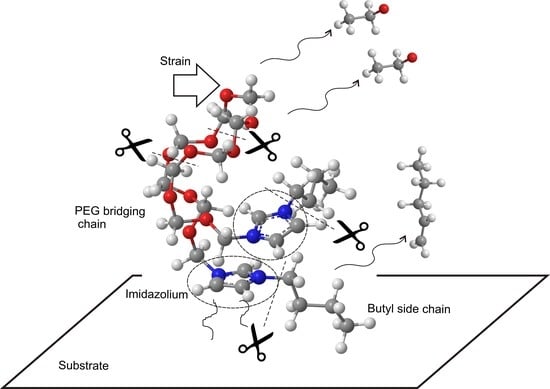
Time-Resolved Characterization of Dynamic Tribochemical Processes for Dicationic Imidazolium Ionic Liquid
Abstract
:1. Introduction
2. Experimental
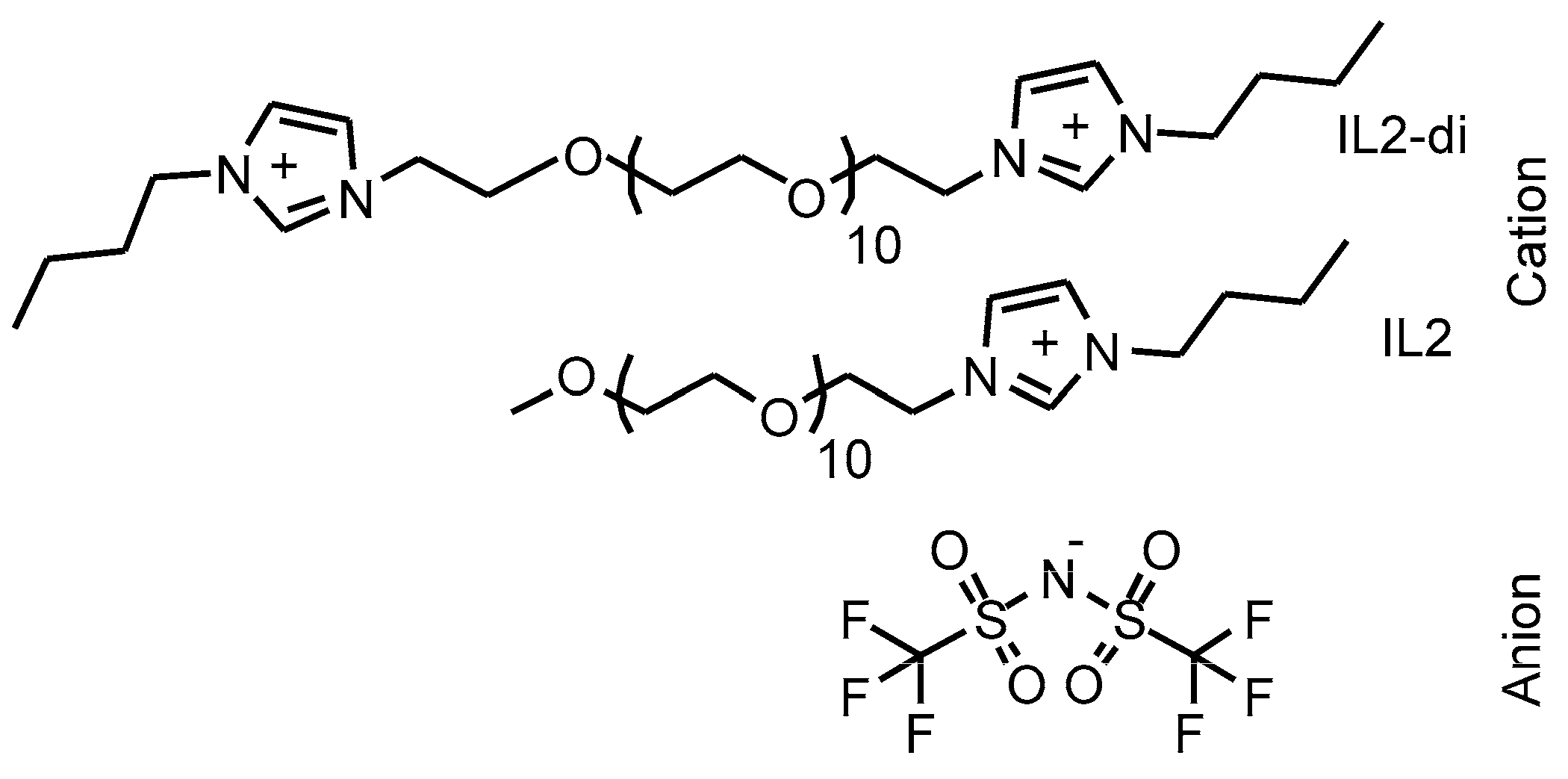
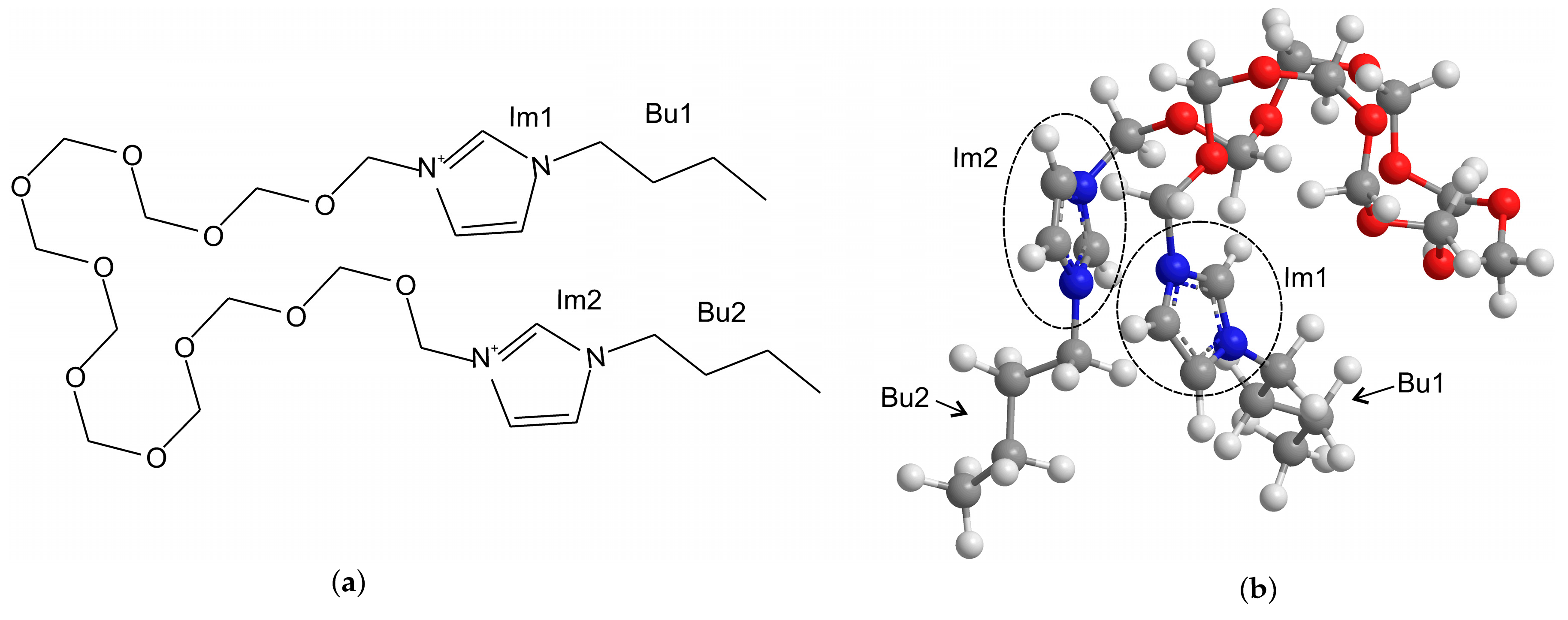
2.1. Materials
2.2. Experimental Techniques and Procedures
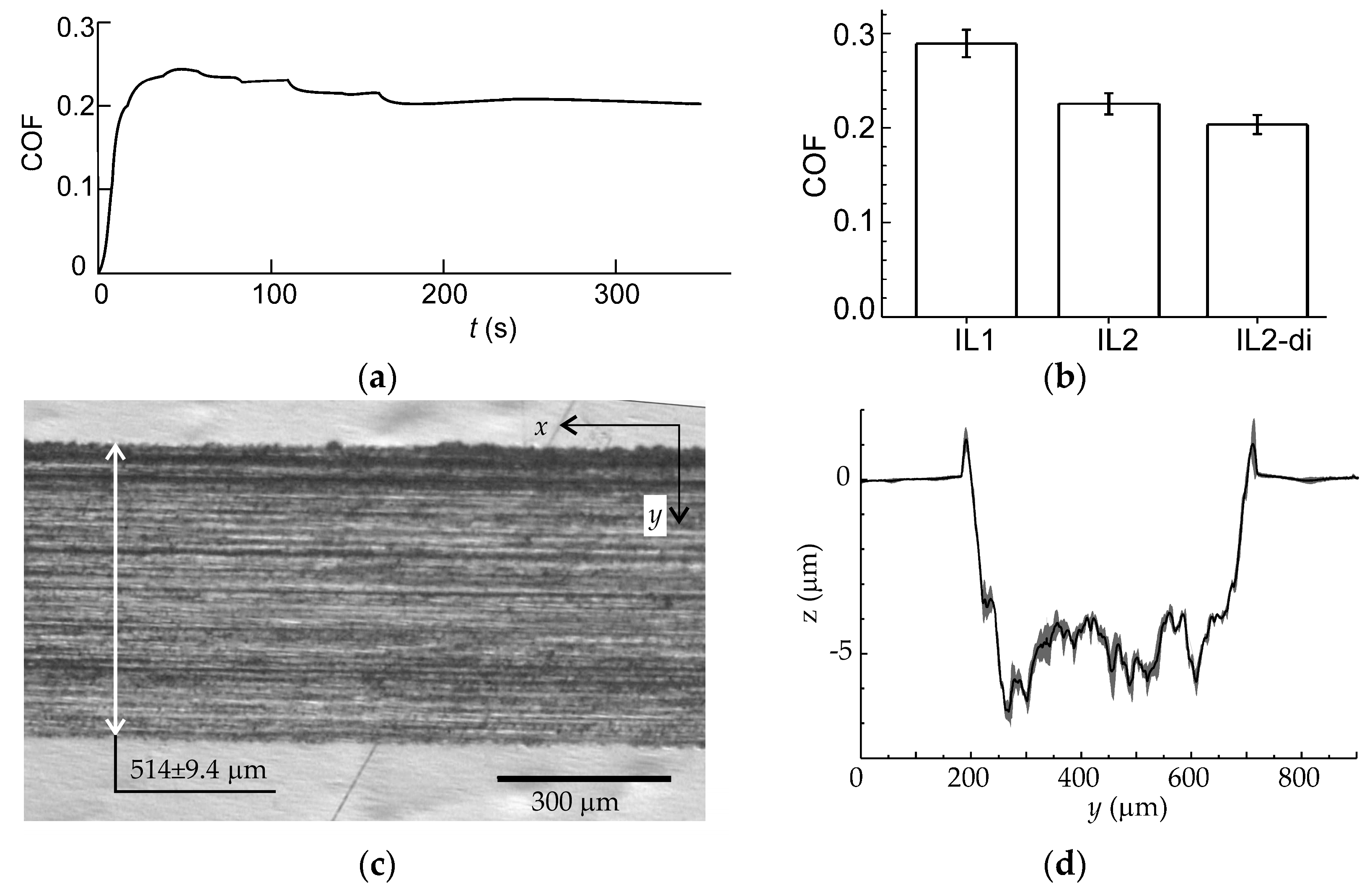
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arias-Pardilla, J.; Espinosa, T.; Bermúdez, M.D. Applications: Ionic liquids in surface protection. In Electrochemistry in Ionic Liquids: Volume 2: Applications; Springer: Berlin, Germany, 2015; pp. 530–561. [Google Scholar]
- Somers, A.; Howlett, P.; MacFarlane, D.; Forsyth, M. A review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Mordukhovich, G.; Qu, J.; Howe, J.Y.; Bair, S.; Yu, B.; Luo, H.; Smolenski, D.J.; Blau, P.J.; Bunting, B.G.; Dai, S. A low-viscosity ionic liquid demonstrating superior lubricating performance from mixed to boundary lubrication. Wear 2013, 301, 740–746. [Google Scholar] [CrossRef]
- Gutierrez, M.; Haselkorn, M.; Iglesias, P. The lubrication ability of ionic liquids as additives for wind turbine gearboxes oils. Lubricants 2016, 4, 14. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-miscible and non-corrosive phosphonium-based ionic liquids as candidate lubricant additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Somers, A.; Yunis, R.; Armand, M.; Pringle, J.; MacFarlane, D.; Forsyth, M. Towards phosphorus free ionic liquid anti-wear lubricant additives. Lubricants 2016, 4, 22. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Ma, Z.; Cai, M.; Liu, W. Probing the lubricating mechanism of oil-soluble ionic liquids additives. Tribol. Int. 2017, 107, 152–162. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Yao, M.; Xia, Y.; Zhou, F.; Liu, W. Imidazolium ionic liquids as antiwear and antioxidant additive in poly(ethylene glycol) for steel/steel contacts. ACS Appl. Mater. Interfaces 2010, 2, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Gabler, C.; Dörr, N.; Allmaier, G. Influence of cationic moieties on the tribolayer constitution shown for bis(trifluoromethylsulfonyl)imide based ionic liquids studied by X-ray photoelectron spectroscopy. Tribol. Int. 2014, 80, 90–97. [Google Scholar] [CrossRef]
- Pisarova, L.; Gabler, C.; Dörr, N.; Pittenauer, E.; Allmaier, G. Thermo-oxidative stability and corrosion properties of ammonium based ionic liquids. Tribol. Int. 2012, 46, 73–83. [Google Scholar] [CrossRef]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Morales, W.; Street, K.W.J.; Rychard, R.M.; Valco, D. Tribological testing and thermal analysis of an alkyl sulfate series of ionic liquids for use as aerospace lubricants. Tribol. Trans. 2012, 55, 815–821. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Qiao, D.; Feng, D.; Wang, H. Vacuum tribological performance of phosphonium-based ionic liquids as lubricants and lubricant additives of multialkylated cyclopentanes. Tribol. Int. 2013, 66, 289–295. [Google Scholar] [CrossRef]
- Jin, C.-M.; Ye, C.; Phillips, B.S.; Zabinski, J.S.; Liu, X.; Liu, W.; Shreeve, J.N.M. Polyethylene glycol functionalized dicationic ionic liquids with alkyl or polyfluoroalkyl substituents as high temperature lubricants. J. Mater. Chem. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Jiménez, A.; Bermúdez, M.-D. Ionic liquids as lubricants of titanium—Steel contact. Tribol. Lett. 2009, 33, 111–126. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel—Aluminium contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Jiménez, A.-E.; Bermúdez, M.-D. Imidazolium ionic liquids as additives of the synthetic ester propylene glycol dioleate in aluminium—Steel lubrication. Wear 2008, 265, 787–798. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-organophosphate ionic liquids as lubricant additives: Effects of cation structure on physicochemical and tribological characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef] [PubMed]
- Pejaković, V.; Tomastik, C.; Dörr, N.; Kalin, M. Influence of concentration and anion alkyl chain length on tribological properties of imidazolium sulfate ionic liquids as additives to glycerol in steel–steel contact lubrication. Tribol. Int. 2016, 97, 234–243. [Google Scholar] [CrossRef]
- Mu, Z.; Zhou, F.; Zhang, S.; Liang, Y.; Liu, W. Effect of the functional groups in ionic liquid molecules on the friction and wear behavior of aluminum alloy in lubricated aluminum-on-steel contact. Tribol. Int. 2005, 38, 725–731. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, Z.; Liang, Y.; Zhou, F.; Liu, W. Alkyl imidazolium ionic liquids as friction reduction and anti-wear additive in polyurea grease for steel/steel contacts. Tribol. Lett. 2010, 40, 215–224. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, D.; Liu, S.; Pan, G.; Lu, X. Film thickness of ionic liquids under high contact pressures as a function of alkyl chain length. Tribol. Lett. 2011, 41, 471–477. [Google Scholar] [CrossRef]
- Fan, M.; Song, Z.; Liang, Y.; Zhou, F.; Liu, W. Laxative inspired ionic liquid lubricants with good detergency and no corrosion. ACS Appl. Mater. Interfaces 2014, 6, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 2244–2245. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium—Steel contact. Part 3. Ti6Al4V lubricated with imidazolium ionic liquids with different alkyl chain lengths. Tribol. Lett. 2010, 40, 237–246. [Google Scholar] [CrossRef]
- Mahrova, M.; Conte, M.; Roman, E.; Nevshupa, R. Critical insight into mechanochemical and thermal degradation of imidazolium-based ionic liquids with alkyl and monomethoxypoly(ethylene glycol) side chains. J. Phys. Chem. C 2014, 118, 22544–22552. [Google Scholar] [CrossRef]
- Li, S.; Feng, G.; Bañuelos, J.L.; Rother, G.; Fulvio, P.F.; Dai, S.; Cummings, P.T. Distinctive nanoscale organization of dicationic versus monocationic ionic liquids. J. Phys. Chem. C 2013, 117, 18251–18257. [Google Scholar] [CrossRef]
- Shirota, H.; Ishida, T. Microscopic aspects in dicationic ionic liquids through the low-frequency spectra by femtosecond Raman-induced KERR effect spectroscopy. J. Phys. Chem. B 2011, 115, 10860–10870. [Google Scholar] [CrossRef] [PubMed]
- Bodo, E.; Chiricotto, M.; Caminiti, R. Structure of geminal imidazolium bis(trifluoromethylsulfonyl)imide dicationic ionic liquids: A theoretical study of the liquid phase. J. Phys. Chem. B 2011, 115, 14341–14347. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.A.; Talebi, M.; Xu, C.; Bhawal, S.S.; Armstrong, D.W. Synthesis of thermally stable geminal dicationic ionic liquids and related ionic compounds: An examination of physicochemical properties by structural modification. Chem. Mater. 2016, 28, 4315–4323. [Google Scholar] [CrossRef]
- Pagano, F.; Gabler, C.; Zare, P.; Mahrova, M.; Dörr, N.; Bayon, R.; Fernandez, X.; Binder, W.; Hernaiz, M.; Tojo, E.; et al. Dicationic ionic liquids as lubricants. J. Eng. Tribol. 2012, 226, 952–964. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. Molecularly thick dicationic ionic liquid films for nanolubrication. J. Vac. Sci. Technol. A 2009, 27, 986–995. [Google Scholar] [CrossRef]
- Czerniak, K.; Walkiewicz, F. Synthesis and antioxidant properties of dicationic ionic liquids. New J. Chem. 2017, 41, 530–539. [Google Scholar] [CrossRef]
- Chang, J.-C.; Ho, W.-Y.; Sun, I.W.; Tung, Y.-L.; Tsui, M.-C.; Wu, T.-Y.; Liang, S.-S. Synthesis and characterization of dicationic ionic liquids that contain both hydrophilic and hydrophobic anions. Tetrahedron 2010, 66, 6150–6155. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Improvement of tribological and anti-corrosive performance of titanium surfaces coated with dicationic imidazolium-based ionic liquids. RSC Adv. 2016, 6, 78795–78802. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Arvina, A.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Muhammad, N. Dicationic imidazolium based ionic liquids: Synthesis and properties. J. Mol. Liq. 2017, 227, 98–105. [Google Scholar] [CrossRef]
- Serva, A.; Migliorati, V.; Lapi, A.; Aquilanti, G.; Arcovito, A.; D’Angelo, P. Structural properties of geminal dicationic ionic liquid/water mixtures: A theoretical and experimental insight. Phys. Chem. Chem. Phys. 2016, 18, 16544–16554. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and properties of high stability geminal dicationic ionic liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between dicationic and monocationic ionic liquids: Liquid density, thermal properties, surface tension, and shear viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Zeng, Z.; Phillips, B.S.; Xiao, J.-C.; Shreeve, J.N.M. Polyfluoroalkyl, polyethylene glycol, 1,4-bismethylenebenzene, or 1,4-bismethylene-2,3,5,6-tetrafluorobenzene bridged functionalized dicationic ionic liquids: Synthesis and properties as high temperature lubricants. Chem. Mater. 2008, 20, 2719–2726. [Google Scholar] [CrossRef]
- Minami, I.; Inada, T.; Sasaki, R.; Nanao, H. Tribo-chemistry of phosphonium-derived ionic liquids. Tribol. Lett. 2010, 40, 225–235. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Short alkyl chain imidazolium ionic liquid additives in lubrication of three aluminium alloys with synthetic ester oil. Tribol. Mater. Surf. Interfaces 2012, 6, 109–115. [Google Scholar] [CrossRef]
- Nevshupa, R.; Conte, M.; del Campo, A.; Roman, E. Analysis of tribochemical decomposition of two imidazolium ionic liquids on Ti–6Al–4V through mechanically stimulated gas emission spectrometry. Tribol. Int. 2016, 102, 19–27. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Zhou, F.; Liu, W. A novel imidazolium salt with antioxidation and anticorrosion dual functionalities as the additive in poly(ethylene glycol) for steel/steel contacts. Wear 2013, 306, 197–208. [Google Scholar] [CrossRef]
- Mahrova, M.; Pagano, F.; Pejakovic, V.; Valea, A.; Kalin, M.; Igartua, A.; Tojo, E. Pyridinium based dicationic ionic liquids as base lubricants or lubricant additives. Tribol. Int. 2015, 82, 245–254. [Google Scholar] [CrossRef]
- Bermúdez, M.-D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Mori, S.; Kobayashi, K.; Nanao, H. Study of tribochemical decomposition of ionic liquids on a nascent steel surface. Appl. Surf. Sci. 2009, 255, 8965–8971. [Google Scholar] [CrossRef]
- Nevchoupa, R.A.; De Segovia, J.L.; Deulin, E.A. An UHV system to study gassing and outgassing of metals under friction. Vacuum 1999, 52, 73–81. [Google Scholar] [CrossRef]
- Nevshupa, R.A.; Conte, M.; Igartua, A.; Roman, E.; de Segovia, J.L. Ultrahigh vacuum system for advanced tribology studies: Design principles and applications. Tribol. Int. 2015, 86, 28–35. [Google Scholar] [CrossRef]
- Okubo, H.; Sasaki, S. In situ raman observation of structural transformation of diamond-like carbon films lubricated with modtc solution: Mechanism of wear acceleration of DLC films lubricated with MoDTC solution. Tribol. Int. 2017, 113, 399–410. [Google Scholar] [CrossRef]
- Merkle, A.P.; Erdemir, A.; Eryilmaz, O.L.; Johnson, J.A.; Marks, L.D. In situ TEM studies of tribo-induced bonding modifications in near-frictionless carbon films. Carbon 2010, 48, 587–591. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Kondo, Y.; Tsuboi, R.; Sasaki, S. Tribochemical reactions of ionic liquids under vacuum conditions. Tribol. Lett. 2014, 54, 309–315. [Google Scholar] [CrossRef]
- Nevshupa, R.; Grinkevich, K.E.; Martinez, I.; Roman, E. Trides—A new tool for the design, development and non-destructive evaluation of advanced construction steels. Mater. Constr. 2016, 66, e099. [Google Scholar] [CrossRef]
- Zare, P.; Mahrova, M.; Tojo, E.; Stojanovic, A.; Binder, W.H. Ethylene glycol-based ionic liquids via azide/alkyne click chemistry. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 190–202. [Google Scholar] [CrossRef]
- Nevshupa, R.A.; Roman, E.; de Segovia, J.L. Origin of hydrogen desorption during friction of stainless steel by alumina in ultrahigh vacuum. J. Vac. Sci. Technol. A 2008, 26, 1218–1223. [Google Scholar] [CrossRef]
- Rusanov, A.; Nevshupa, R.; Fontaine, J.; Martin, J.-M.; Le Mogne, T.; Elinson, V.; Lyamin, A.; Roman, E. Probing the tribochemical degradation of hydrogenated amorphous carbon using mechanically stimulated gas emission spectroscopy. Carbon 2015, 81, 788–799. [Google Scholar] [CrossRef]
- Roman, E.; Conte, M.; Nevshupa, R. Proceedings of Lubmat: Lubrication, Maintenance and Tribology, Bilbao, Spain, 7–8 June 2016; Aranzabe, A., Ed.; IK4-Tekniker: Bilbao, Spain, 2016; p. 620. [Google Scholar]
- Stein, S.E. “Mass spectra” by nist mass spec data center. In NIST Chemistry Webbook NIST, Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Rusanov, A.; Nevshupa, R.; Martin, J.-M.; Garrido, M.Á.; Roman, E. Tribochemistry of hydrogenated amorphous carbon through analysis of mechanically stimulated gas eission. Diam. Relat. Mater. 2015, 55, 32–40. [Google Scholar] [CrossRef]
- Köddermann, T.; Wertz, C.; Heintz, A.; Ludwig, R. Ion-pair formation in the ionic liquid 1-ethyl-3-methylimidazolium bis(triflyl)imide as a function of temperature and concentration. ChemPhysChem 2006, 7, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Kroon, M.C.; Buijs, W.; Peters, C.J.; Witkamp, G.-J. Quantum chemical aided prediction of the thermal decomposition mechanisms and temperatures of ionic liquids. Thermochim. Acta 2007, 465, 40–47. [Google Scholar] [CrossRef]
- Zhang, X.-M. Homolytic bond dissociation enthalpies of the c-h bonds adjacent to radical centers. J. Org. Chem. 1998, 63, 1872–1877. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Non-oxidative thermal degradation of poly(ethylene oxide): Kinetic and thermoanalytical study. J. Anal. Appl. Pyrolysis 2005, 73, 131–138. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177–190. [Google Scholar] [CrossRef]
- Ribas-Arino, J.; Marx, D. Covalent mechanochemistry: Theoretical concepts and computational tools with applications to molecular nanomechanics. Chem. Rev. 2012, 112, 5412–5487. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, Y.; Takahashi, S.; Fujii, T. Thermal analysis of polyethylene glycol: Evolved gas analysis with ion attachment mass spectrometry. Chemosphere 2012, 88, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Keppler, A.; Himmerlich, M.; Ikari, T.; Marschewski, M.; Pachomow, E.; Hofft, O.; Maus-Friedrichs, W.; Endres, F.; Krischok, S. Changes of the near-surface chemical composition of the 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide room temperature ionic liquid under the influence of irradiation. Phys. Chem. Chem. Phys. 2011, 13, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Nainaparampil, J.J.; Phillips, B.S.; Eapen, K.C.; Zabinski, J.S. Micro–nano behaviour of dmbi-pf 6 ionic liquid nanocrystals: Large and small-scale interfaces. Nanotechnology 2005, 16, 2474. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Holderich, W.F. Immobilisation of ionic liquids on solid supports. Green Chem. 2002, 4, 88–93. [Google Scholar] [CrossRef]
- Buslaev, Y.A.; Bochkareva, V.A.; Nikolaev, N.S. The reaction of titanium dioxide with hydrofluoric acid. Russ. Chem. Bull. 1962, 11, 361–364. [Google Scholar] [CrossRef]
- Walton, A.J. Triboluminescence. Adv. Phys. 1977, 26, 887–948. [Google Scholar] [CrossRef]
- Nevshupa, R. The role of athermal mechanisms in the activation of tribodesorption and triboluminisence in miniature and lightly loaded friction units. J. Frict. Wear 2009, 30, 118–126. [Google Scholar] [CrossRef]
- Nevshupa, R.A.; Roman, E.; de Segovia, J.L. Model of the effect of local frictional heating on the tribodesorbed gases from metals in ultra-high vacuum. Int. J. Mater. Prod. Technol. 2010, 38, 57–65. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Gindri, I.M.; Bender, C.R.; Tier, A.Z.; Villetti, M.A.; Rodrigues, D.C.; Machado, G.; Martins, M.A.P. Effect on aggregation behavior of long-chain spacers of dicationic imidazolium-based ionic liquids in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 468, 285–294. [Google Scholar] [CrossRef]


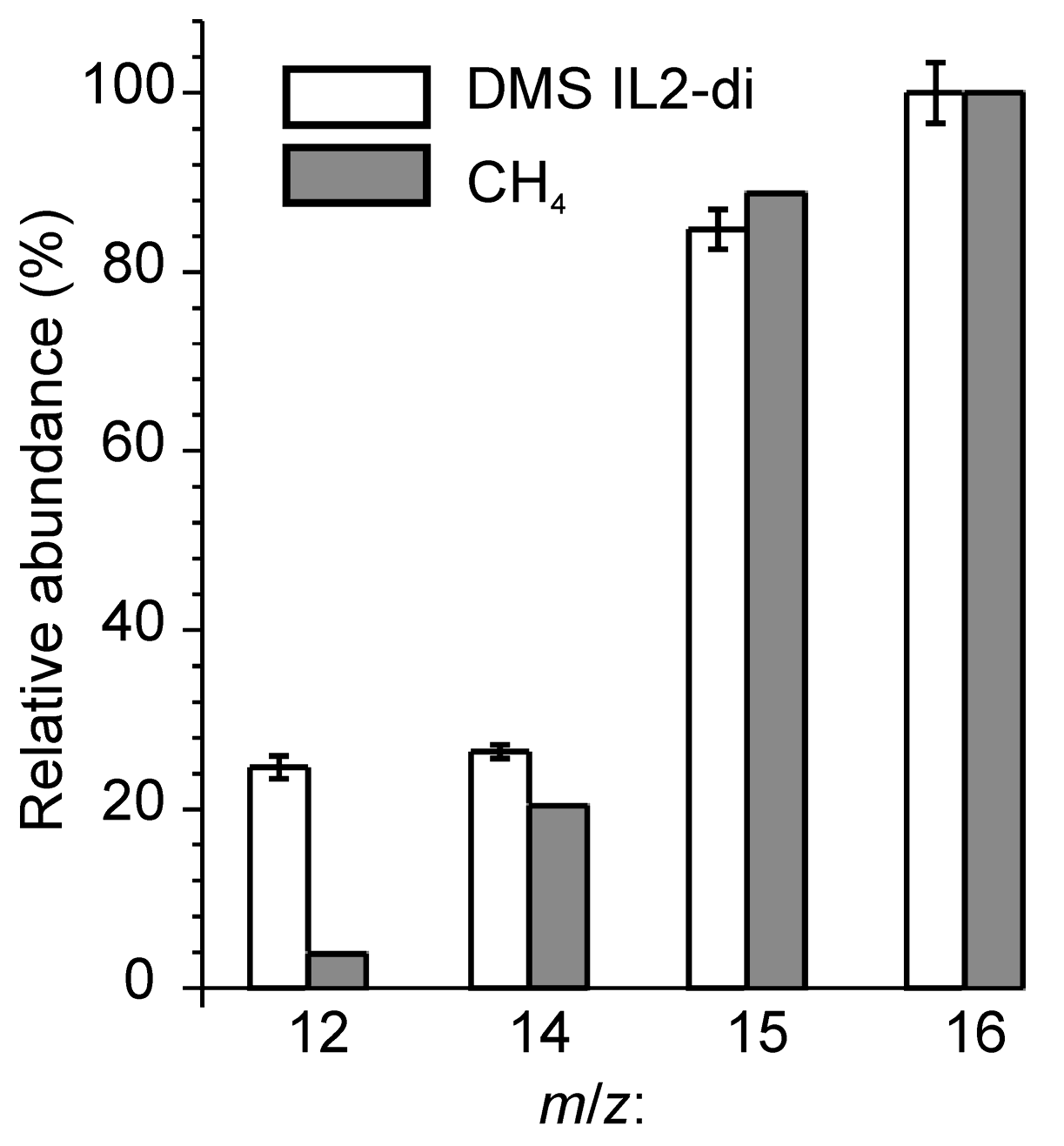

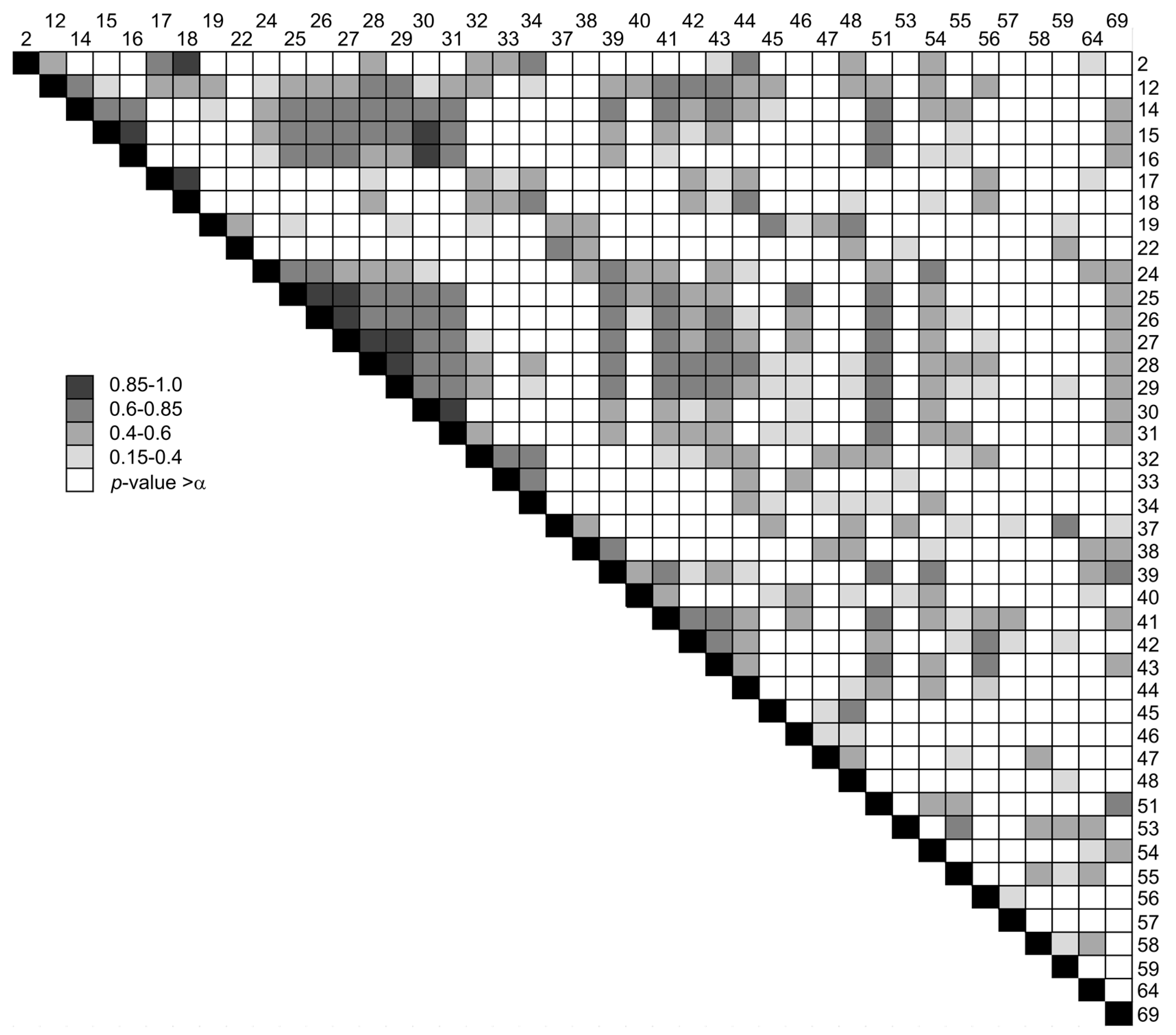
| Group | m/z | Possible Precursors |
|---|---|---|
| A | 19, 31, 32, 51, 69 | CF3, CFxHy 1), PEG fragments (31, 32) |
| B | 12–16 | CH4, CH3*, CH3+ |
| C | 24–34, 39–45, 55–58 | PEG, alkanes |
| D | 2, 17, 18 | H2, H2O |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevshupa, R.; Conte, M.; Guerra, S.; Roman, E. Time-Resolved Characterization of Dynamic Tribochemical Processes for Dicationic Imidazolium Ionic Liquid. Lubricants 2017, 5, 27. https://doi.org/10.3390/lubricants5030027
Nevshupa R, Conte M, Guerra S, Roman E. Time-Resolved Characterization of Dynamic Tribochemical Processes for Dicationic Imidazolium Ionic Liquid. Lubricants. 2017; 5(3):27. https://doi.org/10.3390/lubricants5030027
Chicago/Turabian StyleNevshupa, Roman, Marcello Conte, Silvia Guerra, and Elisa Roman. 2017. "Time-Resolved Characterization of Dynamic Tribochemical Processes for Dicationic Imidazolium Ionic Liquid" Lubricants 5, no. 3: 27. https://doi.org/10.3390/lubricants5030027