Volatiles from Different Instars of Honeybee Worker Larvae and Their Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments
2.2. SPDE-GC-MS System
2.3. Qualitative and Quantitative Analysis
2.4. Statistical Analysis
3. Results
3.1. Volatiles
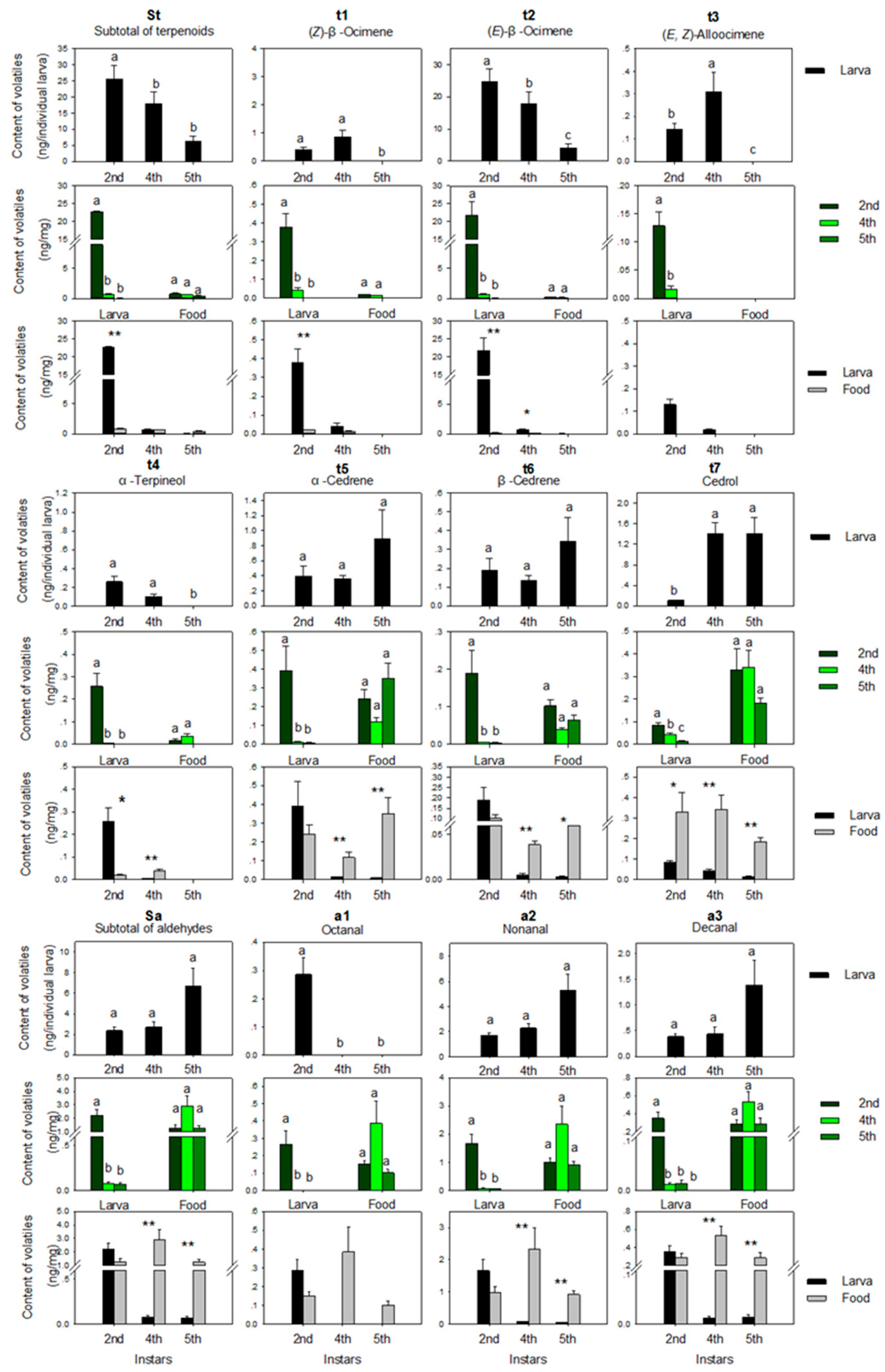
3.1.1. Terpenoids
3.1.2. Aldehydes
3.1.3. Hydrocarbons
3.1.4. Ester
3.1.5. Ketone
3.2. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carroll, M.J.; Duehl, A.J. Collection of volatiles from honeybee larvae and adults enclosed on brood frames. Apidologie 2012, 43, 715–730. [Google Scholar] [CrossRef]
- Breed, M.D.; Guzman-Novoa, E.; Hunt, G.J. Defensive behavior of honey bees: Organization, genetics, and comparisons with other bees. Annu. Rev. Entomol. 2004, 49, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Janson, S.; Middendorf, M.; Beekman, M. Honeybee swarms: How do scouts guide a swarm of uninformed bees? Anim. Behav. 2005, 70, 349–358. [Google Scholar] [CrossRef]
- Gilley, D.C.; DeGrandi-Hoffman, G.; Hooper, J.E. Volatile compounds emitted by live European honey bee (Apis mellifera L.) queens. J. Insect Physiol. 2006, 52, 520–527. [Google Scholar] [CrossRef]
- Le Conte, Y.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B. Identification of a brood pheromone in honeybees. Naturwissenschaften 1990, 77, 334–336. [Google Scholar] [CrossRef]
- Rickli, M.; Diehl, P.A.; Guerin, P.M. Cuticle alkanes of honeybee larvae mediate arrestment of bee parasite Varroa jacobsoni. J. Chem. Ecol. 1994, 20, 2437–2453. [Google Scholar] [CrossRef] [PubMed]
- Aumeier, P.; Rosenkranz, P.; Francke, W. Cuticular volatiles, attractivity of worker larvae and invasion of brood cells by Varroa mites. A comparison of Africanized and European honey bees. Chemoecology 2002, 12, 65–75. [Google Scholar] [CrossRef]
- Maisonnasse, A.; Lenoir, J.-C.; Costagliola, G.; Beslay, D.; Choteau, F.; Crauser, D.; Becard, J.-M.; Plettner, E.; Le Conte, Y. A scientific note on E-β-ocimene, a new volatile primer pheromone that inhibits worker ovary development in honey bees. Apidologie 2009, 40, 562–564. [Google Scholar] [CrossRef]
- He, X.J.; Zhang, X.C.; Jiang, W.J.; Barron, A.B.; Zhang, J.H.; Zeng, Z.J. Starving honey bee (Apis mellifera) larvae signal pheromonally to worker bees. Sci. Rep. 2016, 6, 22359. [Google Scholar] [CrossRef] [PubMed]
- Sammataro, D.; Finley, J.; LeBlancz, B.; Wardell, G.; Ahumada-Segura, F.; Carroll, M.J. Feeding essential oils and 2-heptanone in sugar syrup and liquid protein diets to honey bees (Apis mellifera L.) as potential Varroa mite (Varroa destructor) controls. J. Apic. Res. 2009, 48, 256–262. [Google Scholar] [CrossRef]
- Nazzi, F.; Milani, N.; Della Vedova, G. A semiochemical from larval food influences the entrance of Varroa destructor into brood cells. Apidologie 2004, 35, 403–410. [Google Scholar] [CrossRef]
- Maisonnasse, A.; Lenoir, J.C.; Beslay, D.; Crauser, D.; Le Conte, Y. E-beta-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE 2010, 5, e13531. [Google Scholar] [CrossRef]
- Castro, L.F.; Ross, C.F.; Vixie, K.R. Optimization of a solid phase dynamic extraction (SPDE) method for beer volatile profiling. Food Anal Method 2015, 8, 2115–2124. [Google Scholar] [CrossRef]
- Trhlin, M.; Rajchard, J. Chemical communication in the honeybee (Apis mellifera L.): A review. Vet. Med. 2011, 56, 265–273. [Google Scholar] [CrossRef]
- Teal, P.E.A.; Meredith, J.A.; Gomez-Simuta, Y. Isolation and identification of terpenoid sex pheromone components from extracts of hemolymph of males of the Caribbean fruit fly. Arch. Insect Biochem. Physiol. 1999, 42, 225–232. [Google Scholar] [CrossRef]
- Sant’Ana, J.; Da Silva, R.F.P.; Dickens, J.C. Olfactory reception of conspecific aggregation pheromone and plant odors by nymphs of the predator, Podisus maculiventris. J. Chem. Ecol. 1999, 25, 1813–1826. [Google Scholar] [CrossRef]
- Eguaras, M.J.; Fuselli, S.; Gende, L.; Fritz, R.; Ruffinengo, S.R.; Clemente, G.; Gonzalez, A.; Bailac, P.N.; Ponzi, M.I. An in vitro evaluation of Tagetes minuta essential oil for the control of the honeybee pathogens Paenibacillus larvae and Ascosphaera apis, and the parasitic mite Varroa destructor. J. Essent. Oil Res. 2005, 17, 336–340. [Google Scholar] [CrossRef]
- Peng, G.; Kashio, M.; Morimoto, T.; Li, T.; Zhu, J.; Tominaga, M.; Kadowaki, T. Plant-derived tick repellents activate the honey bee ectoparasitic mite TRPA1. Cell Rep. 2015, 12, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.H.; Birkett, M.A.; Bruce, T.J.A.; Chamberlain, K.; Field, L.M.; Huttly, A.K.; Martin, J.L.; Parker, R.; Phillips, A.L.; Pickett, J.A.; et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. USA 2006, 103, 10509–10513. [Google Scholar] [CrossRef]
- Lebedeva, K.V.; Vendilo, N.V.; Ponomarev, V.L.; Pletnev, V.A.; Mitroshin, D.B. Identification of pheromone of the greater wax moth Galleria mellonella from the different regions of Russia. IOBC/WPRS Bull. 2002, 25, 229–231. [Google Scholar]
- Torto, B.; Suazo, A.; Alborn, H.; Tumlinson, J.H.; Teal, P.E.A. Response of the small hive beetle (Aethina tumida) to a blend of chemicals identified from honeybee (Apis mellifera) volatiles. Apidologie 2005, 36, 523–532. [Google Scholar] [CrossRef]
- Huang, M.H.; DeGrandi-Hoffman, G.; LeBlanc, B. Comparisons of the queen volatile compounds of instrumentally inseminated versus naturally mated honey bee (Apis mellifera) queens. Apidologie 2009, 40, 464–471. [Google Scholar] [CrossRef]
- Torto, B.; Arbogast, R.T.; Alborn, H.; Suazo, A.; van Engelsdorp, D.; Boucias, D.; Tumlinson, J.H.; Teal, P.E.A. Composition of volatiles from fermenting pollen dough and attractiveness to the small hive beetle Aethina tumida, a parasite of the honeybee Apis mellifera. Apidologie 2007, 38, 380–389. [Google Scholar] [CrossRef]
- Del Piccolo, F.; Nazzi, F.; Della Vedova, G.; Milani, N. Selection of Apis mellifera workers by the parasitic mite Varroa destructor using host cuticular hydrocarbons. Parasitology 2010, 137, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Hajong, S.R.; Gevar, J.; Lenoir, A.; Darrouzet, E. Cuticular hydrocarbon compounds in worker castes and their role in nestmate recognition in Apis cerana indica. J. Chem. Ecol. 2016, 42, 444–451. [Google Scholar] [CrossRef]
- Nazzi, F.; Della Vedova, G.; D’Agaro, M. A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie 2004, 35, 65–70. [Google Scholar] [CrossRef][Green Version]
- Pernal, S.F.; Baird, D.S.; Birmingham, A.L.; Higo, H.A.; Slessor, K.N.; Winston, M.L. Semiochemicals influencing the host-finding behaviour of Varroa destructor. Exp. Appl. Acarol. 2005, 37, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Carr-Markell, M.; Hefetz, A.; Grozinger, C.M.; Mattila, H.R. Queen-produced volatiles change dynamically during reproductive swarming and are associated with changes in honey bee (Apis mellifera) worker behavior. Apidologie 2015, 46, 679–690. [Google Scholar] [CrossRef]
- Breed, M.D.; Stiller, T.M. Honey bee, Apis mellifera, nestmate discrimination: Hydrocarbon effects and the evolutionary implications of comb choice. Anim. Behav. 1992, 43, 875–883. [Google Scholar] [CrossRef]
- Minaeimoghadam, M.; Askarianzadeh, A.; Imani, S.; Shojaei, M.; Larijani, K.; Abbasipour, H. Identification of chemical compounds of the pheromone in different ages of female adults of the clearwing moth, Paranthrene diaphana Dalla Torre & Strand. Arch. Phytopatho. Plant Prot. 2017, 50, 1019–1033. [Google Scholar]
- Wakonigg, G.; Eveleigh, L.; Arnold, G.; Crailsheim, K. Cuticular hydrocarbon profiles reveal age-related changes in honey bee drones (Apis mellifera carnica). J. Apic. Res. 2000, 39, 137–141. [Google Scholar] [CrossRef]
- Jang, E.B.; Light, D.M.; Binder, R.G.; Flath, R.A.; Carvalho, L.A. Attraction of female Mediterranean fruit flies to the five major components of male-produced pheromone in a laboratory flight tunnel. J. Chem. Ecol. 1994, 20, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, A.; Khalil, A.; Chaline, N.; Hefetz, A. New chemical data on the ant Myrmecina graminicola (Formicidae, Myrmicinae): Unusual abundance of alkene hydrocarbons and esters. Biochem. Syst. Ecol. 2018, 80, 39–42. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Heppelthwaite, V.J.; Manning, L.M.; Gibb, A.R.; Suckling, D.M. Volatile constituents of fermented sugar baits and their attraction to Lepidopteran species. J. Agric. Food Chem. 2005, 53, 953–958. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Vidal, D.M.; Zarbin, P.H.G. Male-produced sex pheromone of the cerambycid beetle Hedypathes betulinus: Chemical identification and biological activity. J. Chem. Ecol. 2010, 36, 1132–1139. [Google Scholar] [CrossRef]
- Halloran, S.T.; Collignon, R.M.; McElfresh, J.S.; Millar, J.G. Fuscumol and geranylacetone as pheromone components of Californian longhorn beetles (Coleoptera: Cerambycidae) in the subfamily Spondylidinae. Environ. Entomol. 2018, 47, 1300–1305. [Google Scholar] [CrossRef] [PubMed]




| RT a | Compound | Code b | Peak | LRI Calc c | LRI Lit d | Identify e | Diagnostic Ions | |
|---|---|---|---|---|---|---|---|---|
| DB5 | BPX5 | |||||||
| Terpenoids | ||||||||
| 11.75 | (Z)-β-Ocimene | t1 | 2 | 1040 | 1229 | 976 | S. N. L | 93, 41, 79 |
| 12.06 | (E)-β-Ocimene | t2 | 3 | 1050 | 1250 | 976 | S. N. L | 93, 79, 105 |
| 14.61 | (E, Z)-Alloocimene | t3 | 5 | 1134 | 1371 | 1088 | N. L | 119, 91, 134 |
| 16.56 | α-Terpineol | t4 | 6 | 1199 | 1143 | S. N. L | 59, 93, 121 | |
| 22.62 | α-Cedrene | t5 | 8 | 1421 | 1556 | 1403 | N. L | 119, 93, 105 |
| 22.82 | β-Cedrene | t6 | 9 | 1429 | 1560 | 1403 | N. L | 161, 69, 204 |
| 27.34 | Cedrol | t7 | 13 | 1615 | 2112 | 1543 | N. L | |
| Aldehydes | ||||||||
| 10.73 | Octanal | a1 | 1 | 1008 | 1005 | S. N. L | 43, 56, 84 | |
| 13.88 | Nonanal | a2 | 4 | 1109 | 1104 | S. N. L | 57, 41, 70 | |
| 16.91 | Decanal | a3 | 7 | 1212 | 1204 | S. N. L | 43, 57, 70 | |
| Hydrocarbons | ||||||||
| 24.80 | Pentadecane | h1 | 12 | 1508 | 1498 | 1512 | S. N. L | 57, 43, 71, 85 |
| 29.48 | Heptadecane | h2 | 14 | 1709 | 1699 | 1711 | S. N. L | 57, 43, 71, 86 |
| 31.77 | Octadecane | h5 | 15 | 1816 | 1799 | 1852 | S. N. L | 57, 71, 85, 43 |
| Ester | ||||||||
| 24.03 | Ethyl 2(E)-decenoate | e1 | 11 | 1489 | 1758 | 1389 | N. L | 43, 55, 73 |
| Ketone | ||||||||
| 23. 65 | (E)-Geranylacetone | k1 | 10 | 1454 | 1420 | N. L | 43, 41, 69 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Hou, C.; Dai, P.; Liu, Y.; Wu, Y.; Pang, Y.; Diao, Q. Volatiles from Different Instars of Honeybee Worker Larvae and Their Food. Insects 2019, 10, 118. https://doi.org/10.3390/insects10040118
Zhang H, Hou C, Dai P, Liu Y, Wu Y, Pang Y, Diao Q. Volatiles from Different Instars of Honeybee Worker Larvae and Their Food. Insects. 2019; 10(4):118. https://doi.org/10.3390/insects10040118
Chicago/Turabian StyleZhang, Haohao, Chunsheng Hou, Pingli Dai, Yongjun Liu, Yanyan Wu, Yonggang Pang, and Qingyun Diao. 2019. "Volatiles from Different Instars of Honeybee Worker Larvae and Their Food" Insects 10, no. 4: 118. https://doi.org/10.3390/insects10040118
APA StyleZhang, H., Hou, C., Dai, P., Liu, Y., Wu, Y., Pang, Y., & Diao, Q. (2019). Volatiles from Different Instars of Honeybee Worker Larvae and Their Food. Insects, 10(4), 118. https://doi.org/10.3390/insects10040118







