High-Performance Ni-SiC Coatings Fabricated by Flash Heating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Characterization
3. Results
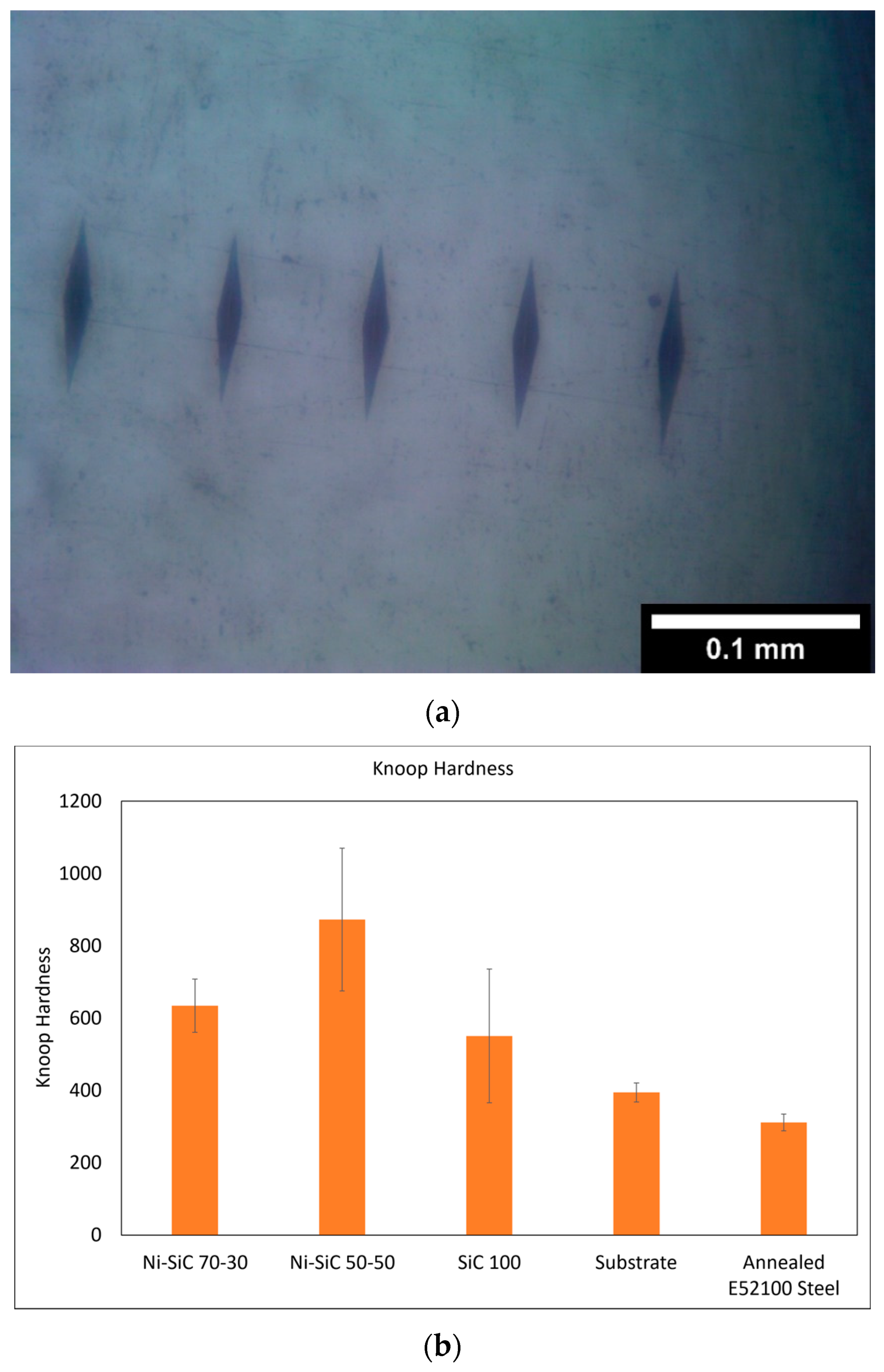
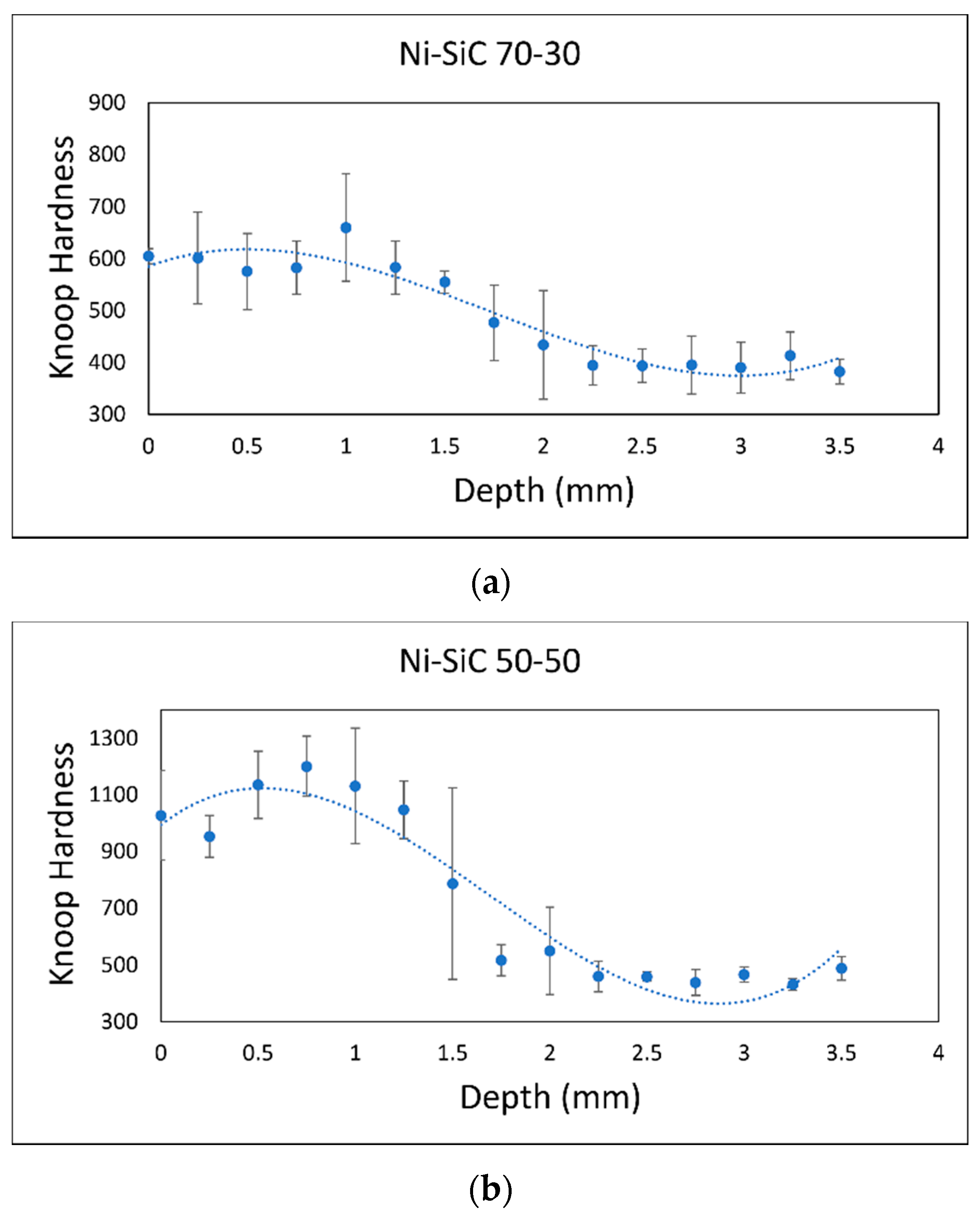
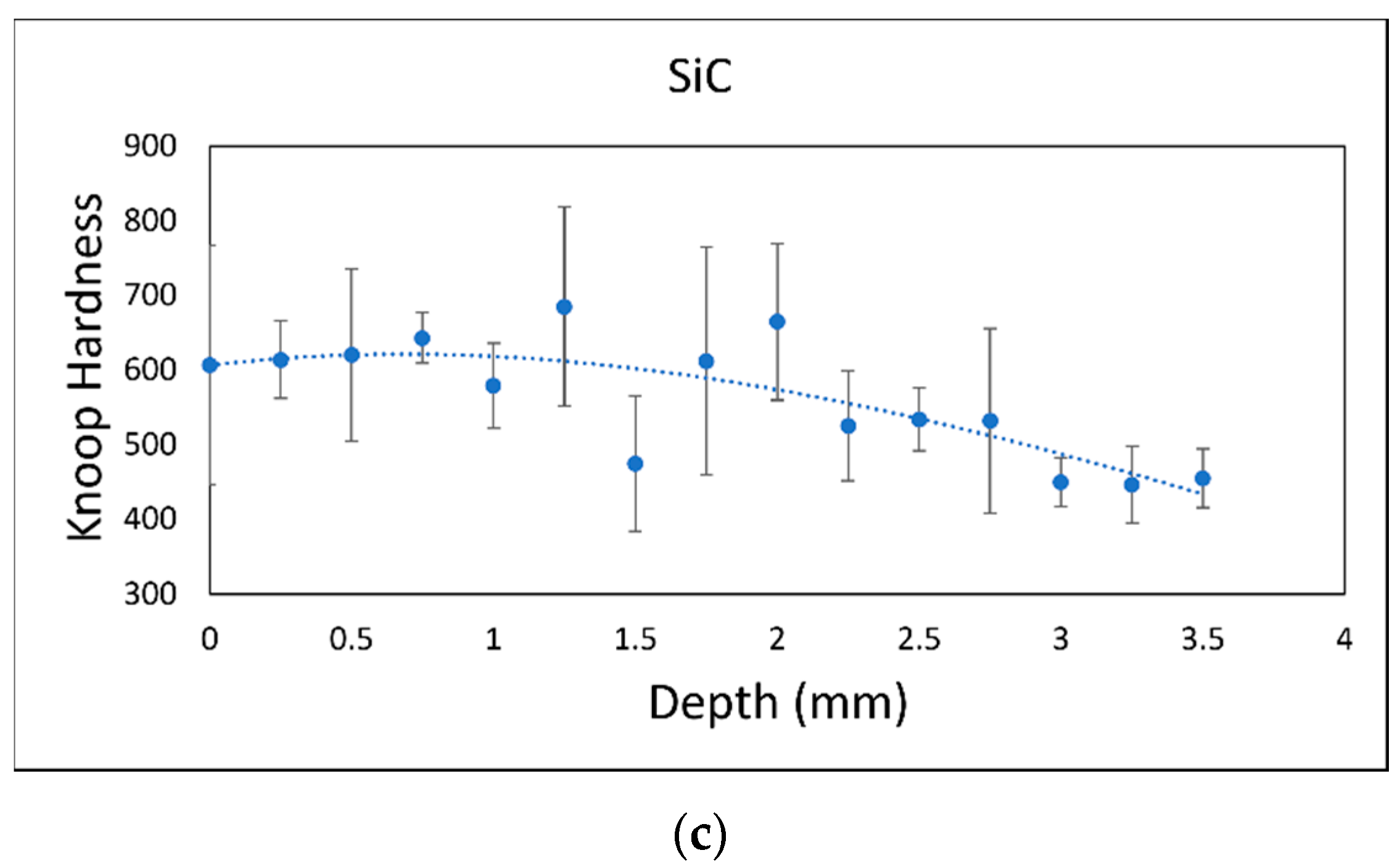
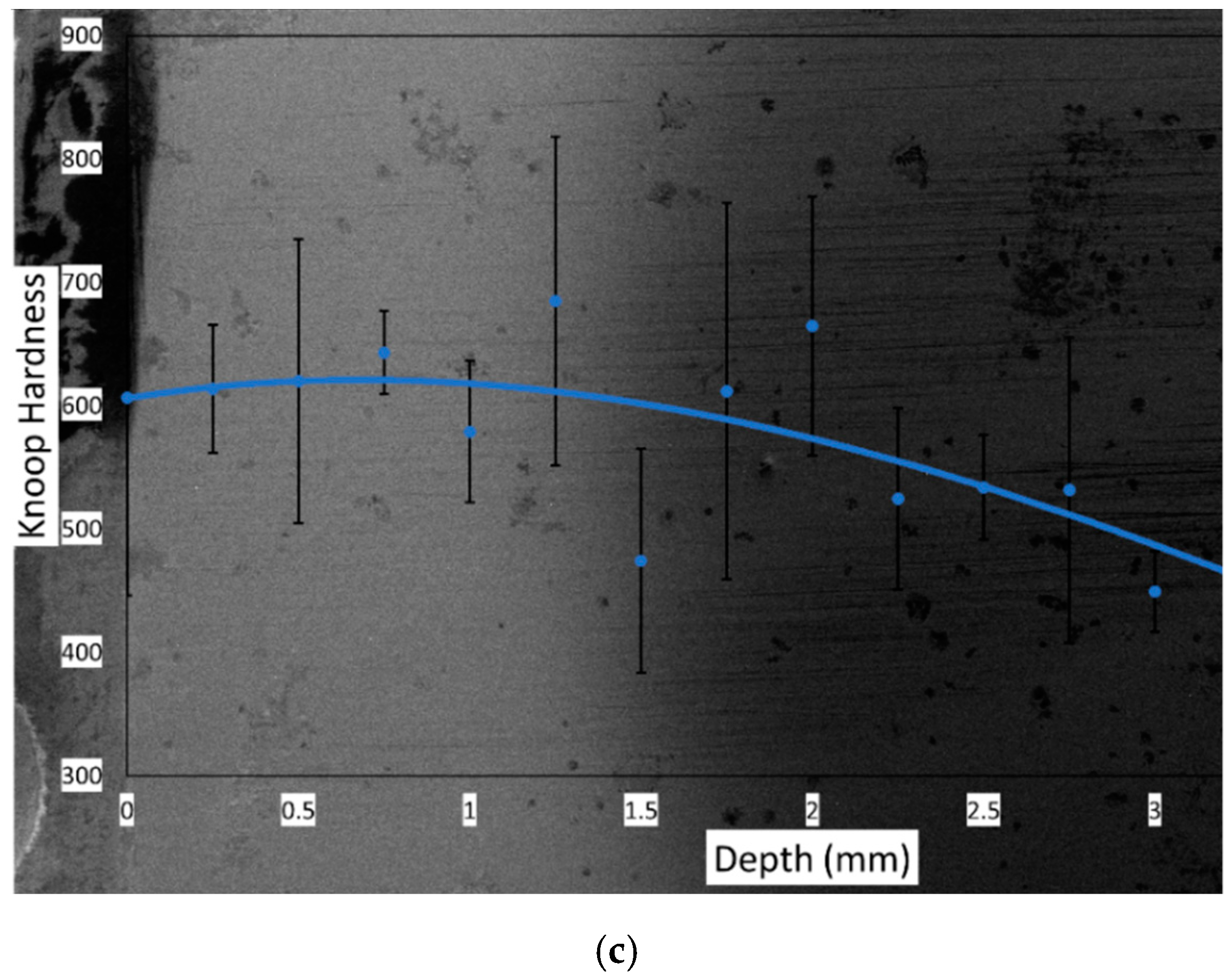
3.1. Increased Hardness Due to High-Hardness Carbides
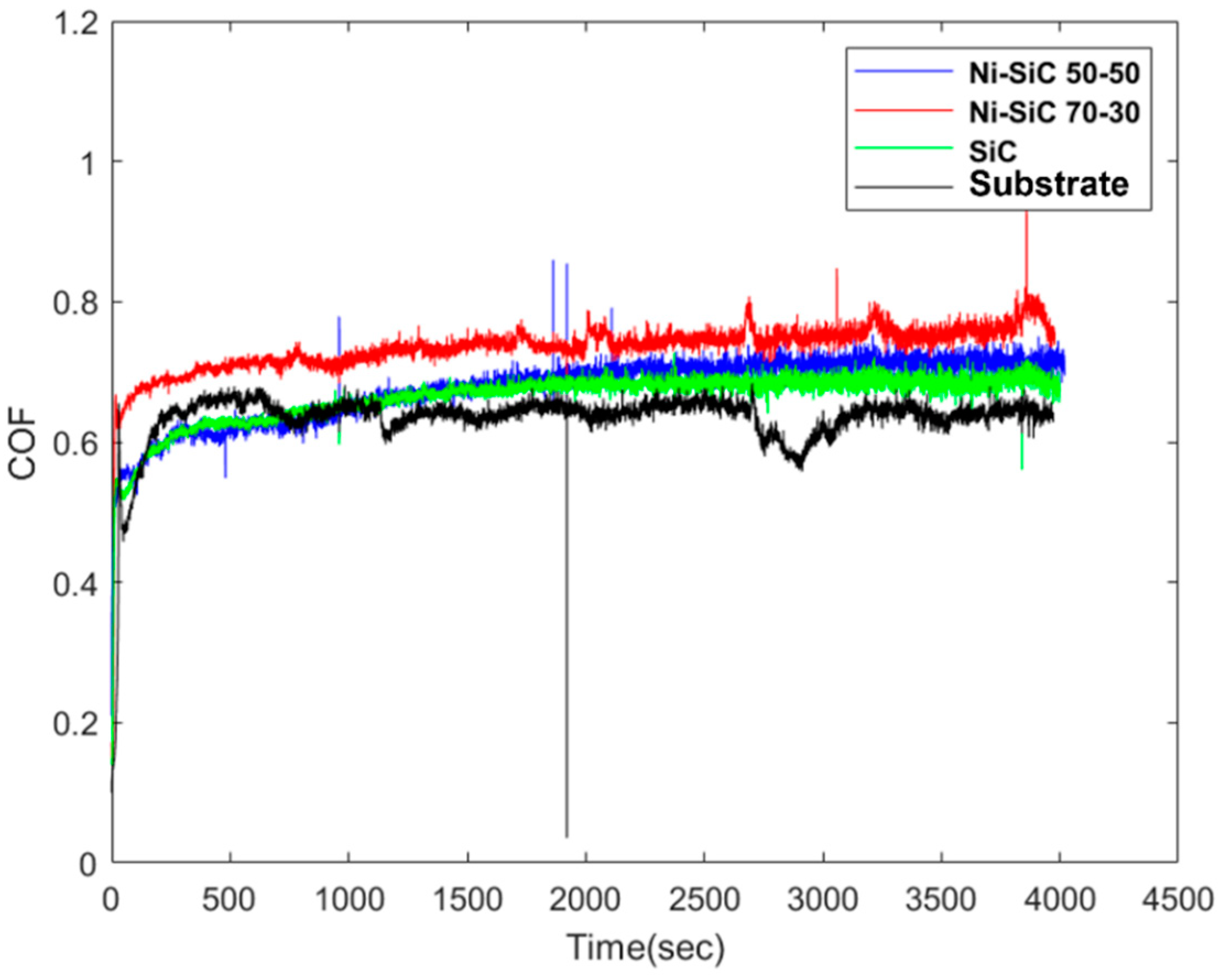
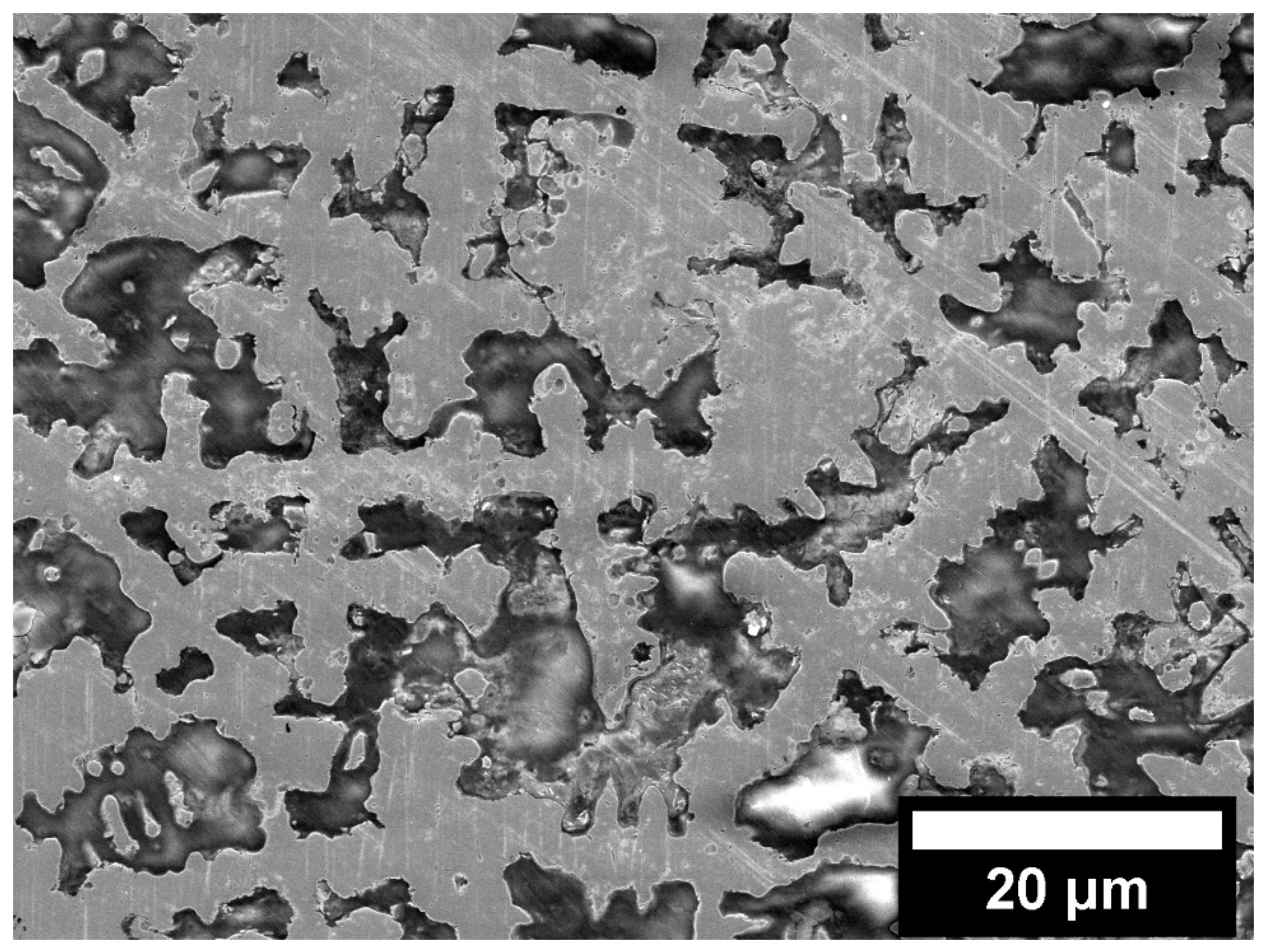
3.2. Wear Performance
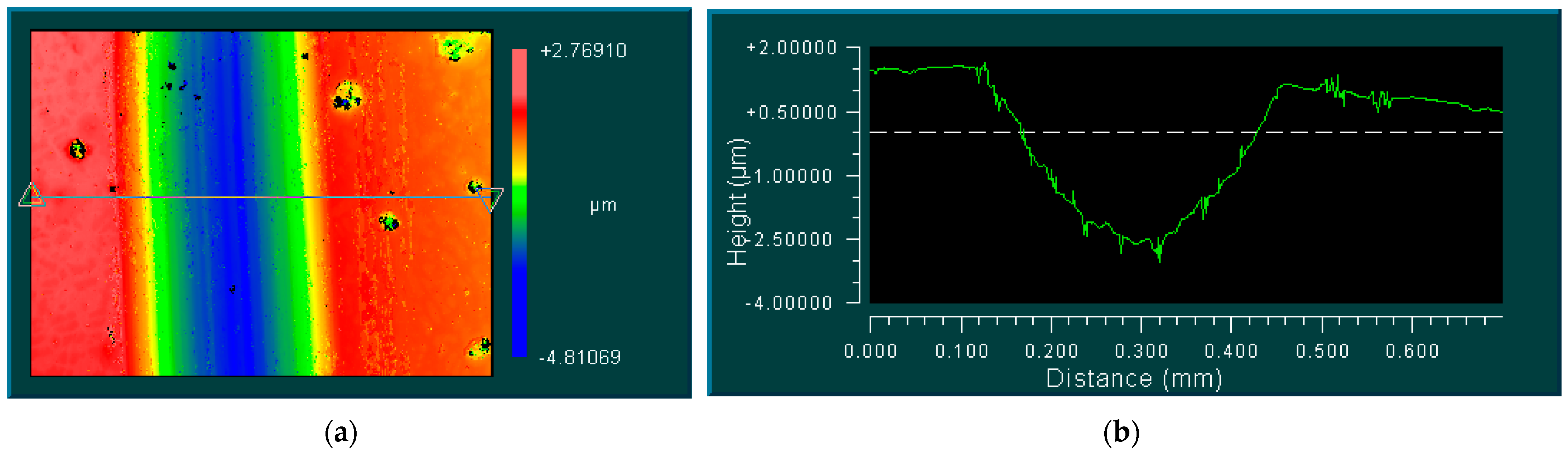
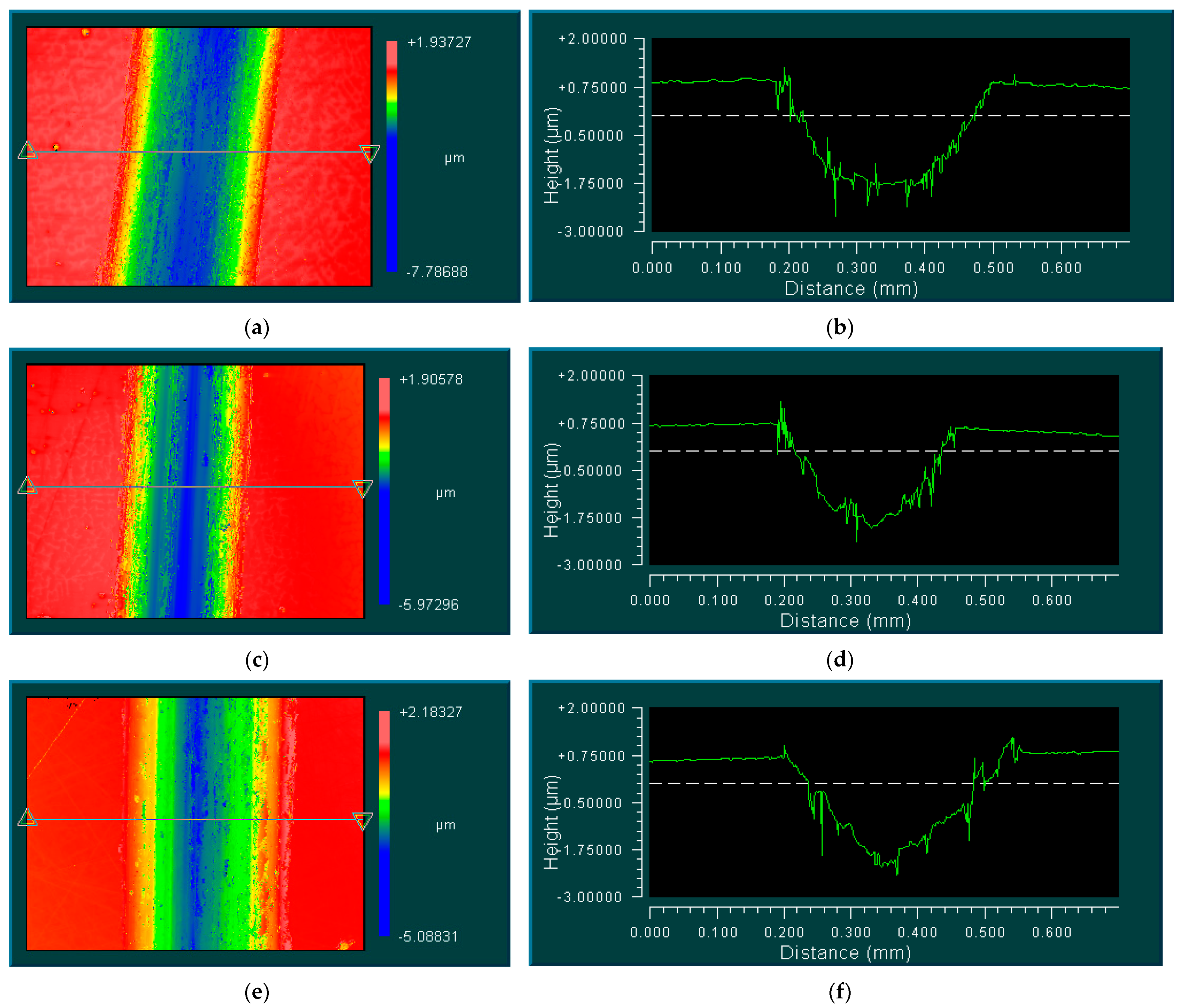
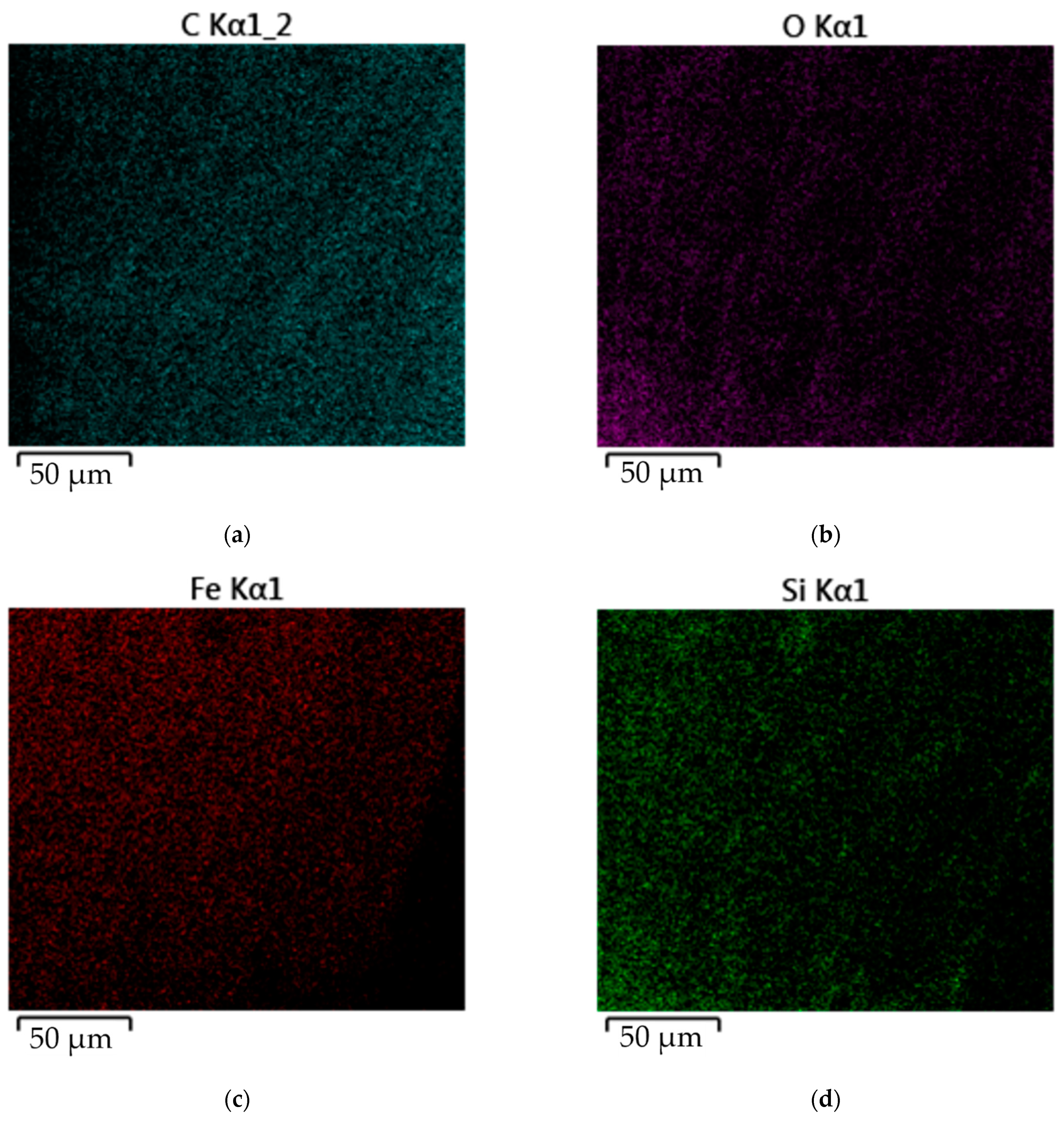
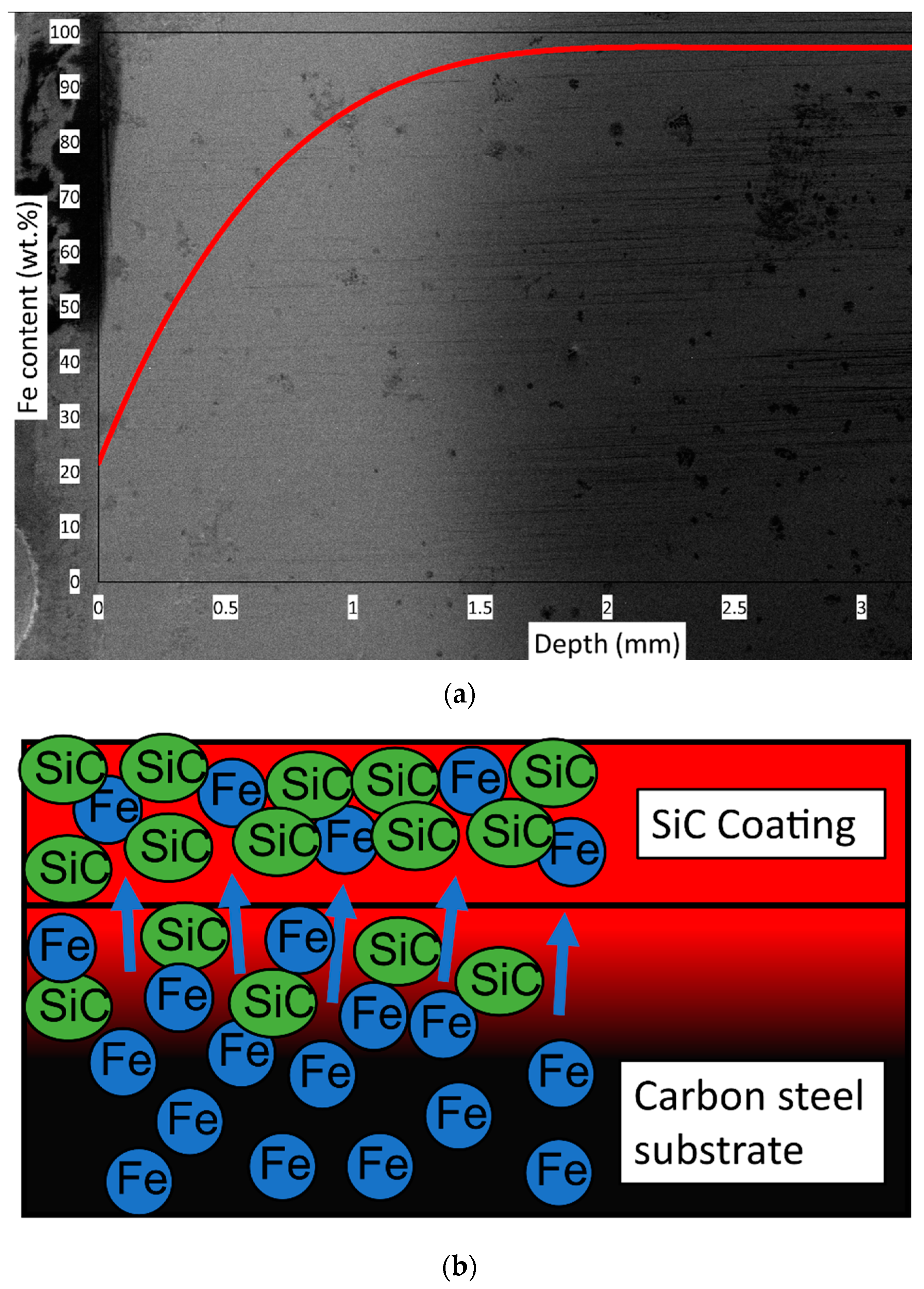
3.3. Mechanisms of Coating Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, I.; Fransaer, J.; Celis, J.-P. Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf. Coat. Technol. 2001, 148, 171–178. [Google Scholar] [CrossRef]
- Hou, K.; Ger, M.; Wang, L.; Ke, S. The wear behaviour of electro-codeposited Ni–SiC composites. Wear 2002, 253, 994–1003. [Google Scholar] [CrossRef]
- Fini, M.H.; Amadeh, A. Improvement of wear and corrosion resistance of AZ91 magnesium alloy by applying Ni–SiC nanocomposite coating via pulse electrodeposition. Trans. Nonferrous Met. Soc. China 2013, 23, 2914–2922. [Google Scholar] [CrossRef]
- Donnet, C.; Grill, A. Friction control of diamond-like carbon coatings. Surf. Coat. Technol. 1997, 94, 456–462. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L.; Sehgal, A.; Lu, D.; McCreery, R.; Frankel, G. Effects of chromate and chromate conversion coatings on corrosion of aluminum alloy 2024-T3. Surf. Coat. Technol. 2001, 140, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Fischer-Cripps, A.; Karvánková, P.; Veprek, S. On the measurement of hardness of super-hard coatings. Surf. Coat. Technol. 2006, 200, 5645–5654. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, X. Ni60-SiC coating prepared by plasma spraying, plasma re-melting and plasma spray welding on surface of hot forging die. Mater. Sci. Ed. 2011, 26, 715–718. [Google Scholar] [CrossRef]
- Fu, Y.; Loh, N.L.; Batchelor, A.W.; Liu, D.; Zhu, X.; He, J.; Xu, K. Improvement in fretting wear and fatigue resistance of Ti–6Al–4V by application of several surface treatments and coatings. Surf. Coat. Technol. 1998, 106, 193–197. [Google Scholar] [CrossRef]
- Schwarzacher, W. Electrodeposition: A technology for the future. Electrochem. Soc. Interface 2006, 15, 32–33. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Wang, M. Composite coatings for implants and tissue engineering scaffolds. In Biomedical Composites; Elsevier: Amsterdam, The Netherlands, 2010; Volume 3, pp. 127–177. [Google Scholar]
- Vaezi, M.; Sadrnezhaad, S.; Nikzad, L. Electrodeposition of Ni–SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Jha, S.; Chen, Y.; Renner, P.; Likhari, R.; Stewart, W.; Gharib, M.; Liang, H. Design of Anti-frictional Ceramic-Based Composite Coatings. J. Mater. Eng. Perform. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Renner, P.; Parkinson, D.; Fang, A.; Liang, H. Synthesis and Morphological Characterization of Electroless-Deposited Ni-P Coatings on Diamond Abrasives. Lubricants 2021, 9, 20. [Google Scholar] [CrossRef]
- He, X.; Xiao, H.; Ozaydin, M.F.; Balzuweit, K.; Liang, H. Low-temperature boriding of high-carbon steel. Surf. Coat. Technol. 2015, 263, 21–26. [Google Scholar] [CrossRef]
- He, X.; Chiu, C.; Esmacher, M.J.; Liang, H. Nanostructured photocatalytic coatings for corrosion protection and surface repair. Surf. Coat. Technol. 2013, 237, 320–327. [Google Scholar] [CrossRef]
- Cardinal, M.F.; Castro, P.; Baxi, J.; Liang, H.; Williams, F.J. Characterization and frictional behavior of nanostructured Ni–W–MoS2 composite coatings. Surf. Coat. Technol. 2009, 204, 85–90. [Google Scholar] [CrossRef]
- Xu, G.H.; Lee, J.H.; Liang, H.; Georing, D. Tribological properties of solid-lubricating coatings on cylinder bore at low temperature. Wear 2004, 257, 59–65. [Google Scholar] [CrossRef]
- Özkan, S.; Hapçı, G.; Orhan, G.; Kazmanlı, K. Electrodeposited Ni/SiC nanocomposite coatings and evaluation of wear and corrosion properties. Surf. Coat. Technol. 2013, 232, 734–741. [Google Scholar] [CrossRef]
- Temam, H.B.; Chala, A.; Rahmane, S. Microhardness and corrosion behavior of Ni–SiC electrodeposited coatings in presence of organic additives. Surf. Coat. Technol. 2011, 205, S161–S164. [Google Scholar] [CrossRef]
- Temam, H.B.; Zeroual, L.; Chala, A.; Rahmane, S.; Nouveau, C. Microhardness and Corrosion Behavior of Ni-SiC Electrodeposited Coatings. Plasma Processes Polym. 2007, 4, S618–S621. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, J.; Chen, S.; Wang, H.; He, Y.; Ma, C. Ni–SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2021, 47, 9437–9446. [Google Scholar] [CrossRef]
- Hu, F.; Chan, K.C.; Song, S.; Yang, X. Enhancement of corrosion resistance of electrocodeposited Ni-SiC composites by magnetic field. J. Solid State Electrochem. 2007, 11, 745–750. [Google Scholar] [CrossRef]
- Ger, M.-D. Electrochemical deposition of nickel/SiC composites in the presence of surfactants. Mater. Chem. Phys. 2004, 87, 67–74. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Takadoum, J.; Bercot, P. Hardness, friction and wear characteristics of nickel-SiC electroless composite deposits. Surf. Coat. Technol. 2001, 137, 92–96. [Google Scholar] [CrossRef]
- Pavlatou, E.; Stroumbouli, M.; Gyftou, P.; Spyrellis, N. Hardening effect induced by incorporation of SiC particles in nickel electrodeposits. J. Appl. Electrochem. 2006, 36, 385–394. [Google Scholar] [CrossRef]
- Balaraju, J.; Narayanan, T.S.; Seshadri, S. Electroless Ni–P composite coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Qian, B. Friction and wear properties of the co-deposited Ni–SiC nanocomposite coating. Appl. Surf. Sci. 2007, 253, 8335–8339. [Google Scholar] [CrossRef]
- Huang, P.-C.; Hou, K.-H.; Hong, J.-J.; Lin, M.-H.; Wang, G.-L. Study of fabrication and wear properties of Ni–SiC composite coatings on A356 aluminum alloy. Wear 2021, 477, 203772. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Qiu, M.; Xu, M.; Tian, Z. Microhardness, wear, and corrosion resistance of Ni–SiC composite coating with magnetic-field-assisted jet electrodeposition. Mater. Res. Express 2018, 5, 096407. [Google Scholar] [CrossRef]
- Li, R.; Chu, Q.; Liang, J. Electrodeposition and characterization of Ni–SiC composite coatings from deep eutectic solvent. RSC Adv. 2015, 5, 44933–44942. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, K.; Li, Q.; Ma, C.; Xia, F. Jet Pulse Electrodeposited Ni-SiC Thin Coatings by Using Experimental System Designed for Potential Industrial Application. Int. J. Electrochem. Sci. 2021, 16, 21044. [Google Scholar] [CrossRef]
- Bratu, F.; Benea, L.; Celis, J.-P. Tribocorrosion behaviour of Ni–SiC composite coatings under lubricated conditions. Surf. Coat. Technol. 2007, 201, 6940–6946. [Google Scholar] [CrossRef]
- Singh, S.K.; Samanta, S.; Das, A.K.; Sahoo, R.R. Electrodeposited SiC-graphene oxide composite in nickel matrix for improved tribological applications. Surf. Topogr. Metrol. Prop. 2019, 7, 035004. [Google Scholar] [CrossRef]
- Renner, P.; Chen, Y.; Huang, Z.; Raut, A.; Liang, H. Tribocorrosion Influenced Pitting of a Duplex Stainless Steel. Lubricants 2021, 9, 52. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Jie, T.; Ling, W.; He, P.-T.; Tao, W. HA coating on titanium with nanotubular anodized TiO2 intermediate layer via electrochemical deposition. Trans. Nonferrous Met. Soc. China 2008, 18, 631–635. [Google Scholar] [CrossRef]
- Wang, J.; Chao, Y.; Wan, Q.; Zhu, Z.; Yu, H. Fluoridated hydroxyapatite coatings on titanium obtained by electrochemical deposition. Acta Biomater. 2009, 5, 1798–1807. [Google Scholar] [CrossRef]
- Xiao, X.F.; Liu, R.F. Effect of suspension stability on electrophoretic deposition of hydroxyapatite coatings. Mater. Lett. 2006, 60, 2627–2632. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Zhang, W.; Huan, Y. Preparation of Ni-W/SiC nanocomposite coatings by electrochemical deposition. J. Alloy. Compd. 2017, 702, 38–50. [Google Scholar] [CrossRef]
- Niu, Z.-X.; Cao, F.; Wei, W.; Zhang, Z.; Zhang, J.; Cao, C.-N. Electrodeposition of Ni-SiC nanocomposite film. Trans. Nonferrous Met. Soc. China 2007, 17, 9–15. [Google Scholar] [CrossRef]
- Gyawali, G.; Hamal, K.; Joshi, B.; Rajbhandari, A.; Lee, S.W. Microstructural and electrochemical analysis of Ni–SiC composite coatings prepared in presence of additives. Mater. Lett. 2014, 126, 228–231. [Google Scholar] [CrossRef]
- Wang, H.; Yao, S.; Matsumura, S. Electrochemical preparation and characterization of Ni/SiC gradient deposit. J. Mater. Processing Technol. 2004, 145, 299–302. [Google Scholar] [CrossRef]
- Baron, A.; Guerrero, J.; González, J.; Gómez, A.; Jurado, A.; Sthepa, H.S. Effect of Ni content on the microstructure, thermal properties, and morphology of Ni-SiC composites produced by mechanical alloying. Ceram. Int. 2017, 43, 2592–2597. [Google Scholar] [CrossRef]
- Abubakar, A.A.; Arif, A.F.M.; Al-Athel, K.S.; Akhtar, S.S.; Mostaghimi, J. Modeling residual stress development in thermal spray coatings: Current status and way forward. J. Therm. Spray Technol. 2017, 26, 1115–1145. [Google Scholar] [CrossRef]
- Abbas, R.A.; Ajeel, S.A.; Bash, M.A.A.; Kadhim, M.J. Effect of plasma spray distance on the features and hardness reliability of YSZ thermal barrier coating. Mater. Today Proc. 2021, 42, 2553–2560. [Google Scholar] [CrossRef]
- Marple, B.; Voyer, J.; Bisson, J.-F.; Moreau, C. Thermal spraying of nanostructured cermet coatings. J. Mater. Processing Technol. 2001, 117, 418–423. [Google Scholar] [CrossRef]
- La Barbera-Sosa, J.; Santana, Y.; Moreno, E.; Cuadrado, N.; Caro, J.; Renault, P.; Le Bourhis, E.; Staia, M.; Puchi-Cabrera, E. Effect of spraying distance on the microstructure and mechanical properties of a Colmonoy 88 alloy deposited by HVOF thermal spraying. Surf. Coat. Technol. 2010, 205, 1799–1806. [Google Scholar] [CrossRef]
- Chang, C. Thermal Spray: International Advances in Coatings Technology; Materials Park—ASM International: Novelty, OH, USA, 1992; p. 79398. [Google Scholar]
- Pierlot, C.; Pawlowski, L.; Bigan, M.; Chagnon, P. Design of experiments in thermal spraying: A review. Surf. Coat. Technol. 2008, 202, 4483–4490. [Google Scholar] [CrossRef]
- Mubarok, F.; Espallargas, N. Tribological behaviour of thermally sprayed silicon carbide coatings. Tribol. Int. 2015, 85, 56–65. [Google Scholar] [CrossRef]
- Draper, C.; Poate, J. Laser surface alloying. Int. Met. Rev. 1985, 30, 85–108. [Google Scholar] [CrossRef]
- Selvan, J.S.; Subramanian, K.; Nath, A.K. Laser surface alloying of (pre-placed) SiC and Ni-SiC coating on commercially pure titanium. Mater. Manuf. Processes 1999, 14, 285–296. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Z.; Yang, G.; Wang, Y. Study of In-situ Synthesis TiCp/Ti Composite Coating on Alloy Ti6Al4 V by TIG Cladding. Procedia Eng. 2012, 36, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Song, G. Interfacial structure of the joints between magnesium alloy and mild steel with nickel as interlayer by hybrid laser-TIG welding. Mater. Des. 2010, 31, 605–609. [Google Scholar] [CrossRef]
- Zhang, G.; Tian, C.; Liu, L.; Xu, R. Interfacial Structure of the Joints between Magnesium Alloy andmild Steel with Nickel as Interlayer. In Proceedings of the International Conference on Logistics Engineering, Management and Computer Science (LEMCS 2014), Shenyang, China, 24–26 May 2014; Atlantis Press: Amsterdam, The Netherlands, 2014; pp. 335–338. [Google Scholar]
- Ghadami, F.; Sohi, M.H.; Ghadami, S. Effect of TIG surface melting on structure and wear properties of air plasma-sprayed WC–Co coatings, Surface and Coatings Technology. In Proceedings of the International Conference on Logistics Engineering, Management and Computer Science (LEMCS 2014), Shenyang, China, 24–26 May 2014; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 108–113. [Google Scholar]
- Dong, T.; Zheng, X.; Li, G.; Wang, H.; Liu, M.; Zhou, X.; Li, Y. Effect of Tungsten Inert Gas Remelting on Microstructure, Interface and Wear Resistance of Fe-Based Coating. J. Eng. Mater. Technol. 2018, 140, 041007. [Google Scholar] [CrossRef]
- Li, J.; Lin, O.; Cheng, C.; Wang, W.; Xu, C.; Ren, L. Fabrication of a Ni/SiC composite coating on steel surface with excellent corrosion inhibition performance. J. Mater. Processing Technol. 2021, 290, 116987. [Google Scholar] [CrossRef]
- Babu, A.; Arora, H.; Singh, R.; Grewal, H. Slurry Erosion Resistance of Microwave Derived Ni-SiC Composite Claddings. Silicon 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Babu, A.; Arora, H.; Singh, H.; Grewal, H. Microwave synthesized composite claddings with enhanced cavitation erosion resistance. Wear 2019, 422, 242–251. [Google Scholar] [CrossRef]
- Ozimina, D.; Madej, M.; Kałdoński, T. The wear resistance of HVOF sprayed composite coatings. Tribol. Lett. 2011, 41, 103–111. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Sun, G.; Xiao, J.; Zhang, L.; Zhang, G. Role of SiC nanoparticles on tribological properties of atmospheric plasma sprayed 5 wt% SiC–Ni60 coatings. Tribol. Int. 2020, 146, 106220. [Google Scholar] [CrossRef]
- Huang, R.-S.; Liu, L.-M.; Song, G. Infrared temperature measurement and interference analysis of magnesium alloys in hybrid laser-TIG welding process. Mater. Sci. Eng. A 2007, 447, 239–243. [Google Scholar] [CrossRef]
- Choo, R.T.C.; Szekely, J.; Westhoff, R.C. On the calculation of the free surface temperature of gas-tungsten-arc weld pools from first principles: Part I. Modeling the welding arc. Metall. Mater. Trans. B 1992, 23, 357–369. [Google Scholar] [CrossRef]
- Choo, R.T.C.; Szekely, J.; David, S.A. On the calculation of the free surface temperature of gas-tungsten-arc weld pools from first principles: Part II. Modeling the weld pool and comparison with experiments. Metall. Mater. Trans. B 1992, 23, 371–384. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zhang, R.; Guo, Q.; Wang, P.; Chen, R.; Zeng, D.; Li, F.; Guo, J.; Li, L. An investigation into the microstructure and tribological properties of rail materials with plasma selective quenching. Tribol. Int. 2020, 146, 106032. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.-W.; Wang, M.; Zhao, Q.-Z. Manipulation of tribological properties of stainless steel by picosecond laser texturing and quenching. Tribol. Int. 2016, 99, 14–22. [Google Scholar] [CrossRef]
- Xia, Y.-Q.; Liu, W.-M.; Xue, Q.-J. Comparative study of the tribological properties of various modified mild steels under boundary lubrication condition. Tribol. Int. 2005, 38, 508–514. [Google Scholar] [CrossRef]
- Liang, H.; Kaufman, F.; Sevilla, R.; Anjur, S. Wear phenomena in chemical mechanical polishing. Wear 1997, 211, 271–279. [Google Scholar] [CrossRef]
- Liang, H.; Xu, G.H. Lubricating behavior in chemical–mechanical polishing of copper. Scr. Mater. 2002, 46, 343–347. [Google Scholar] [CrossRef]
- Chekina, O.G.; Keer, L.M.; Liang, H. Wear-Contact Problems and Modeling of Chemical Mechanical Polishing. J. Electrochem. Soc. 1998, 145, 2100. [Google Scholar] [CrossRef]
- Estragnat, E.; Tang, G.; Liang, H.; Jahanmir, S.; Pei, P.; Martin, J.M. Experimental investigation on mechanisms of silicon chemical mechanical polishing. J. Electron. Mater. 2004, 33, 334–339. [Google Scholar] [CrossRef]
- Knoop, F.; Peters, C.; Emerson, W. A sensitive pyramidal-diamond tool for indentation measurements. J. Res. Natl. Bur. Stand. 1939, 23, 39. [Google Scholar] [CrossRef]
- Ritter, J.E.; Mahoney, F.M.; Jakus, K. A comparison of Vickers and Knoop indentations in soda-lime glass. In Fracture Mechanics of Ceramics; Springer: Berlin/Heidelberg, Germany, 1986; pp. 213–223. [Google Scholar]
- Shahdad, S.A.; McCabe, J.F.; Bull, S.; Rusby, S.; Wassell, R.W. Hardness measured with traditional Vickers and Martens hardness methods. Dent. Mater. 2007, 23, 1079–1085. [Google Scholar] [CrossRef]
- Ferro, D.; Barinov, S.; Rau, J.; Latini, A.; Scandurra, R.; Brunetti, B. Vickers and Knoop hardness of electron beam deposited ZrC and HfC thin films on titanium. Surf. Coat. Technol. 2006, 200, 4701–4707. [Google Scholar] [CrossRef]
- Standard, A. Standard test method for microindentation hardness of materials. ASTM Int. 2017, E384. [Google Scholar]
- Delaunay, B. Sur la sphere vide. Izv. Akad. Nauk. SSSR Otd. Mat. I Estestv. Nauk. 1934, 7, 1–2. [Google Scholar]
- Lee, D.T.; Schachter, B.J. Two algorithms for constructing a Delaunay triangulation. Int. J. Comput. Inf. Sci. 1980, 9, 219–242. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Shewmon, P. Diffusion in Solids; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids; Oxford University Press: Oxford, UK, 1959. [Google Scholar]
- Gyftou, P.; Stroumbouli, M.; Pavlatou, E.; Spyrellis, N. Electrodeposition of Ni/SiC composites by pulse electrolysis. Trans. IMF 2002, 80, 88–91. [Google Scholar] [CrossRef]
- Winchell, H. The Knoop microhardness tester as a mineralogical tool. Am. Mineral. J. Earth Planet. Mater. 1945, 30, 583–595. [Google Scholar]
- Zhang, Z.; Li, X.; Almandoz, E.; Fuentes, G.G.; Dong, H. Sliding friction and wear behaviour of Titanium-Zirconium-Molybdenum (TZM) alloy against Al2O3 and Si3N4 balls under several environments and temperatures. Tribol. Int. 2017, 110, 348–357. [Google Scholar] [CrossRef]
- Živić, F.; Babić, M.; Mitrović, S.; Todorović, P. Interpretation of the friction coefficient during reciprocating sliding of Ti6Al4V alloy against Al2O3. Tribol. Ind. 2011, 33, 36–42. [Google Scholar]
- Parchovianský, M.; Balko, J.; Švančárek, P.; Sedláček, J.; Dusza, J.; Lofaj, F.; Galusek, D. Mechanical properties and sliding wear behaviour of Al2O3-SiC nanocomposites with 3–20 vol% SiC. J. Eur. Ceram. Soc. 2017, 37, 4297–4306. [Google Scholar] [CrossRef]
- Archard, J.F.; Hirst, W. The wear of metals under unlubricated conditions. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1956, 236, 397–410. [Google Scholar]
- Renner, P.; Gharib, M.; Liang, H. Tribological Evaluation of a High-Performance Composite Coating. In Proceedings of the ASME 2021 International Mechanical Engineering Conference and Exhibition, Virtual Online, 1–5 November 2021. [Google Scholar]
- Archard, J. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Adachi, K.; Kato, K.; Chen, N. Wear map of ceramics. Wear 1997, 203, 291–301. [Google Scholar] [CrossRef]
- Vaughn, W.L.; Maahs, H.G. Active-to-passive transition in the oxidation of silicon carbide and silicon nitride in air. J. Am. Ceram. Soc. 1990, 73, 1540–1543. [Google Scholar] [CrossRef]
- Park, D.J.; Jung, Y.I.; Kim, H.G.; Park, J.Y.; Koo, Y.H. Oxidation behavior of silicon carbide at 1200 C in both air and water–vapor-rich environments. Corros. Sci. 2014, 88, 416–422. [Google Scholar] [CrossRef]
- Keys, L. The oxidation of silicon carbide. In Symposium on Properties of High Temperature Alloys with Emphasis on Environmental Effects, Las Vegas; The Electrochemical Society: Princeton, NJ, USA, 1976; Volume 1976. [Google Scholar]
- Gulbransen, E.A.; Andrew, K.F.; Brassart, F.A. The oxidation of silicon carbide at 1150 to 1400 °C and at 9 × 10−3 to 5 × 10−1 torr oxygen pressure. J. Electrochem. Soc. 1966, 113, 1311. [Google Scholar] [CrossRef]
- Sahoo, P.; Wilkins, C.; Yeager, J. Threshold selection using Renyi’s entropy. Pattern Recognit. 1997, 30, 71–84. [Google Scholar] [CrossRef]


| C | Mn | P | S | Si | Cu | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.81 | 0.98 | 0.012 | 0.013 | 0.28 | 0.3 | 0.11 | 0.23 | Balance |
| Coating | Ni (wt%) | SiC (wt%) |
|---|---|---|
| 1 | 70 | 30 |
| 2 | 50 | 50 |
| 3 | 0 | 100 |
| Coating | Ra (µm) | RMS (µm) |
|---|---|---|
| 1 (Ni-SiC 70-30) | 0.063 | 0.080 |
| 2 (Ni-SiC 50-50) | 0.060 | 0.083 |
| 3 (SiC) | 0.067 | 0.097 |
| Substrate | 0.056 | 0.073 |
| Coating | Ni-SiC 70-30 | Ni-SiC 50-50 | SiC | Substrate |
|---|---|---|---|---|
| Wear rate (mm3/N·m) | 2.91 × 10−6 | 1.13 × 10−6 | 4.53 × 10−6 | 5.34 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renner, P.; Raut, A.; Liang, H. High-Performance Ni-SiC Coatings Fabricated by Flash Heating. Lubricants 2022, 10, 42. https://doi.org/10.3390/lubricants10030042
Renner P, Raut A, Liang H. High-Performance Ni-SiC Coatings Fabricated by Flash Heating. Lubricants. 2022; 10(3):42. https://doi.org/10.3390/lubricants10030042
Chicago/Turabian StyleRenner, Peter, Ajinkya Raut, and Hong Liang. 2022. "High-Performance Ni-SiC Coatings Fabricated by Flash Heating" Lubricants 10, no. 3: 42. https://doi.org/10.3390/lubricants10030042





