-CNx | RF Sputtering PECVD | 250–400 | 0.1–0.18 | 0.1 | ||
| DLC [54] | 0.315 | 0.108 | ||||
| DLC:Al2O3-TiC ceramic (AlTiC), DLC:Si (1 0 0), | PECVD | 250 | 0.099 | 0.062 | 0.06 | 150 |
| DLC:fused silica and | PECVD | 0.687 | 0.1625 | 0.052 | ||
| DLC:SU8 photoresist [55] | PECVD | 4.88 | 0.4333 | 0.045 | ||
| PECVD | 7.29 | 0.9 | 0.037 | |||
| a-C:F:H | RF-PECVD | 400 | 0.111 | 0.1053 | 0.15 | 0.01–0.12 |
| a-C:F:N:H [56] | RF-PECVD | 0.047 | 0.079 | 0.13 | ||
| DLC:c-Si [57] | PECVD | 0.2/0.3 | ||||
| a-C (H: 28.1 GPa) | DC Magnetron Sputtering | 1 to 1.6 μm | 0.15 | |||
| bias-graded a-C (H: 25.1 GPa) | 0.14 | |||||
| nc-TiC/a-C (H: 27.4 GPa) | 0.22 | |||||
| nc-TiC/a-C(Al) (H: 19.6 GPa) [29] | 0.18 | |||||
| Si-DLC (0 at.%) | RFPACVD | 1000 | 1.6632 | 2000 | ||
| Si-DLC (1.0 at.%) | 1000 | 1.0189 | ||||
| Si-DLC (2.0 at.%) [33] | 1000 | 0.5895 | ||||
| Cr/a-C [58] | Unbalanced Magnetron Sputter | 2000 | 0.1766 | 0.097 0.111 | ||
| 149 | 0.2298 | |||||
| DLC/C40 | PVD-CVD | 0.15 | ||||
| DLC/Ni 50% Cr | PVD-CVD | 0.1 | 1000 | |||
| DLC/Al2O3-13% TiO2 | PVD-CVD | 0.65 | ||||
| DLC/WC-Co [59] | PVD-CVD | 0.1 | ||||
| DLC [60] | Linear Ion Beam | 2.2 | 0.0222 | 0.0666 | 0.2–12.8 | |
| nc-Ti (N,C)/a-C:H | Pulsed DC | 400 | ||||
| 31.1 H at.% | magnetron | 0.137 | 0.099 | 0.21 | ||
| 42.6 H at.% | Sputtering | 0.0774 | 0.0957 | 0.25 | ||
| 47.3 H at.% [61] | 0.0359 | 0.0847 | 0.35 | |||
| Ti-DLC/alumina | ClosefieldUnbalanced MS | 1000 | 0.2944 | 0.1056 | 0.12–0.25 | 5–10 |
| Ti-DLC/steel [43] | ||||||
| a-CNx-TiNx/G/Ti ratio | PLD | 1200 | ||||
| Pure Ti | 0.1613 | 0.0872 | 0.34 | |||
| 0.5 | 0.1587 | 0.0821 | 0.24 | |||
| 1 | 0.1968 | 0.08651 | 0.19 | 980 | ||
| 2 | 0.2071 | 0.0874 | 0.17 | |||
| Pure graphite [62] | 0.2021 | 0.0858 | 0.11 | |||
| a-C:H polymerlike | ECR-CVD | 630–1150 | 0.007 | 0.05–0.08 | 0.4 | 900–2000 |
| a-C:H fullerenelike [63] | 0.012 | 0.06–0.12 | 0.1 | |||
| aCNx/TiN [64] | Pulsed laser deposition | aCNx TiN | ||||
| 25 28 | 0.2636 | 0.0938 | 0.28 | |||
| 33 21 | 0.1924 | 0.0877 | 0.16 | |||
| 42 12 | 0.1799 | 0.0884 | 0.13 | |||
| a-CNx on Ti-TiN/CNx gradient underlayer | Direct Current Magnetron Sputtering | 180 | 500–8500 | |||
| 0.32308 | 0.11042 | 0.123 | ||||
| M1 | 0.30977 | 0.11043 | 0.115 | |||
| M2 | 0.19287 | 0.09471 | 0.109 | |||
| M3 | 0.14146 | 0.08698 | 0.108 | |||
| M4 | ||||||
| a-CNx on Ti interlayer | 0.22401 | 0.09956 | 0.223 | |||
| P1 | 0.15602 | 0.08945 | 0.218 | |||
| P2 | 0.07756 | 0.07097 | 0.205 | |||
| P3 | 0.04585 | 0.06131 | 0.207 | |||
| M4 [65] | ||||||
| DLC:glass | RF-PECVD | 100 | 0.1814 | 0.12 | ||
| DLC:silicon [66] | 0.13 | 0.1 | ||||
2.3. Applied Load Effect
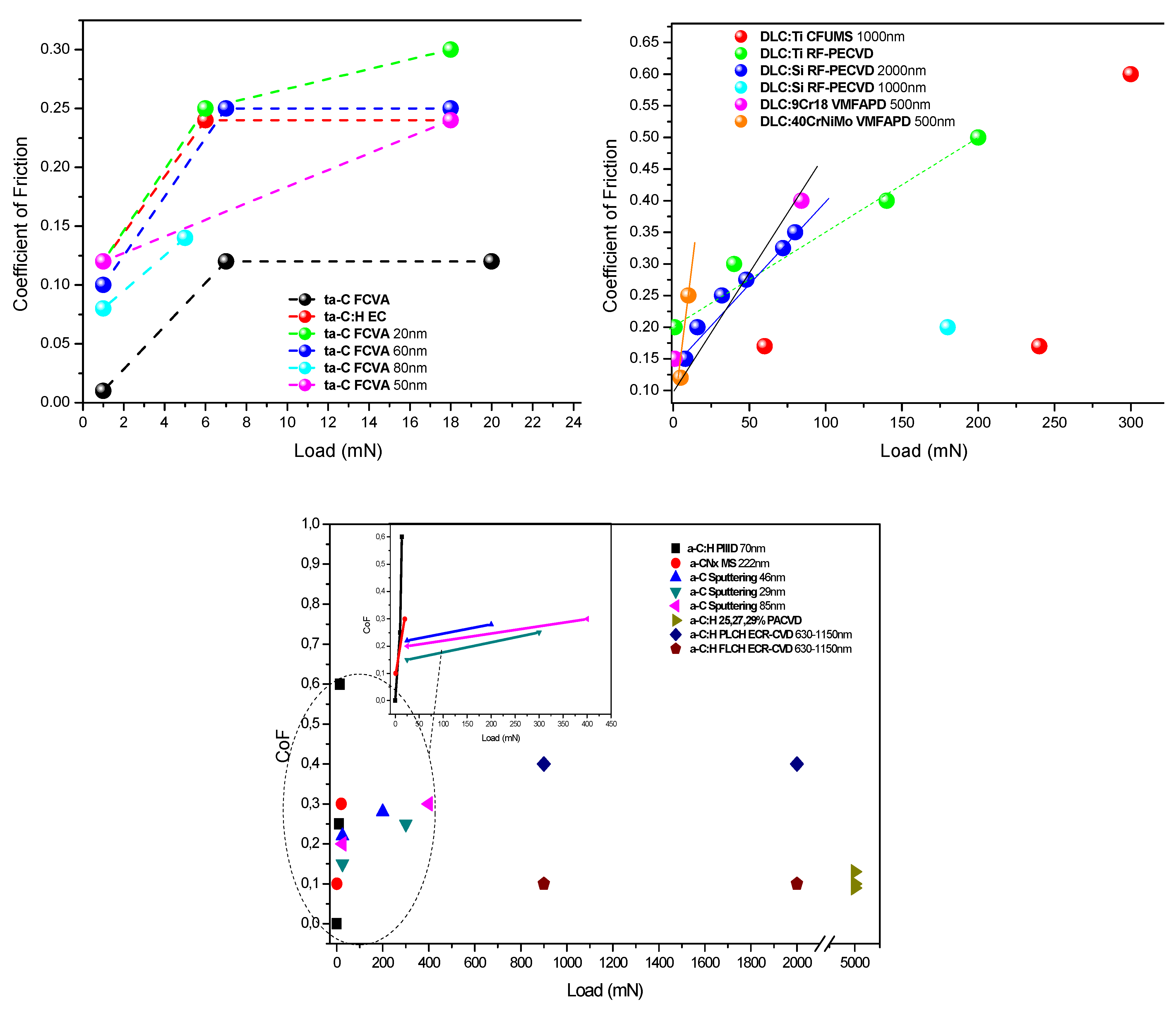
2.4. Orientation Effect in Crystalline Diamond
2.5. Film Thickness Effect
2.6. Film Roughness Effect—Lubricant Use
2.7. Graphite—Superlubricity Effect
2.8. Effect of Environment
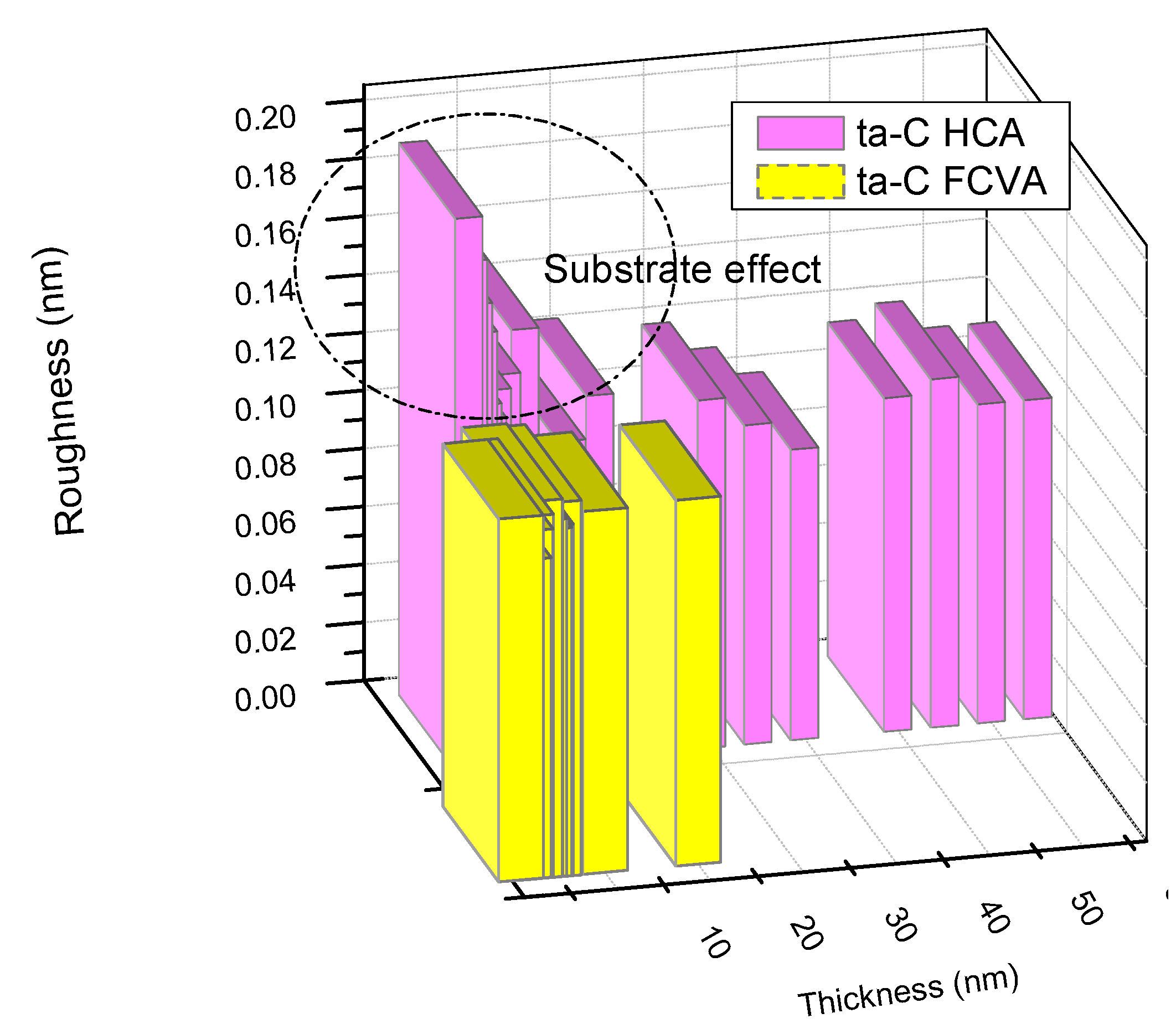
2.9. Effect of Substrate Material, Thickness and Roughness
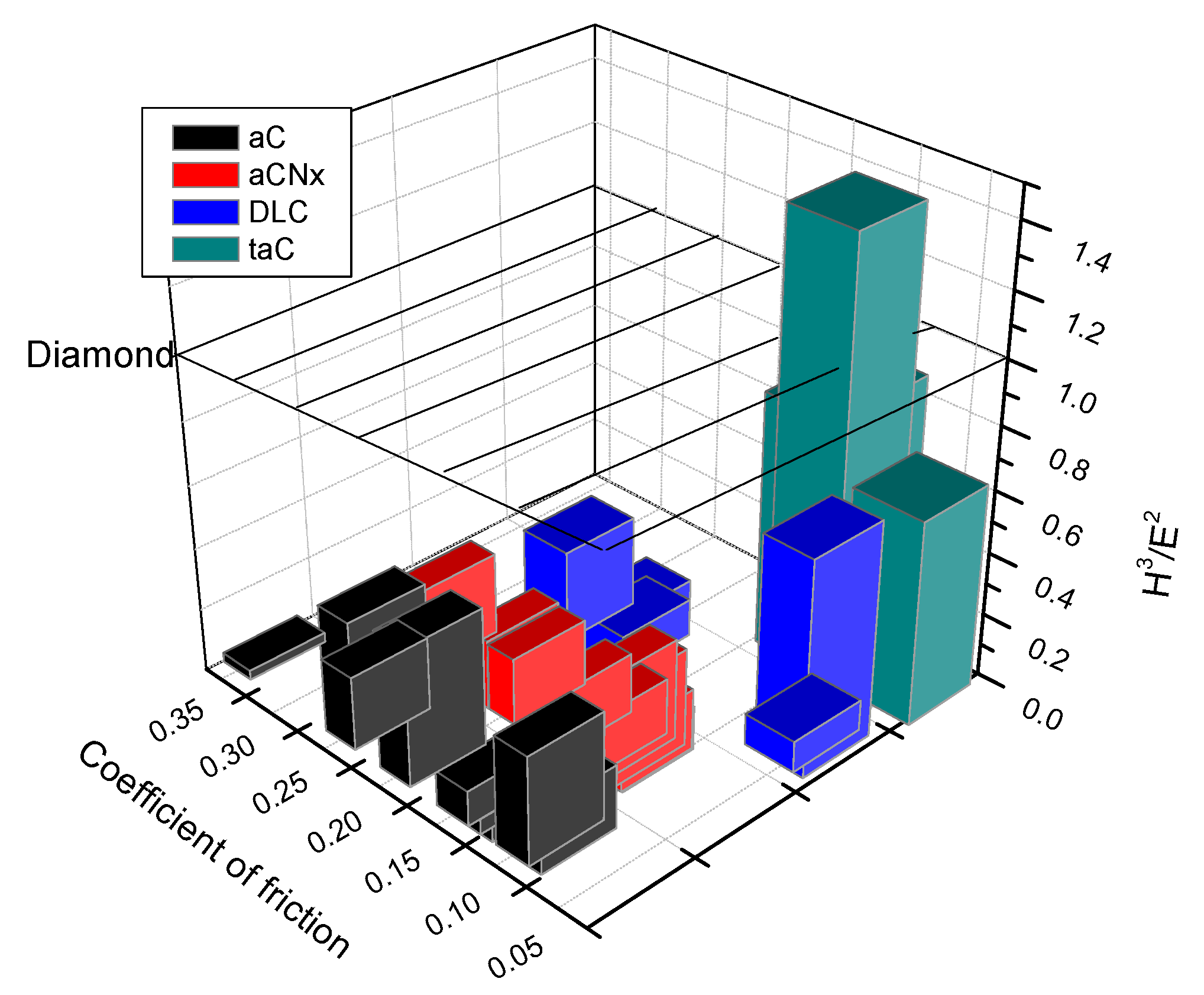
2.10. Tribological Properties: The Significance of Ratio H/E

2.11. Nanoscale Contact Mechanisms Using Atomistic Simulations
3. Conclusions
| Year | Simulation condition | Used materials | Results |
|---|---|---|---|
| 1999 Cagin et al. [115] | Brenner’s potential | Bare diamond hydrogenated diamond | Dangling bond of surface caused higher friction force |
| 2002 Gao et al. [27] | Brenner’s reactive empirical bond-order potential (REBO) | Hydrogen-terminated diamond (111) counterfaces are in sliding contact with diamond (111) surfaces coated with amorphous, hydrogen-free carbon films | Effects of film thickness, adhesion, and long-range interactions |
| 2004 Sulin Zhang et al. [113] | Tersoff-Brenner form | Hydrogenated carbon films (CHx) | Determine how surface hydrogenation affects friction coefficient |
| 2010 Pastewka et al. [114] | Modified Brenner’s reactive empirical bond-order potential (REBO) | Diamond-like carbon (DLC) coatings | Atomistic insights into the running-in, lubrication, and failure of hydrogenated diamond-like carbon coatings |
| 2010 Mylvaganam et al. [110] | Many-body Tersoff-Brenner potential | Carbon-diamond, graphite and carbon nanotube | Nanotubes are the best solid lubricant as it has a low coefficient of friction that can be maintained across any dimensional scales from nano to macro scales due to the large aspect ratio of length to diameter |
| 2012 Bucholz et al. [116] | Brenner’s reactive empirical bond-order potential (REBO) coupled with a Lennard-Jones (LJ) potential | Carbon nano-onions | The ability of the nano-onions to roll is inhibited both by increased contact pressure and the presence of a diamond core within the nanoparticles that enhances the formation of interfacial bonds during friction |
References
- Charitidis, C.A. Nanomechanical and nanotribological properties of carbon-based thin films: A review. Int. J. Refract. Metals Hard Mater. 2010, 28, 51. [Google Scholar] [CrossRef]
- Yoon, E.S.; Singh, R.A.; Oh, H.J.; Kong, H. The effect of contact area on nano/micro-scale friction. Wear 2005, 259, 1424. [Google Scholar] [CrossRef]
- Lafaye, S.; Gauthier, C.; Schirrer, R. Ploughing friction of a conical tip with blunted spherical extremity: Analytic model with elastic recovery. Tribol. Lett. 2006, 21, 95. [Google Scholar] [CrossRef]
- Goddard, J.; Wilman, H. A theory of friction and wear during the abrasion of metals. Wear 1962, 5, 114. [Google Scholar] [CrossRef]
- Müser, M.H. Structural lubricity: Role of dimension and symmetry. Europhys. Lett. 2004, 66, 97. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.E. Nano-scale Friction: A Review. Int. J. Precis. Eng. Manuf. 2009, 10, 141. [Google Scholar]
- Carpick, R.W.; Salmeron, M. Scratching the surface: Fundamental investigations of tribology with atomic force microscopy. Chem. Rev. 1997, 97, 1163. [Google Scholar] [CrossRef]
- Urbakh, M.; Klafter, J.; Gourdon, D.; Israelachvili, J. The nonlinear nature of friction. Nature 2004, 430, 525. [Google Scholar] [CrossRef]
- Bhushan, B.; Israelachvili, J.N.; Landman, U. Nanotribology: Friction, wear and lubrication at the atomic scale. Nature 1995, 374, 607. [Google Scholar] [CrossRef]
- Hölscher, H.; Schirmeisen, A.; Schwarz, U.D. Principles of atomic friction: from sticking atoms to superlubric sliding. Phil. Trans. R. Soc. A 2008, 336, 1383. [Google Scholar]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R: Rep. 2008, 3, 129. [Google Scholar]
- Ferrari, A.C. Tribologyof Diamond-Like Carbon Films; Chapter 2; Donnet, C., Erdemir, A., Eds.; Springer: New York, NY, USA, 2008; p. 25. [Google Scholar]
- Jacob, W.; Moller, W. On the structure of thin hydrocarbon films. Appl. Phys. 1993, 63, 1771. [Google Scholar]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 1999, 8, 428. [Google Scholar] [CrossRef]
- Ronkainen, H.; Varjus, S.; Koskinen, J.; Holmberg, K. Differentiating the tribological performance of hydrogenated and hydrogen-free DLC coatings. Wear 2001, 249, 260. [Google Scholar] [CrossRef]
- Liu, Y.; Erdemir, A.; Meletis, E.I. A study of the wear mechanism of ond-like carbon, films. Surf. Coat. Technol. 1996, 82, 48. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications; Imperial College Press: London, UK, 2004; pp. 391–392. [Google Scholar]
- Kim, S.H.; Asay, D.B.; Dugger, M.T. Nanotribology and MEMS. Nanotoday 2007, 2, 22. [Google Scholar]
- Bhushan, B. Micro/nanotribology and its applications to magnetic storage devices and MEMS. Tribol. Int. 1995, 28, 85. [Google Scholar] [CrossRef]
- Kim, D.; Cao, D.; Bryant, M.D.; Meng, W.J.; Ling, F.F. Tribological study of microbearings for MEMS applications. Tribology 2005, 127, 537. [Google Scholar] [CrossRef]
- Achanta, S.; Drees, D.; Celis, J.-P. Friction and nanowear of hard coatings in reciprocating sliding at milli-Newtons loads. Wear 2005, 259, 719. [Google Scholar] [CrossRef]
- Schonherr, H.; Vancsob, G.J. Molecular resolution imaging and friction anisotropy of highly oriented polyethylene and poly(tetrafluoroethylene) by scanning force microscopy with chemically modified probes. Macromolecules 1997, 30, 6391. [Google Scholar] [CrossRef]
- Wei, B.; Komvopoulos, K. Nanoscale indentation hardness and wear characterization of hydrogenated carbon thin films. ASME J. Tribol. 1996, 118, 431. [Google Scholar] [CrossRef]
- Bhushan, B.; Dandavate, C. Thin-film friction and adhesion studies using atomic force microscopy. J. Appl. Phys. 2000, 87, 1201. [Google Scholar] [CrossRef]
- Deng, H.; Scharf, T.W.; Barnard, J.A. Adhesion assessment of silicon carbide, carbon, and carbon nitride ultrathin overcoats by nanoscratch techniques. J. Appl. Phys. 1997, 81, 5396. [Google Scholar] [CrossRef]
- Charitidis, C.; Logothetidis, S.; Gioti, M. A comparative study of the nanoscratching behavior of amorphous carbon films grown under various deposition conditions. Surf. Coat. Technol. 2000, 125, 201. [Google Scholar] [CrossRef]
- Gao, G.T.; Mikulski, P.T.; Harrison, J.A. Molecular-scale tribology of amorphous carbon coatings: Effects of film thickness, adhesion, and long-range interactions. J. Am. Chem. Soc. 2002, 124, 7202–7209. [Google Scholar] [CrossRef]
- Wong, C.H. Friction at Nanoscale. J. Appl. Mech. Eng. 2012, 1, 1–2. [Google Scholar]
- Gardos, M.N.; Gabelich, S.A. Atmospheric effects of friction, friction noise and wear with silicon and diamond. Part III. SEM tribometry of polycrystalline diamond in vacuum and hydrogen. Tribol. Lett. 1999, 6, 103–112. [Google Scholar] [CrossRef]
- Grierson, D.S.; Carpick, R.W. Nanotribology of carbon-based materials. Nanotoday 2007, 2, 12–21. [Google Scholar] [CrossRef]
- Fontaine, J.; Le Mogne, T.; Loubet, J.L.; Belin, M. Achieving superlow friction with hydrogenated amorphous carbon: Some key requirements. Thin Solid Films 2005, 482, 99–108. [Google Scholar] [CrossRef]
- Riedo, E.; Chevrier, J.; Comin, F.; Brune, H. Nanotribology of carbon-based thin films: The influence of film structure and surface morphology. Surf. Sci. 2001, 477, 25. [Google Scholar] [CrossRef]
- Kim, H.G.; Ahn, S.H.; Kim, J.G.; Park, S.J.; Lee, K.R. Effect of Si-incorporation on wear—Corrosion properties of diamond-like carbon films. Thin Solid Films 2005, 482, 299–304. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, C.J.; Bogy, D.B.; Bhatia, C.S.; Miyamoto, T. Nanotribological characterization of hydrogenated carbon films by scanning probe microscopy. Thin Solid Films 1995, 258, 75–81. [Google Scholar] [CrossRef]
- Xuan, S.Z.; Bui, L.; Zeng, X.T.; Li, X. Towards high adherent and tough a-C coatings. Thin Solid Films 2005, 482, 138. [Google Scholar] [CrossRef]
- Prioli, R.; Jacobsohn, L.G.; Maia da Costa, M.E.H.; Freire, F.L. Nanotribological properties of amorphous carbon-fluorine films. Tribol. Lett. 2003, 15, 177–180. [Google Scholar] [CrossRef]
- Logothetidis, S.; Charitidis, C.; Patsalas, P. Engineering properties of fully sp3- to sp2-bonded carbon films and their modifications after post-growth ion irradiation. Diam. Relat. Mater. 2002, 11, 1095–1099. [Google Scholar] [CrossRef]
- Lu, W.; Komvopoulos, K.; Patsalas, P.; Charitidis, C.; Gioti, M.; Logothetidis, S. Microstructure and nanomechanical and optical properties of single- and multi-layer carbon films synthesized by radio frequency sputtering. Surf. Coat. Technol. 2003, 168, 12–22. [Google Scholar] [CrossRef]
- Bruno, P.; Cicala, G.; Losacco, A.M.; Decuzzi, P. Mechanical properties of PECVD hydrogenated amorphous carbon coatings via nanoindentation and nanoscratching techniques. Surf. Coat. Technol. 2004, 180–181, 259–264. [Google Scholar] [CrossRef]
- Jahanmir, S.; Deckman, D.E.; Ives, L.K.; Feldman, A.; Farrabaugh, E. Tribological characteristics of synthesized diamond films on silicon carbide. Wear 1989, 133, 73–81. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J.; Beghi, M.G.; Bottani, C.E.; Ferulano, R.; Pastorelli, R. Elastic constants of tetrahedral amorphous carbon films by surface Brillouin scattering. Appl. Phys. Lett. 1999, 75, 1893–1895. [Google Scholar] [CrossRef]
- Bonelli, M.; Ferrari, A.C.; Fioravanti, A.; Li Bassi, A.; Miotello, A.; Ossi, P.M. Structure and mechanical properties of low stress tetrahedral amorphous carbon films prepared by pulsed laser deposition. Eur. Phys. J. B—Condens. Matter Complex Syst. 2002, 25, 269–280. [Google Scholar]
- Ouyang, J.H.; Sasaki, S.; Murakami, T. Properties of titanium containing diamond like carbon coatings. Wear 2009, 266, 96–102. [Google Scholar] [CrossRef]
- Malaczynski, G.W.; Elmoursi, A.A.; Leung, C.H.; Hamdi, A.H.; Campbell, A.B. Improved adhesion of diamondlike coatings using shallow carbon implantation. J. Mater. Res. 2000, 15, 590–592. [Google Scholar] [CrossRef]
- Lemoine, P.; Zhao, J.F.; Quinn, J.P.; McLaughlin, J.A.; Maguire, P. Hardness measurements at shallow depths on ultra-thin amorphous carbon films deposited onto silicon and Al2O3-TiC substrates. Thin Solid Films 2000, 379, 166–172. [Google Scholar] [CrossRef]
- Quinn, J.P.; Lemoine, P.; Maguire, P.; Mc Laughlin, J.A. Ultra-thin tetrahedral amorphous carbon films with strong adhesion, as measured by nanoscratch testing. Diam. Relat. Mater. 2004, 13, 1385–1390. [Google Scholar] [CrossRef]
- Teo, E.H.T.; Chua, D.H.C.; Tay, B.K. Mechanical properties of alternating high-low sp3 content thick non-hydrogenated diamond-like amorphous carbon films. Diam. Relat. Mater. 2007, 16, 1882–1886. [Google Scholar] [CrossRef]
- Xu, M.; Cai, X.; Zhao, J.; Chen, Q.; Chu, P.K. Comparative studies on influence of acetylene to argon flow rate ratios on nano-scratch behavior of a-C:H films produced on steel substrates by plasma immersion ion implantation and deposition. Thin Solid Films 2007, 516, 252–256. [Google Scholar] [CrossRef]
- Huang, L.Y.; Xu, K.W.; Lu, J.; Guelorget, B. Nano-scratching process and fracture mechanism of amorphous carbon films. Wear 2003, 254, 1032–1036. [Google Scholar] [CrossRef]
- Druza, B.; Yevtukhov, Y.; Novotny, V.; Zaritsky, I.; Kanarov, V.; Polyakov, V.; Rukavishnikov, A. Nitrogenated carbon films deposited using filtered cathodic arc. Diam. Relat. Mater. 2000, 9, 668–674. [Google Scholar] [CrossRef]
- Ma, X.-G.; Komvopoulos, K.; Wan, D.; Bogy, D.B.; Kim, Y.-S. Effects of film thickness and contact load on nanotribological properties of sputtered amorphous carbon thin films. Wear 2003, 254, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, T.H.; Huan, Y. Nanoindentation and nanoscratch behaviors of DLC coatings on different steel substrates. Compos. Sci. Technol. 2005, 65, 1409–1413. [Google Scholar] [CrossRef]
- Bandorf, R.; Paulkowski, D.M.; Schiffmann, K.I.; Küster, R.L.A. Tribological improvement of moving microparts by application of thin films and micropatterning. J. Phys.: Condens. Matter 2008, 20. [Google Scholar] [CrossRef]
- Bandorf, R.; Luthje, H.; Schiffmann, K.; Staedler, T.; Wortmann, A. Sub-micron coatings with low friction and wear for micro actuators. Microsyst. Technol. 2002, 8, 51–54. [Google Scholar] [CrossRef]
- Staedler, T.; Schiffmann, K. Correlation of nanomechanical and nanotribological behavior of thin DLC coatings on different substrates. Surf. Sci. 2001, 482, 1125–1129. [Google Scholar] [CrossRef]
- Maia da Costa, M.E.H.; Sanchez, C.M.T.; Jacobsohn, L.G.; Freire, F.L., Jr. Structural, mechanical, and nanoscaletribological properties of nitrogen-incorporated fluorine-carbon films. Thin Solid Films 2005, 482, 109–114. [Google Scholar] [CrossRef]
- Corbella, C.; Polo, M.C.; Oncins, G.; Pascual, E.; Andújar, J.L.; Bertran, E. Time-resolved electrical measurements of a pulsed-dc methane discharge used in diamond-like carbon films. Thin Solid Films 2005, 482, 172–176. [Google Scholar] [CrossRef]
- Wilson, G.M.; Sullivan, J.L. An investigation into the effect of film thickness on nanowear with amorphous carbon-based coatings. Wear 2009, 266, 1039–1043. [Google Scholar] [CrossRef]
- Bolelli, G.; Lusvarghi, L.; Mantini, F.P.; Pitacco, F.; Volz, H. Enhanced tribological properties of PECVD DLC coated thermally sprayed coatings. Surf. Coat. Technol. 2008, 202, 4382–4386. [Google Scholar] [CrossRef]
- Crombez, R.; Mc Minis, J.; Veerasamy, V.S.; Shen, W. Experimental study of mechanical properties and scratch resistance of ultra-thin diamond-like-carbon (DLC) coatings deposited on glass. Tribol. Int. 2011, 44, 55–62. [Google Scholar] [CrossRef]
- Tsotsos, C.; Polychronopoulou, K.; Demas, N.G.; Constantinides, G.; Gravani, S.; Böbel, K.; Baker, M.A.; Polycarpou, A.A.; Rebholz, C. Mechanical and high pressure tribological properties of nanocrystalline Ti(N,C) and amorphous C:H nanocomposite coatings. Diam. Relat. Mater. 2010, 19, 960–963. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.H.; Tu, J.P.; Song, R.G. Microstructure and tribological performance of CNx-TiNx composite films prepared by pulsed laser deposition. Mater. Design 2010, 31, 1716–1719. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Camero, M.; Vázquez, L.; Agulló-Rueda, F.; Gago, R.; Jiménez, I.; Gómez-Aleixandre, C.; Albella, J.M. Tribological study of hydrogenated amorphous carbon films with tailored microstructure and composition produced by bias-enhanced plasma chemical vapour deposition. Diam. Relat. Mater. 2010, 19, 1093–1102. [Google Scholar] [CrossRef]
- Zheng, X.H.; Tu, J.P.; Song, R.G. Fabrication, microstructure and tribological behavior of pulsed laser deposited a-CNx/TiN multilayer films. Surf. Coat. Technol. 2010, 205, 902–908. [Google Scholar] [CrossRef]
- Liu, D.G.; Tu, J.P.; Hong, C.F.; Gu, C.D.; Mai, Y.J.; Chen, R. Improving mechanical properties of a-CNx films by Ti-TiN/CNx gradient multilayer. App. Surf. Sci. 2010, 257, 487–494. [Google Scholar] [CrossRef]
- Borrero-Lopez, O.J.; Hoffman, M.J.; Bendavid, A.; Martin, P.J. Substrate effects on the mechanical properties and contact damage of diamond-like carbon thin films. Diam. Relat. Mater. 2010, 19, 1273–1280. [Google Scholar] [CrossRef]
- Gao, G.; Cannara, R.J.; Carpick, R.W.; Harrison, J.A. Atomic-scale friction on diamond: A comparison of different sliding directions on (001) and (111) surfaces using MD and AFM. Langmuir 2007, 23, 5394–5405. [Google Scholar] [CrossRef]
- Charitidis, C.; Logothetidis, S. Effects of normal load on nanotribological properties of sputtered carbon nitride films. Diam. Relat. Mater. 2005, 14, 98. [Google Scholar] [CrossRef]
- Enomoto, Y.; Tabor, D. The frictional anisotropy of diamond. Proc. R. Soc. Lond. A 1981, 373, 405. [Google Scholar] [CrossRef]
- Germann, G.J.; Cohen, S.R.; Neubauer, G.; McClelland, G.M.; Seki, H. Atomic scale friction of a diamond tip on diamond (100) and (111) surfaces. Appl. Phys. 1993, 73, 163. [Google Scholar] [CrossRef]
- Gogotsi, Y.G.; Kailer, A.; Nickel, K.G. Pressure-induced phase transformations in diamond. J. Appl. Phys. 1998, 84, 1299–1304. [Google Scholar] [CrossRef]
- Lee, E.H.; Hembree, D.M., Jr.; Rao, G.R.; Mansur, L.K. Raman scattering from ion-implanted diamond, graphite, and polymers. Phys. Rev. B 1993, 48, 15540–15551. [Google Scholar]
- Erdemir, A.; Halter, M.; Fenske, G.R.; Zuiker, C.; Csencsits, R.; Krauss, A.R.; Gruen, D.M. Friction and wear mechanisms of smooth diamond films during sliding in air and dry nitrogen. Tribol. Trans. 1997, 40, 667–673. [Google Scholar] [CrossRef]
- Carpinteri, A.; Paggi, M. Size-scale effects on the friction coefficient. Int. J. Solids Struct. 2005, 42, 2901–2910. [Google Scholar] [CrossRef]
- Tomala, A.; Roy, M.; Franek, F. Nanotribology of Mo–Se–C films. Philos. Mag. 2010, 90, 3827–3843. [Google Scholar] [CrossRef]
- Hayward, I.P.; Singer, I.L.; Seitzman, L.E. The effect of roughness on the friction of diamond on CVD diamond coatings. Wear 1991, 157, 215. [Google Scholar] [CrossRef]
- Bull, S.J.; Chalkar, P.R.; Johnston, C.; Moore, V. The effect of roughness on the friction and wear of diamond thin film. Surf. Coat. Technol. 1994, 68, 603–610. [Google Scholar] [CrossRef]
- Schneider, A.; Steinmueller-Nethl, D.; Roy, M.; Franek, F. Enhanced tribological performances of nanocrystalline diamond film. Int. J. Refract. Metals Hard Mater. 2010, 28, 40–50. [Google Scholar] [CrossRef]
- Enachescu, M.; Van Den Oetelaar, R.J.A.; Carpick, R.W.; Ogletree, D.F.; Flipse, C.F.J.; Salmeron, M. Atomic force microscopy study of an ideally hard contact: The diamond (111)/tungsten carbide interface. Phys. Rev. Lett. 1998, 81, 1877–1880. [Google Scholar] [CrossRef]
- Medyanik, S.N.; Liu, W.K.; Sung, I.H.; Carpick, R.W. Predictions and observations of multiple slip modes in atomic-scale friction. Phys. Rev. Lett. 2006, 97, 1361061-4. [Google Scholar]
- Socoliuc, A.; Bennewitz, R.; Gnecco, E.; Meyer, E. Transition from stick-slip to continuous sliding in atomic friction: Entering a new regime of ultralow friction. Phys. Rev. Lett. 2004, 92. [Google Scholar] [CrossRef]
- Krylov, S.Y.; Dijksman, J.A.; Van Loo, W.A.; Frenken, J.W.M. Stick-slip motion in spite of a slippery contact: Do we get what we see in atomic friction? Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef]
- Erdemir, A.; Eryilmaz, O.L.; Fenske, G. Synthesis of diamondlike carbon films with superlow friction and wear properties. J. Vac. Sci. Technol. A: Vac. Surf. Films 2000, 18, 1987–1992. [Google Scholar] [CrossRef]
- Donnet, C.; Belin, M.; Auge, J.C.; Martin, J.M.; Grill, A.; Patel, V. Tribochemistry of diamond-like carbon coatings in various environments. Surf. Coat. Technol. 1994, 68, 626–631. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146, 292–297. [Google Scholar] [CrossRef]
- Donnet, C.; Mogne, T.L.; Ponsonnet, L.; Belin, M.; Grill, A.; Patel, V.; Jahnes, C. The respective role of oxygen and water vapor on the tribology of hydrogenated diamond-like carbon coatings. Tribol. Lett. 1998, 4, 259–265. [Google Scholar] [CrossRef]
- Erdemir, A. Superlubricity and wearless sliding in diamond-like carbon films. Mater. Res. Soc. Symp. Proc. 2002, 697, 391–403. [Google Scholar]
- Erdemir, A.; Eryilmaz, O.L.; Nilufer, I.B.; Fenske, G.R. Synthesis of superlow-friction carbon films from highly hydrogenated methane plasmas. Surf. Coat. Technol. 2000, 133, 448–454. [Google Scholar] [CrossRef]
- Erdemir, A.; Nilufer, I.B.; Eryilmaz, O.L.; Beschliesser, M.; Fenske, G.R. Friction and wear performance of diamond-like carbon films grown in various source gas plasmas. Surf. Coat. Technol. 1999, 120, 589–593. [Google Scholar] [CrossRef]
- Erdemir, A.; Eryilmaz, O.L.; Nilufer, I.B.; Fenske, G.R. Effect of source gas chemistry on tribological performance of diamond-like carbon films. Diam. Relat. Mater. 2000, 9, 632–637. [Google Scholar] [CrossRef]
- Donnet, C.; Grill, A. Friction control of diamond-like carbon coatings. Surf. Coat. Technol. 1997, 94, 456–462. [Google Scholar] [CrossRef]
- Donnet, C.; Fontaine, J.; Grill, A.; Le Mogne, T. The role of hydrogen on the friction mechanism of diamond-like carbon films. Tribol. Lett. 2001, 9, 137–142. [Google Scholar] [CrossRef]
- Fontaine, J.; Belin, M.; Le Mogne, T.; Grill, A. How to restore superlow friction of DLC: The healing effect of hydrogen gas. Tribol. Int. 2004, 37, 869–877. [Google Scholar] [CrossRef]
- Erdemir, A.; Donnet, C. Tribology of diamond-like carbon films: Recent progress and future prospects. J. Phys. D: Appl. Phys. 2006, 39, R311. [Google Scholar] [CrossRef]
- Uchidate, M.; Liu, H.; Iwabuchi, A.; Yamamoto, K. Effects of water environment on tribological properties of DLC rubbed against brass. Wear 2009, 267, 1589–1594. [Google Scholar] [CrossRef]
- Sundararajan, S.; Bhushan, B. Micro/Nanotribology of ultra-thin hard amorphous carbon coatings using atomic force/friction force microscopy. Wear 1999, 225, 678–689. [Google Scholar] [CrossRef]
- Lu, W.; Komvopoulos, K. Nanomechanical and nanotribological properties of carbon, chromium, and titanium carbide ultrathin films. J. Tribol. 2001, 123, 717–724. [Google Scholar] [CrossRef]
- Beake, B.D.; Lau, S.P. Nanotribological and nanomechanical properties of 5–80 nm tetrahedral amorphous carbon films on silicon. Diam. Relat. Mater. 2005, 14, 1535–1542. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, C.J.; Bogy, D.B.; Bhatia, C.S.; Miyamoto, T. Nanotribological evaluations of hydrogenated carbon films as thin as 5 nm on magnetic rigid disks. IEEE Trans. Magn. 1995, 31, 3015–3017. [Google Scholar] [CrossRef]
- Singh, R.K.; Xie, Z.H.; Bendavid, A.; Martin, P.J.; Munroe, P.; Hoffman, M. Effect of substrate roughness on the contact damage of DLC coatings. Diam. Relat. Mater. 2008, 17, 975–979. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1985; p. 155. [Google Scholar]
- Charitidis, C.; Logothetidis, S.; Douka, P. Nanoindentation and nanoscratching studies of amorphous carbon films. Diam. Relat. Mater. 1999, 8, 558–562. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite film approach to optimisedtribological behavior. Wear 2000, 246, 1. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. Design criteria for wear-resistant nanostructured and glassy-metal coatings. Surf. Coat. Technol. 2004, 177, 317–324. [Google Scholar] [CrossRef]
- Sham, T.-L.; Tichy, J. A scheme for hybrid molecular dynamics/finite element analysis of thin film lubrication. Wear 1997, 207, 100–106. [Google Scholar] [CrossRef]
- Mo, Y.; Turner, K.T.; Szlufarska, I. Friction laws at the nanoscale. Nature 2009, 457, 1116–1119. [Google Scholar] [CrossRef]
- Johnson, K.L. Adhesion and friction between a smooth elastic spherical asperity and a plane surface. Proc. R. Soc. Lond. Ser. A 1997, 453, 163–179. [Google Scholar] [CrossRef]
- Luan, B.; Robbins, M.O. Contact of single asperities with varying adhesion: Comparing continuum mechanics to atomistic simulations. Phys. Rev. E 2006, 74. [Google Scholar] [CrossRef]
- Brenner, D.W.; Shenderova, O.A.; Harrison, J.A.; Stuart, S.J.; Ni, B.; Sinnott, S.B. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Condens. Matter 2002, 14, 783. [Google Scholar] [CrossRef]
- Mylvaganam, K.; Zhang, L.C. Nano-Friction of some carbon allotropes. J. Comput. Theor. Nanosci. 2010, 7, 2199–2202. [Google Scholar] [CrossRef]
- Mishra, M.; Egberts, P.; Bennewitz, R.; Szlufarska, I. Friction model for single-asperity elastic-plastic contacts. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology, nanomechanics and nanomaterials characterization. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2008, 366, 1351–1381. [Google Scholar] [CrossRef]
- Zhang, S.; Wagner, G.; Medyanik, S.N.; Liu, W.K.; Yu, Y.H.; Chung, Y.W. Experimental and molecular dynamics simulation studies of friction behavior of hydrogenated carbon films. Surf. Coat. Technol. 2004, 177, 818–823. [Google Scholar] [CrossRef]
- Pastewka, L.; Moser, S.; Moseler, M. Atomistic insights into the running-in, lubrication, and failure of hydrogenated diamond-like carbon coatings. Tribol. Lett. 2010, 39, 49–61. [Google Scholar] [CrossRef]
- agin, T.; Che, J.; Gardos, M.N.; Fijany, A.; Goddard, W.A., III. Simulation and experiments on friction and wear of diamond: A material for MEMS and NEMS application. Nanotechnology 1999, 10, 278. [Google Scholar] [CrossRef]
- Bucholz, E.W.; Phillpot, S.R.; Sinnott, S.B. Molecular dynamics investigation of the lubrication mechanism of carbon nano-onions. Comput. Mater. Sci. 2012, 54, 91–96. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Charitidis, C.A.; Koumoulos, E.P.; Dragatogiannis, D.A. Nanotribological Behavior of Carbon Based Thin Films: Friction and Lubricity Mechanisms at the Nanoscale. Lubricants 2013, 1, 22-47. https://doi.org/10.3390/lubricants1020022
Charitidis CA, Koumoulos EP, Dragatogiannis DA. Nanotribological Behavior of Carbon Based Thin Films: Friction and Lubricity Mechanisms at the Nanoscale. Lubricants. 2013; 1(2):22-47. https://doi.org/10.3390/lubricants1020022
Chicago/Turabian StyleCharitidis, Costas A., Elias P. Koumoulos, and Dimitrios A. Dragatogiannis. 2013. "Nanotribological Behavior of Carbon Based Thin Films: Friction and Lubricity Mechanisms at the Nanoscale" Lubricants 1, no. 2: 22-47. https://doi.org/10.3390/lubricants1020022