Biochemical Effects of Petroselinum crispum (Umbellifereae) Essential Oil on the Pyrethroid Resistant Strains of Aedes aegypti (Diptera: Culicidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of P. crispum Oil
2.2. Chemicals and Reagents
2.3. Mosquito Test Population and Colonization
2.4. Determination of Lethal Threshold Time for the Lethal Effect of P. crispum Oil
2.5. Preparation of Whole Body Homogenates Used for Enzyme Assay
2.6. Total Protein Assay
2.7. Glutathione S-Transferases (GSTs) Assay
2.8. α-/β-Esterases (α-/β-ESTs) Assays
2.9. Acetylcholinesterase (AChE) Assay
2.10. Acid and Alkaline Phosphatases (ACP and ALP) Assays
2.11. Mixed-Function Oxidases (MFO) Assay
2.12. Statistical Analysis
3. Results
3.1. Enzyme Activity Levels in the Three Strains of Ae. aegypti Prior to Treatment (0 h Time Point)
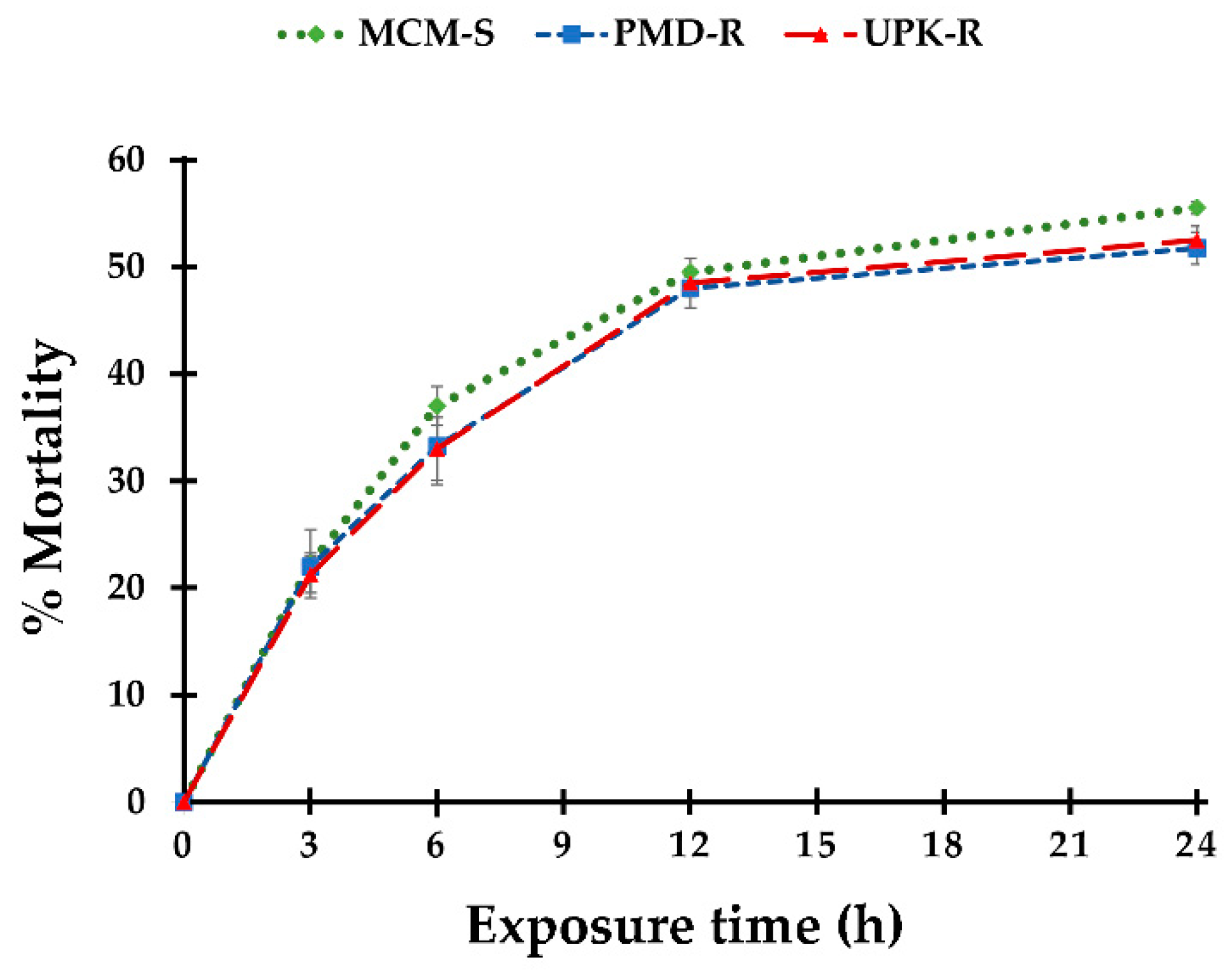
3.2. Threshold Time for the Lethal Effect of P. crispum Oil on Ae. aegypti Larvae
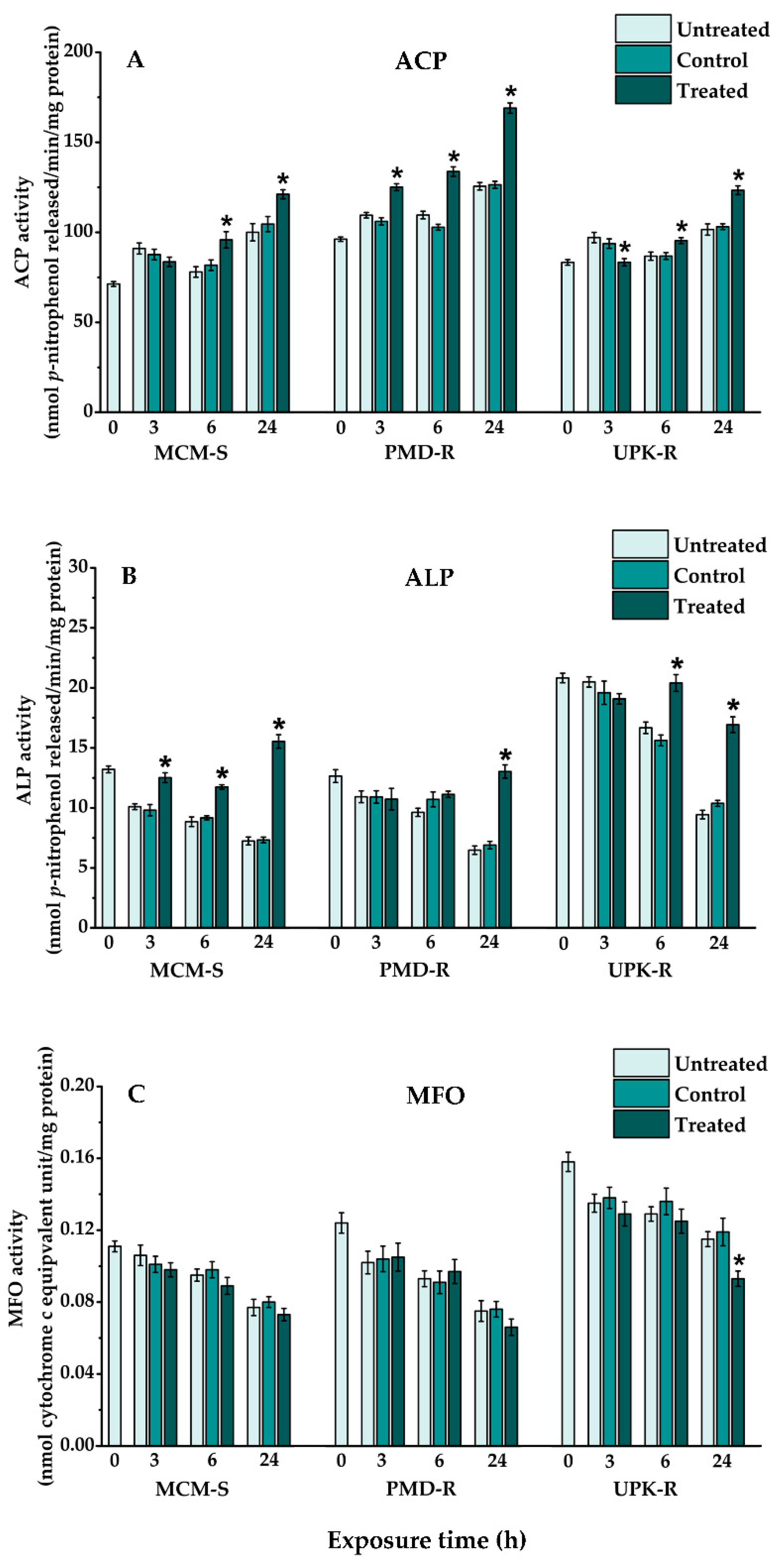
3.3. Effects of P. crispum Oil on Biochemical Features of Ae. aegypti Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knudsen, A.B.; Sloof, R. Vector-borne disease problems in rapid urbanization: NEW approaches to vector control. Bull. World Health Organ. 1992, 70, 1–6. [Google Scholar] [PubMed]
- World Health Organization (WHO). Global Strategy for Dengue Prevention and Control, 2012–2020; WHO Press: Geneva, Switzerland, 2012; ISBN 978-9-241-50403-4. [Google Scholar]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue: GUIDELINES for Diagnosis, Treatment, Prevention and Control; WHO Press: Paris, France, 2009; ISBN 978-9-241-54787-1. [Google Scholar]
- World Health Organization (WHO). Dengue Fact Sheet. 2016. Available online: http://www.searo.who.int/entity/vector_borne_tropical_diseases/data/data_factsheet/en/ (accessed on 5 May 2018).
- World Health Organization (WHO). Dengue and Severe Dengue. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue/ (accessed on 17 May 2018).
- World Health Organization (WHO). Dengue Vaccine Research. 2016. Available online: https://www.who.int/immunization/research/development/dengue_vaccines/en/ (accessed on 17 May 2018).
- Champakaew, D.; Junkum, A.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Sanghong, R.; Intirach, J.; Muangmoon, R.; Chansang, A.; Tuetun, B.; et al. Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol. Res. 2015, 114, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Tetravalent Dengue Vaccine: A review in the prevention of dengue disease. Drugs 2016, 76, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Badolo, A.; Ilboudo-Sanogo, E.; Ouédraogo, A.P.; Costantini, C. Evaluation of the sensitivity of Aedes aegypti and Anopheles gambiae complex mosquitoes to two insect repellents: DEET and KBR 3023. Trop. Med. Int. Health 2004, 9, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Waliwitiya, R.; Kennedy, C.J.; Lowenberger, C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 2008, 65, 241–248. [Google Scholar] [CrossRef]
- Monnerat, R.; Dumas, V.; Ramos, F.; Pimentel, L.; Nunes, A.; Sujii, E.; Praca, L.; Vilarinhos, P. Evaluation of different larvicides for the control of Aedes aegypti (Linnaeus) (Diptera: Culicidae) under simulated field conditions. Bioassay 2012, 7, 3. [Google Scholar] [CrossRef]
- Zaim, M.; Aitio, A.; Nakashima, N. Safety of pyrethroid-treated mosquito nets. Med. Vet. Entomol. 2000, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors 2013, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Grieco, J.P.; Achee, N.L.; Chareonviriyaphap, T. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE 2007, 2, e716. [Google Scholar] [CrossRef]
- Thanispong, K.; Sathantriphop, S.; Chareonviriyaphap, T. Insecticide resistance of Aedes aegypti and Culex quinquefasciatus in Thailand. J. Pestic. Sci. 2008, 33, 351–356. [Google Scholar] [CrossRef]
- Ranson, H.; Burhani, J.; Lumjuan, N.; Black, W.C. Insecticide resistance in dengue vectors. TropIKAnet J. 2010, 1, 1–12. [Google Scholar]
- Karunamoorthi, K.; Sabesan, S. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: A potential threat to global public health. Health Scope 2013, 2, 4–18. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Monitoring and Managing Insecticide Resistance in Aedes aegypti Populations, Interim Guidance for Entomologist. 2016. Available online: http://www.who.int/csr/resources/publications/zika/insecticide-resistance/en/ (accessed on 20 March 2016).
- Chansang, A.; Champakaew, D.; Junkum, A.; Jitpakdi, A.; Amornlerdpison, D.; Aldred, A.K.; Riyong, D.; Wannasan, A.; Intirach, J.; Muangmoon, R.; et al. Synergy in the adulticidal efficacy of essential oils for the improvement of permethrin toxicity against Aedes aegypti L. (Diptera: Culicidae). Parasit. Vectors 2018, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Bloomquist, J.R. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Pitasawat, B.; Champakaew, D.; Choochote, W.; Jitpakdi, A.; Chaithong, U.; Kanjanapothi, D.; Rattanachanpichai, E.; Tippawangkosol, P.; Riyong, D.; Tuetun, B.; et al. Aromatic plant-derived essential oil: AN alternative larvicide for mosquito control. Fitoterapia 2007, 78, 205–210. [Google Scholar] [CrossRef]
- Kweka, E.J.; Nyindo, M.; Mosha, F.; Sila, A.G. Insecticidal activity of the essential oil from fruits and seeds of Schinus terebinthifolia Raddi against African malaria vectors. Parasit. Vectors 2011, 4, 129. [Google Scholar] [CrossRef]
- Champakaew, D.; Choochote, W.; Pongpaibul, Y.; Chaithong, U.; Jitpakdi, A.; Tuetun, B.; Pitasawat, B. Larvicidal efficacy and biological stability of a botanical natural product, zedoary oil-impregnated sand granules, against Aedes aegypti (Diptera, Culicidae). Parasitol. Res. 2007, 100, 729–737. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Nicholson, R.A.; Kennedy, C.J.; Lowenberger, C.A. The synergistic effects of insecticidal essential oils and piperonyl butoxide on biotransformational enzyme activities in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 614–623. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.S.; Ponsankar, A.; Selin-Rani, S.; Chellappandian, M.; Kalaivani, K.; Senthil-Nathan, S.; Benelli, G. Development of an eco-friendly mosquitocidal agent from Alangium salvifolium against the dengue vector Aedes aegypti and its biosafety on the aquatic predator. Environ. Sci. Pollut. Res. Int. 2018, 25, 10340–10352. [Google Scholar] [CrossRef]
- Tehri, K.; Singh, N. The role of botanicals as green pesticides in integrated mosquito management—A review. Int. J. Mosq. Res. 2015, 2, 18–23. [Google Scholar]
- Bekele, D. Review on insecticidal and repellent activity of plant products for malaria mosquito control. Biomed. Res. Rev. 2018. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system-A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Intirach, J.; Junkum, A.; Lumjuan, N.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Wannasan, A.; Champakaew, D.; Muangmoon, R.; Chansang, A.; et al. Antimosquito property of Petroselinum crispum (Umbellifereae) against the pyrethroid resistant and susceptible strains of Aedes aegypti (Diptera: Culicidae). Environ. Sci. Pollut. Res. Int. 2016, 23, 23994–24008. [Google Scholar] [CrossRef]
- Okumu, F.O.; Knols, B.G.J.; Fillinger, U. Larvicidal effects of a neem (Azadirachta indica) oil formulation on the malaria vector Anopheles gambiae. Malar. J. 2007, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Liu, Y.; Wu, X.W.; Hua, R.M.; Wang, G.R.; Cao, H.Q. Insecticidal activity of Melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS ONE 2016, 11, e0167748. [Google Scholar] [CrossRef]
- Brengues, C.; Hawkes, N.J.; Chandre, F.; McCarroll, L.; Duchon, S.; Guillet, P.; Manguin, S.; Morgan, J.C.; Hemingway, J. Pyrethroid and DDT cross-resistance in Aedes aegypti correlated with novel mutations in the voltage-gated sodium channel gene. Med. Vet. Entomol. 2003, 17, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Polson, K.; Brogdon, W.G.; Rawlins, S.C.; Chadee, D.D. Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Trop. 2011, 117, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Koodalingam, A.; Mullainadhan, P.; Arumugam, M. Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae). Acta Trop. 2011, 118, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sutthanont, N.; Choochote, W.; Tuetun, B.; Junkum, A.; Jitpakdi, A.; Chaithong, U.; Riyong, D.; Pitasawat, B. Chemical composition and larvicidal activity of edible plant-derived essential oils against the pyrethroid-susceptible and -resistant strains of Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 2010, 35, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Prapanthadara, L.; Promtet, N.; Koottathep, S.; Somboon, P.; Suwonkerd, W.; McCarroll, L.; Hemingway, J. Mechanisms of DDT and permethrin resistance in Aedes aegypti from Chiang Mai, Thailand. Dengue Bull. 2002, 26, 185–189. [Google Scholar]
- Plernsub, S.; Saingamsook, J.; Yanola, J.; Lumjuan, N.; Tippawangkosol, P.; Sukontason, K.; Somboon, P. Additive effect of knockdown resistance mutations, S989P, V1016G and F1534C, in a heterozygous genotype conferring pyrethroid resistance in Aedes aegypti in Thailand. Parasit. Vectors 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Yanola, J.; Somboon, P.; Walton, C.; Nachaiwieng, W.; Prapanthadara, L. A novel F1552/C1552 point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. Pest Biochem. Physiol. 2010, 96, 127–131. [Google Scholar] [CrossRef]
- Somwang, P.; Yanola, J.; Suwan, W.; Walton, C.; Lumjuan, N.; Prapanthadara, L.A.; Somboon, P. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol. Res. 2011, 109, 531–537. [Google Scholar] [CrossRef]
- Lumjuan, N.; Wicheer, J.; Leelapat, P.; Choochote, W.; Somboon, P. Identification and characterisation of Aedes aegypti aldehyde dehydrogenases involved in pyrethroid metabolism. PLoS ONE 2014, 9, e102746. [Google Scholar] [CrossRef]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticide on Treated Surfaces: Report of the WHO Informal Consultation; WHO Press: Geneva, Switzerland, 1998. [Google Scholar]
- World Health Organization. Instruction for Determining the Susceptibility or Resistance of Mosquito Larvae to Insecticide; WHO/VBC/81.807; WHO Press: Geneva, Switzerland, 1981. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Asakura, K. Phosphatase activity in the larva of the euryhalinemosquito, Aedes togoi Theobold, with special reference to sea water adaptation. J. Exp. Mar. Biol. Ecol. 1978, 31, 325–337. [Google Scholar] [CrossRef]
- Brogdon, W.G.; McAllister, J.C.; Vulule, J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for insecticide resistance. J. Am. Mosq. Control Assoc. 1997, 13, 233–237. [Google Scholar] [PubMed]
- Mazzarri, M.B.; Georghiou, G.P. Characterization of resistance to organophosphate, carbamate, and pyrethroid insecticides in field populations of Aedes aegypti from Venezuela. J. Am. Mosq. Control Assoc. 1995, 11, 315–322. [Google Scholar]
- Grant, D.F. Evolution of glutathione S-transferase subunits in Culicidae and related Nematocera: ELECTROPHORETIC and immunological evidence for conserved enzyme structure and expression. Insect Biochem. 1991, 21, 435–445. [Google Scholar] [CrossRef]
- Grant, D.F.; Dietze, E.C.; Hammock, B.D. Glutathione S-transferase isozymes in Aedes aegypti: PURIFICATION, characterization, and isozyme specific regulation. Insect Biochem. 1991, 4, 421–433. [Google Scholar] [CrossRef]
- Macoris, M.L.G.; Andrighetti, L.; Takaku, L.; Glasser, C.M.; Garbeloto, V.V.; Bracco, J.E. Resistance of Aedes aegypti from the state of Sao Paulo, Brazil, to organophosphate insecticides. Mem. Inst. Oswaldo Cruz 2003, 98, 703–708. [Google Scholar] [CrossRef]
- Yaicharoen, R.; Kiatfuengfoo, R.; Chareonviriyaphap, T.; Rongnoparut, P. Characterization of deltamethrin resistance in field populations of Aedes aegypti in Thailand. J. Vector Ecol. 2005, 30, 144–150. [Google Scholar] [PubMed]
- Jagadeshwaran, U.; Vijayan, V. Biochemical characterization of deltamethrin resistance in a laboratory-selected strain of Aedes aegypti. Parasitol. Res. 2009, 104, 1431–1438. [Google Scholar]
- Pimsamarn, S.; Sornpengb, W.; Akksilp, S.; Paeporn, P.; Limpawitthayakul, M. Detection of insecticide resistance in Aedes aegypti to organophosphate and synthetic pyrethroid compounds in the north-east of Thailand. Dengue Bull. 2009, 33, 194–202. [Google Scholar]
- Awan, D.A.; Saleem, M.A.; Nadeem, M.S.; Shakoori, A.R. Toxicological and biochemical studies on spinosad and synergism with piperonyl butoxide in susceptible and resistant strains of Tribolium castaneum. Pak. J. Zool. 2012, 44, 649–662. [Google Scholar]
- Hariprasad, T.P.N.; Shetty, N.J. Biochemical basis of alphamethrin resistance in different life stages of Anopheles stephensi strains of Bangalore, India. Pest Manag. Sci. 2016, 72, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M. Preliminary investigation of the mechanisms of DDT and pyrethroid resistance in Culex quinquefasciatus Say. (Diptera: Culicidae) from Saudi Arabia. Bull. Entomol. Res. 1989, 79, 361–366. [Google Scholar] [CrossRef]
- Putra, R.E.; Ahmad, I.; Prasetyo, D.B.; Susanti, S.; Rahayu, R.; Hariani, N. Detection of insecticide resistance in the larvae of some Aedes aegypti (Diptera: Culicidae) strains from Java, Indonesia to temephos, malathion and permethrin. Int. J. Mosq. Res. 2016, 3, 23–28. [Google Scholar]
- Choovattanapakorn, N.; Yanola, J.; Lumjuan, N.; Saingamsook, J.; Somboon, P. Characterization of metabolic detoxifying enzymes in an insecticide resistant strain of Aedes aegypti harboring homozygous S989P and V1016G kdr mutations. Med. Entomol. Zool. 2017, 68, 19–26. [Google Scholar] [CrossRef]
- Hemingway, J.; Karunaratne, S.H.P.P. Mosquito carboxylesterases: A review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med. Vet. Entomol. 1998, 12, 1–12. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Rongnoparut, P.; Chantarumporn, P.; Bangs, M.J. Biochemical detection of pyrethroid resistance mechanisms in Anopheles minimus in Thailand. J. Vector Ecol. 2003, 28, 108–116. [Google Scholar]
- Ahmad, I.; Astari, S.; Tan, M. Resistance of Aedes aegypti (Diptera: Culicidae) in 2006 to pyrethroid insecticides in Indonesia and its association with oxidase and esterase levels. Pak. J. Biol. Sci. 2007, 10, 3688–3692. [Google Scholar]
- Braga, I.A.; Valle, D. Aedes aegypti: INSETICIDAS, mecanismos de ação e resistência. Epidemiol. Serv. Saúde 2007, 16, 279–293. [Google Scholar] [CrossRef]
- Diniz, D.F.A.; de Melo-Santos, M.A.V.; de Mendonca Santos, E.A.; Beserra, E.B.; Helvecio, E.; de Carvalho-Leandro, D.; dos Santos, B.S.; de Menezes Lima, V.L.; Ayres, C.V.J. Fitness cost in field and laboratory Aedes aegypti populations associated with resistance to the insecticide temephos. Parasit. Vectors 2015, 8, 662. [Google Scholar] [CrossRef]
- Koodalingam, A.; Mullainadhan, P.; Rajalakshmi, A.; Deepalakshmi, R.; Ammua, M. Effect of a Bt-based product (Vectobar) on esterases and phosphatases from larvae of the mosquito Aedes aegypti. Pest Biochem. Physiol. 2012, 104, 267–272. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, M.; Rup, P.J.; Singh, J. Effects of Indian coral tree, Erythrina indica lectin on eggs and larval development of melon fruit fly, Bactrocera cucurbitae. J. Environ. Biol. 2009, 30, 509–514. [Google Scholar] [PubMed]
- Berge, J.B.; Feyereisen, R.; Amichot, M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998, 353, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulule, J.M.; Beach, R.F.; Atieli, F.K.; McAllister, J.C.; Brogdon, W.G.; Roberts, J.M.; Mwangi, R.W.; Hawley, W.A. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin-impregnated nets. Med. Vet. Entomol. 1999, 3, 239–244. [Google Scholar] [CrossRef]
- Vontas, J.; Ranson, H.; Alphey, L. Transcriptomics and disease vector control. BMC Biol. 2010, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Sarita, N.; Moharil, M.P.; Ghodki, B.S.; Lande, G.K.; Bisane, K.D.; Thakare, A.S.; Barkhade, U.P. Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. J. Asia-Pac. Entomol. 2010, 13, 91–95. [Google Scholar]
- Zibaee, A.; Bandani, A.R. A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). J. Plant Protect. Res. 2010, 50, 79–85. [Google Scholar] [CrossRef]
- Wang, K.Y.; Yong, Z.; Yan, W.H.; Ming, X.X.; Xian, L.T. Influence of three diets on susceptibility of selected insecticides and activities of detoxification esterases of Helicoverpa assulta (Lepidoptera: Noctuidae). Pest Biochem. Physiol. 2010, 96, 51–55. [Google Scholar] [CrossRef]
- Gamil, W.E.; Mariy, F.M.; Youssef, L.A.; Abdel Halim, S.M. Effect of Indoxacarb on some biological and biochemical aspects of Spodoptera littoralis (Boisd.) larvae. Ann. Agric. Sci. 2011, 56, 121–126. [Google Scholar] [CrossRef]
- Huron, E.N.; Abbas, A.A.; El-Hamid, N.A.A.; Nada, M.S.; Amin, T.R. Toxicity and acute macromolecular abnormalities induced by some plant extracts against the Cowpea aphid; Aphis carricivora Koch. J. Plant Prot. Path. 2016, 7, 445–449. [Google Scholar]
- Shekari, M.; Sendi, J.J.; Etebari, K.; Zibaee, A.; Shadparvar, A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull. (Coleoptera: Chrysomellidae). Pest Biochem. Physiol. 2008, 91, 66–74. [Google Scholar] [CrossRef]
- Ghoneim, K.; Amer, M.; Al-Daly, A.; Mohammad, A.; Khadrawy, F.; Mahmoud, M.A. Disturbed acid and alkaline phosphatase activities in desert locust Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) by extracts from the khella plant Ammi visnaga L. (Apiaceae). Int. J. Adv. Res. 2014, 2, 584–596. [Google Scholar]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Panini, M.; Manicardi, G.; Moores, G.; Mazzoni, E. An overview of the main pathways of metabolic resistance in insects. Invertebr. Surv. J. 2016, 13, 326–335. [Google Scholar]
- Hayes, J.D.; Wolf, C.R. Role of glutathione transferase in drug resistance. In Glutathione Conjugation: Mechanisms and Biological Significance; Sies, H., Ketterer, B., Eds.; Academic Press: London, UK, 1988; pp. 315–355. [Google Scholar]
- Mannervik, B.; Danielson, U.H. Glutathione transferases-structure and catalytic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef] [PubMed]
- Pickett, C.B.; Lu, A.Y. Glutathione S-transferases: GENE structure, regulation, and biological function. Annu. Rev. Biochem. 1989, 58, 743–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, J.Z.; Singhal, S.S.; Saini, M.; Pandya, U.; Awasthi, S. Role of glutathione S-transferases in protection against lipid peroxidation. J. Biol. Chem. 2001, 276, 19220–19230. [Google Scholar] [CrossRef]
- Dauterman, W.C. Insect metabolism: EXTRAMICROSOMAL. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 713–730. [Google Scholar]
- Callaghan, A. Insecticide resistance: MECHANISM and detection methods. Sci. Progress 1991, 75, 423–438. [Google Scholar]
- Majerus, P.; Kisseleva, M.; Norris, F.A. The role of phosphatases in inositol signaling reactions. J. Biol. Chem. 1999, 274, 10669–10672. [Google Scholar] [CrossRef]
- Chaubey, M.K. Fumigant toxicity of essential oils against rice weevil Sitophilus oryzae L. (Coleoptera: Curculionidae). J. Biol. Sci. 2011, 11, 411–416. [Google Scholar] [CrossRef]
- Seo, S.M.; Jung, C.S.; Kang, J.; Lee, H.R.; Kim, S.W.; Hyun, J.; Park, I.K. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef] [PubMed]
- Mclaughlin, L.A.; Niazi, U.; Bibby, J.; David, J.P.; Vontas, J.; Hemingway, J.; Ranson, H.; Sutcliffe, M.J.; Paine, M.J. Characterization of inhibitors and substrates of Anopheles gambiae CYP6Z2. Insect Mol. Biol. 2008, 17, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Joffe, T.; Gunning, R.V.; Allen, G.R.; Kristensen, M.; Alptekin, S.; Field, L.M.; Moores, G.D. Investigating the potential of selected natural compounds to increase the potency of pyrethrum against houseflies Musca domestica (Diptera:Muscidae). Pest Manag. Sci. 2012, 68, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.I.J.; Logan, J.G.; Loza-Reyes, E.; Stashenko, E.; Moores, G.D. Repellents inhibit P450 enzymes in Stegomyia (Aedes) aegypti. PLoS ONE 2012, 7, e48698. [Google Scholar]
- Helmy, N.; Bakr, R.F.A.; Nawwar, G.A.; Ibrahim, S.E.; Helmy, O.M. Biochemical effects of some agricultural waste extracts against Culex pipiens (Diptera: Culicidae). Egyptian Acad. J. Biol. Sci. 2010, 2, 75–81. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Senthil-Nathana, S.; Ponsankar, A.; Thanigaivel, A.; Edwina, E.S.; Selin-Rani, S.; Chellappandiana, M.; Pradeepaa, V.; Lija-Escalinea, J.; Kalaivani, K.; et al. Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide temephos and plant derived essential oil derived from Piper betle L. Ecotoxicol. Environ. Saf. 2017, 139, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Carreño Otero, A.L.; Palacio-Cortés, A.M.; Navarro-Silva, M.A.; Kouznetsov, V.V.; Duque L, J.E. Behavior of detoxifying enzymes of Aedes aegypti exposed to girgensohnine alkaloid analog and Cymbopogon flexuosus essential oil. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 204, 14–25. [Google Scholar] [CrossRef]
- Wang, J.J.; Cheng, W.X.; Ding, W.; Zhao, Z.M. The effect of insecticide dichlorvos on esterase activity extracted from the psocids, Liposcelis bostrychophila and L. entomophila. J. Insect Sci. 2004, 4, 1–5. [Google Scholar] [CrossRef]
- Harel, M.; Kryger, G.; Rosenberry, T.L.; Mallender, W.D.; Lewis, T.; Fletcher, R.J.; Guss, J.M.; Silman, I.; Sussman, J.L. Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and its complexes with two potent inhibitors. Protein Sci. 2000, 9, 1063–1072. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakall, D.B. Principles of Ecotoxicology, 3rd ed.; Taylor & Francis: London, UK, 2012. [Google Scholar]
- Rebechi, D.; Richardi, V.S.; Vicentini, M.; Guilosky, I.C.; de Assis, H.C.S.; Navarro-Silva, M.A. Low malathion concentrations influence metabolism in Chironomus sancticaroli (Diptera, Chironomidae) in acute and chronic toxicity test. Rev. Bras. Entomol. 2014, 58, 296–301. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Melo-Santos, M.V.; Varjal-Melo, J.J.M.; Araújo, P.; Gomes, T.C.S.; Paiva, M.H.S.; Regis, L.N.; Furtado, F.; Magalhaes, T.; Macoris, M.L.G.; Andrighetti, M.T.M.; et al. Resistance to the organophosphate temephos: MECHANISMS, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010, 113, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, C.B.; Salazar-Terreros, M.J.; Mina, N.J.; McAllister, J.; Brogdon, W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop. 2011, 118, 37–44. [Google Scholar] [CrossRef] [PubMed]
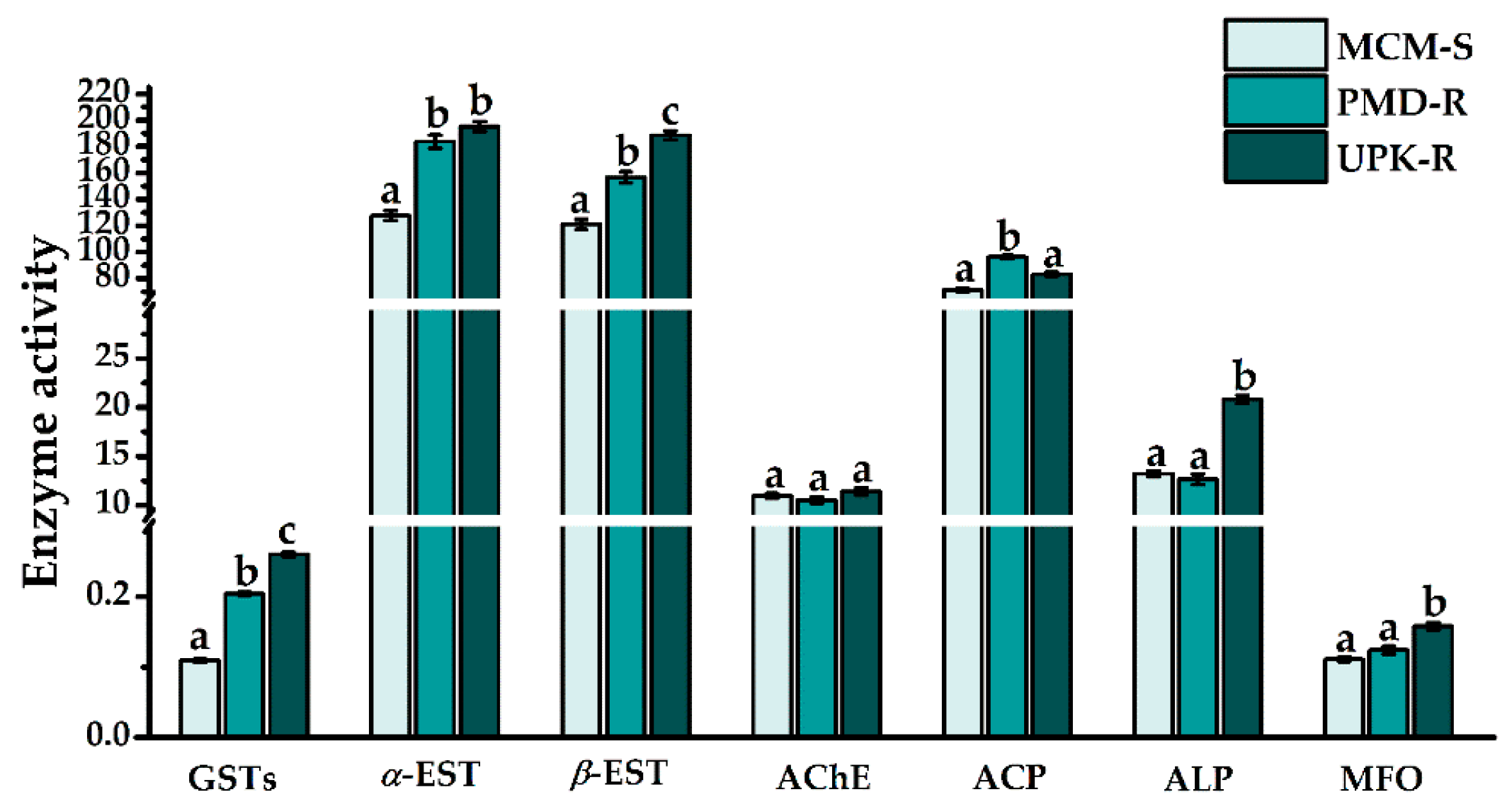

| Enzyme | Enzyme Activity (Mean ± SE) * | |||
|---|---|---|---|---|
| MCM-S | PMD-R | UPK-R | ||
| GSTs 1 | ||||
| 0 h | Untreated | 0.109 ± 0.002 a,A | 0.204 ± 0.002 a,B | 0.260 ± 0.003 a,C |
| 24 h | Untreated | 0.116 ± 0.004 a,A | 0.227 ± 0.004 a,B | 0.276 ± 0.006 a,b,C |
| Control | 0.112 ± 0.004 a,A | 0.242 ± 0.006 a,B | 0.280 ± 0.007 b,C | |
| Treated | 0.210 ± 0.007 b,A | 0.442 ± 0.021 b,B | 0.521 ± 0.007 c,C | |
| α-EST 2 | ||||
| 0 h | Untreated | 127.73 ± 3.73 a,A | 183.82 ± 4.94 a,B | 195.23 ± 3.79 a,B |
| 24 h | Untreated | 133.10 ± 3.52 a,A | 178.64 ± 5.35 a,B | 192.88 ± 3.79 a,B |
| Control | 125.92 ± 5.56 a,A | 174.59 ± 3.40 a,B | 193.33 ± 4.40 a,C | |
| Treated | 202.19 ± 5.08 b,A | 219.44 ± 6.71 b,A | 215.14 ± 3.67 b,A | |
| β-EST 3 | ||||
| 0 h | Untreated | 121.07 ± 3.84 a,A | 156.67 ± 4.10 a,B | 188.44 ± 3.44 a,C |
| 24 h | Untreated | 128.42 ± 4.33 a,A | 158.87 ± 6.04 a,B | 170.79 ± 4.15 b,B |
| Control | 125.28 ± 4.16 a,A | 157.25 ± 2.83 a,B | 169.66 ± 2.92 b,C | |
| Treated | 203.34 ± 8.31 b,A,B | 185.03 ± 7.41 b,A | 212.09 ± 5.99 c,B | |
| AChE 4 | ||||
| 0 h | Untreated | 10.96 ± 0.24 a,A | 10.51 ± 0.33 a,A | 11.40 ± 0.36 a,A |
| 24 h | Untreated | 12.81 ± 0.42 b,A | 12.12 ± 0.60 a,A | 15.06 ± 0.46 b,B |
| Control | 12.90 ± 0.46 b,A | 11.90 ± 0.44 a,A | 15.27 ± 0.35 b,B | |
| Treated | 8.65 ± 0.32 c,A | 8.67 ± 0.37 b,A | 12.93 ± 0.35 c,B | |
| ACP 5 | ||||
| 0 h | Untreated | 71.31 ± 1.34 a,A | 96.21 ± 1.21 a,C | 83.29 ± 1.55 a,B |
| 24 h | Untreated | 100.02 ± 4.81 b,A | 125.55 ± 2.07 b,B | 101.57 ± 3.14 b,A |
| Control | 104.56 ± 4.20 b,A | 126.39 ± 1.94 b,B | 103.13 ± 1.60 b,A | |
| Treated | 121.12 ± 2.52 c,A | 168.97 ± 2.85 c,B | 123.35 ± 2.41c,A | |
| ALP 6 | ||||
| 0 h | Untreated | 13.21 ± 0.28 a,A | 12.65 ± 0.54 a,A | 20.82 ± 0.40 a,B |
| 24 h | Untreated | 7.24 ± 0.33 b,A | 6.48 ± 0.35 b,A | 9.44 ± 0.36 b,B |
| Control | 7.33 ± 0.23 b,A | 6.90 ± 0.31 b,A | 10.38 ± 0.25 b,B | |
| Treated | 15.54 ± 0.56 c,A | 13.03 ± 0.55 a,B | 16.93 ± 0.65 c,A | |
| MFO 7 | ||||
| 0 h | Untreated | 0.111 ± 0.003 a,A | 0.124 ± 0.006 a,A | 0.158 ± 0.005 a,B |
| 24 h | Untreated | 0.077 ± 0.005 b,A | 0.075 ± 0.006 b,A | 0.115 ± 0.004 b,c,B |
| Control | 0.080 ± 0.003 b,A | 0.076 ± 0.004 b,A | 0.119 ± 0.008 b,B | |
| Treated | 0.073 ± 0.003 b,A | 0.066 ± 0.005 b,A | 0.093 ± 0.004 c,B | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intirach, J.; Junkum, A.; Lumjuan, N.; Chaithong, U.; Somboon, P.; Jitpakdi, A.; Riyong, D.; Champakaew, D.; Muangmoon, R.; Chansang, A.; et al. Biochemical Effects of Petroselinum crispum (Umbellifereae) Essential Oil on the Pyrethroid Resistant Strains of Aedes aegypti (Diptera: Culicidae). Insects 2019, 10, 1. https://doi.org/10.3390/insects10010001
Intirach J, Junkum A, Lumjuan N, Chaithong U, Somboon P, Jitpakdi A, Riyong D, Champakaew D, Muangmoon R, Chansang A, et al. Biochemical Effects of Petroselinum crispum (Umbellifereae) Essential Oil on the Pyrethroid Resistant Strains of Aedes aegypti (Diptera: Culicidae). Insects. 2019; 10(1):1. https://doi.org/10.3390/insects10010001
Chicago/Turabian StyleIntirach, Jitrawadee, Anuluck Junkum, Nongkran Lumjuan, Udom Chaithong, Pradya Somboon, Atchariya Jitpakdi, Doungrat Riyong, Danita Champakaew, Roongtawan Muangmoon, Arpaporn Chansang, and et al. 2019. "Biochemical Effects of Petroselinum crispum (Umbellifereae) Essential Oil on the Pyrethroid Resistant Strains of Aedes aegypti (Diptera: Culicidae)" Insects 10, no. 1: 1. https://doi.org/10.3390/insects10010001





