Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.2.1. Ants
2.2.2. Pitfall Traps
2.2.3. Soil Monoliths
2.2.4. Bait Trap
2.2.5. Direct Sampling
2.2.6. Termites
2.3. Identifications
2.3.1. Ants
2.3.2. Termites
2.4. Data Analysis
2.4.1. Ants
2.4.2. Termites
3. Results
3.1. Ants
3.1.1. Specificity of the Two Habitats
3.1.2. Shared Species
3.2. Termites
3.2.1. Specificity of the Two Habitats
3.2.2. Shared Species
4. Discussion
4.1. Ants
Specificity of the Two Habitats and Bio Indicator Species
4.2. Termites
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ingels, J.; Vanreusel, A.; Brandt, A.; Catarino, A.I.; David, B.; de Ridder, C.; Dubois, P.; Gooday, A.J.; Martin, P.; Pasotti, F.; et al. Possible effects of global environmental changes on Antarctic benthos: A synthesis across five major taxa. Ecol. Evol. 2012, 2, 453–485. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Garcia, A.; Ehrlich, P.R. The sixth extinction crisis: Loss of animal populations and species. J. Cosmol. 2010, 8, 1821–1831. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2006, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- McCauley, D.J.; Pinsky, M.L.; Palumbi, S.R.; Estes, J.A.; Joyce, F.H.; Warner, R.R. Marine defaunation: Animal loss in the global ocean. Science 2015, 347, 1255641. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; Garcia, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Ecological science and tomorrow’s world. Philos. Trans. R. Soc. B 2009, 365, 41–47. [Google Scholar] [CrossRef]
- Ehrlich, P.R. The loss of diversity: Causes and consequences. In Biodiversity; Wilson, E.O., Peter, F.M., Eds.; National Academies Press: Washington, DC, USA, 1988; pp. 21–27. ISBN 10-0-309-03783-2. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Fahrig, L. Relative Effects of Habitat Loss and Fragmentation on Population Extinction. J. Wildl. Manag. 1997, 61, 603–610. [Google Scholar] [CrossRef]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Palmer, R.N.; Korb, E.; Bartz, K.K.; Imaki, H. Projected impacts of climate change on salmon habitat restoration. Proc. Natl. Acad. Sci. USA 2007, 104, 6720–6725. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.W.; Budy, P.; Schmidt, J.C. Quantifying Macroinvertebrate Responses to In-Stream Habitat Restoration: Applications of Meta-Analysis to River Restoration. Restor. Ecol. 2010, 18, 8–19. [Google Scholar] [CrossRef]
- Miller, J.R.; Hobbs, R.J. Habitat Restoration-Do We Know What We are Doing? Restor. Ecol. 2007, 15, 382–390. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R.; Canadell, J. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, K.A.; Parker, W.H. Using portfolio theory to guide reforestation and restoration under climate change scenarios. Clim. Chang. 2008, 89, 355–370. [Google Scholar] [CrossRef]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Wangpakapattanawong, P.; Kavinchan, N.; Vaidhayakarn, C.; Schmidt-Vogt, D.; Elliott, S. Fallow to forest: Applying indigenous and scientific knowledge of swidden cultivation to tropical forest restoration. For. Ecol. Manag. 2010, 260, 1399–1406. [Google Scholar] [CrossRef]
- Tyrväinen, L. The amenity value of the urban forest: An application of the hedonic pricing method. Landsc. Urban Plan. 1997, 37, 211–222. [Google Scholar] [CrossRef]
- Vilisics, F.; Hornung, E. Urban areas as hot-spots for introduced and shelters for native isopod species. Urban Ecosyst. 2009, 12, 333–345. [Google Scholar] [CrossRef]
- Shepherd, P.A. A review of plant communities of derelict land in the city of Nottingham, England and their value for nature conservation. Memorab. Zool. 1994, 49, 129–137. [Google Scholar]
- McGeoch, M.A.; Chown, S.L. Scaling up the value of bioindicators. Trends Ecol. Evol. 1998, 13, 46–47. [Google Scholar] [CrossRef]
- Pearson, D.L.; Carroll, S.S. Global Patterns of Species Richness: Spatial Models for Conservation Planning Using Bioindicator and Precipitation Data. Conserv. Biol. 2008, 12, 809–821. [Google Scholar] [CrossRef]
- Siddig, A.A.; Ellison, A.M.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Bohac, J. Staphylinid beetles as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.L.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecol. Indic. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Haneda, N.F.; Kusuma, F.D. The Diversity of Butterfly (Lepidoptera) and Longhorn Beetles (Coleoptera: Cerambycidae) Protection Areas in Kalimantan Barat. IOP Conf. Ser. Earth Environ. Sci. 2018, 197, 012020. [Google Scholar] [CrossRef]
- Pe’Er, G.; Settele, J. The Rare Butterfly Tomares Nesimachus(Lycaenidae) as a Bioindicator for Pollination Services and Ecosystem Functioning in Northern Israel. Isr. J. Ecol. Evol. 2008, 54, 111–136. [Google Scholar] [CrossRef]
- Stefanescu, C.; Peñuelas, J.; Filella, I. Butterflies highlight the conservation value of hay meadows highly threatened by land-use changes in a protected Mediterranean area. Biol. Conserv. 2005, 126, 234–246. [Google Scholar] [CrossRef]
- Blinova, S.V.; Dobrydina, T.I. Study of ants as bioindicators of industrial pollution in Kemerovo Region, Russia. IOP Conf. Ser. Earth Environ. Sci. 2018, 115, 012035. [Google Scholar] [CrossRef]
- Laste, K.C.D.; Durigan, G.; Andersen, A.N. Biodiversity responses to land-use and restoration in a global biodiversity hotspot: Ant communities in Brazilian Cerrado. Austral Ecol. 2018, 44, 313–326. [Google Scholar] [CrossRef]
- Jamison, S.-L.; Robertson, M.; Engelbrecht, I.; Hawkes, P. An assessment of rehabilitation success in an African grassland using ants as bioindicators. Koedoe 2016, 58. [Google Scholar] [CrossRef]
- Viana, J.A.B.; Souza, V.B.; Reis, Y.T.; Marques-Costa, A.P. Termite assemblages in dry tropical forests of Northeastern Brazil: Are termites bioindicators of environmental disturbances? Sociobiology 2016, 61, 324–331. [Google Scholar] [CrossRef]
- Bharti, H.; Barthi, M.; Pfeiffer, M. Ants as bioindicators of ecosystem health in Shivalik Mountains of Himalayas: Assessment of species diversity and invasive species. Asian Myrmecol. 2016, 8, 1–15. [Google Scholar] [CrossRef]
- Lawes, M.J.; Moore, A.M.; Andersen, A.N.; Preece, N.D.; Franklin, D.C. Ants as ecological indicators of rainforest restoration: Community convergence and the development of an Ant Forest Indicator Index in the Australian wet tropics. Ecol. Evol. 2017, 7, 8442–8455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, K.; Konate, S.; Tiho, S.; Camara, S.T. Impacts of land use types on ant communities in a tropical forest margin (Oumé–Côte d’Ivoire). Afr. J. Agric. Res. 2011, 6, 260–274. [Google Scholar] [CrossRef]
- Taylor, B.; Agoinon, N.; Sinzogan, A.; Adandonon, A.; Kouagou, Y.N.; Bello, S.; Wargui, R.; Anato, F.; Ouagoussounon, I.; Houngbo, H.; et al. Records of ants (Hymenoptera: Formicidae) from the Republic of Benin, with particular reference to the mango farm ecosystem. J. Insect Biodivers. 2018, 8, 6–29. [Google Scholar] [CrossRef]
- Kone, M.; Konaté, S.; Yéo, K.; Kouassi, P.K.; Linsenmair, K.E. Changes in ant communities along an age gradient of cocoa cultivation in the Oumé region, central Côte d’Ivoire. Èntomol. Sci. 2012, 15, 324–339. [Google Scholar] [CrossRef]
- Groc, S.; Delabie, J.H.; Fernandez, F.; Petitclerc, F.; Corbara, B.; Leponce, M.; Céréghino, R.; Dejean, A. Litter-dwelling ants as bioindicators to gauge the sustainability of small arboreal monocultures embedded in the Amazonian rainforest. Ecol. Indic. 2017, 82, 43–49. [Google Scholar] [CrossRef]
- Jouquet, P.; Blanchart, E.; Capowiez, Y. Utilization of earthworms and termites for the restoration of ecosystem functioning. Appl. Soil Ecol. 2014, 73, 34–40. [Google Scholar] [CrossRef]
- Alves, W.D.F.; Mota, A.S.; de Lima, R.A.A.; Bellezoni, R.; Vasconcellos, A. Termites as bioindicators of habitat quality in the caatinga, Brazil: Is there agreement between structural habitat variables and the sampled assemblages? Neotrop. Èntomol. 2011, 40, 39–46. [Google Scholar] [CrossRef]
- Agosti, D.; Alonso, L.E. The ALL protocol: A standard protocol for the collection of ground-dwelling ants. In Ants: Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Schultz, T., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 204–206. ISBN 1-56098-885-1. [Google Scholar]
- Jones, D.T.; Eggleton, P. Sampling termite assemblages in tropical forests: Testing a rapid biodiversity assessment protocol. J. Appl. Ecol. 2000, 37, 191–203. [Google Scholar] [CrossRef]
- The Ants of Africa. Available online: http://antsofafrica.org/ant_species_2012/contents.htm (accessed on 25 June 2019).
- Bolton, B. Identification Guide to the Ant Genera of the World; Harvard University Press: Cambridge, MA, USA, 1994; ISBN 0674442806. [Google Scholar]
- Fisher, B.L.; Bolton, B. Ants of Africa and Madagascar. A Guide to Genera; University of California Press: Oakland, CA, USA, 2016; ISBN 0520290895. [Google Scholar]
- Grass, P.P. Recherches sur la Systématique et la Biologie des Termites de l’Afrique occidentale frangaise. Ann. Soc. Entomol. Fr. 1937, 106, 1–100. [Google Scholar]
- Grass, P.P. Recherches sur la biologie des termites champignonnistes (Macrotermitinae). Extr. Ann. Sci. Nat. Zool. 1944, 11, 97–171. [Google Scholar]
- Bouillon, A.; Mathot, G. Quel est ce termite Africain? Zooleo 1965, 1, 1–23. [Google Scholar]
- Sands, W.A. The Identification of Worker Castes of Termites Genera from Soils of Africa and the Middle East; CAB International: Wallingford, UK, 1998; ISBN 0-85199-225-0. [Google Scholar]
- Ruelle, J.E. A revision of the termites of the genus Macrotermes from the Ethiopian region (Isoptera: Termitidae). Bull. Br. Mus. 1970, 24, 365–444. [Google Scholar] [CrossRef]
- Sands, W.A. A revision of the termite subfamily Nasutitermitinae (Isoptera, Termitidae) from the Ethiopian region. Bull. Br. Mus. 1965, 4, 1–172. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9.1.0. 2013. Available online: http://viceroy.colorado.edu/estimates/ (accessed on 24 August 2018).
- Romero, H.; Jaffe, K. A Comparison of Methods for Sampling Ants (Hymenoptera, Formicidae) in Savannas. Biotropica 1989, 21, 348. [Google Scholar] [CrossRef] [Green Version]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Lopes, C.T.; Vasconcelos, H.L. Evaluation of three methods for sampling ground-dwelling Ants in the Brazilian Cerrado. Neotrop. Èntomol. 2008, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A. Functional groups and patterns of organization in North American ant communities: A comparison with Australia. J. Biogeogr. 1997, 24, 433–460. [Google Scholar] [CrossRef]
- Gómez, C.; Casellas, D.; Oliveras, J.; Bas, J.M. Structure of ground-foraging ant assemblages in relation to land-use change in the northwestern Mediterranean region. Biodivers. Conserv. 2003, 12, 2135–2146. [Google Scholar] [CrossRef]
- AntWeb: Ants of All Antweb. Available online: https://www.antweb.org/project.do?name=allantwebants (accessed on 9 July 2019).
- Stork, N.E.; Watt, A.D.; Bolton, B. The diversity and abundance of ants in relation to forest disturbance and plantation establishment in southern Cameroon. J. Appl. Ecol. 2002, 39, 18–30. [Google Scholar] [Green Version]
- Philpott, S.M.; Armbrecht, I. Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol. Èntomol. 2006, 31, 369–377. [Google Scholar] [CrossRef]
- Wetterer, J. Geographic spread of the samsum or sword ant, Pachycondyla (Brachyponera) sennaarensis (Hymenoptera: Formicidae). Myrmecol. News 2013, 18, 13–18. [Google Scholar]
- Al-Khalifa, M.S.; Mashaly, A.M.A.; Siddiqui, M.I.; Al-Mekhlafi, F.A. Samsum ant, Brachyponera sennaarensis (Formicidae: Ponerinae): Distribution and abundance in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 575–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levieux, J.; Diomande, T. La nutrition des fourmis granivores II. Cycle d’activité et régime alimentaire de Brachyponera senaarensis (Mayr) (Hymenoptera Formicidae). Insectes Soc. 1978, 25, 187–196. [Google Scholar] [CrossRef]
- Wetterer, J. Worldwide spread of the longhorn crazy ant, Paratrechina longicornis (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 137–149. [Google Scholar]
- AntWeb. Available online: https://www.antweb.org/description.do?rank=species&genus=trichomyrmex&name=oscaris (accessed on 28 October 2018).
- Pacheco, R.; Vasconcelos, H.L. Invertebrate conservation in urban areas: Ants in the Brazilian Cerrado. Landsc. Urban Plan. 2007, 81, 193–199. [Google Scholar] [CrossRef]
- Yamaguchi, T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan I. Analysis of ant species richness. Ecol. Res. 2004, 19, 209–216. [Google Scholar] [CrossRef]
- Aanen, D.K.; Eggleton, P. Fungus-Growing Termites Originated in African Rain Forest. Curr. Biol. 2005, 15, 851–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepage, M.; Abbadie, L.; Mariotti, A. Food habits of sympatric termite species (Isoptera, Macrotermitinae) as determined by stable carbon isotope analysis in a Guinean savanna (Lamto, Côte d’Ivoire). J. Trop. Ecol. 1993, 9, 303–311. [Google Scholar] [CrossRef]
- Jones, D.T.; Eggleton, P. Global Biogeography of Termites: A Compilation of Sources. In Biology of Termites: A Modern Synthesis; Springer Science and Business Media LLC: Berlin, Germany, 2011; pp. 477–498. [Google Scholar]
- AntWiki. Available online: http://www.antwiki.org/wiki/Togo (accessed on 14 November 2018).
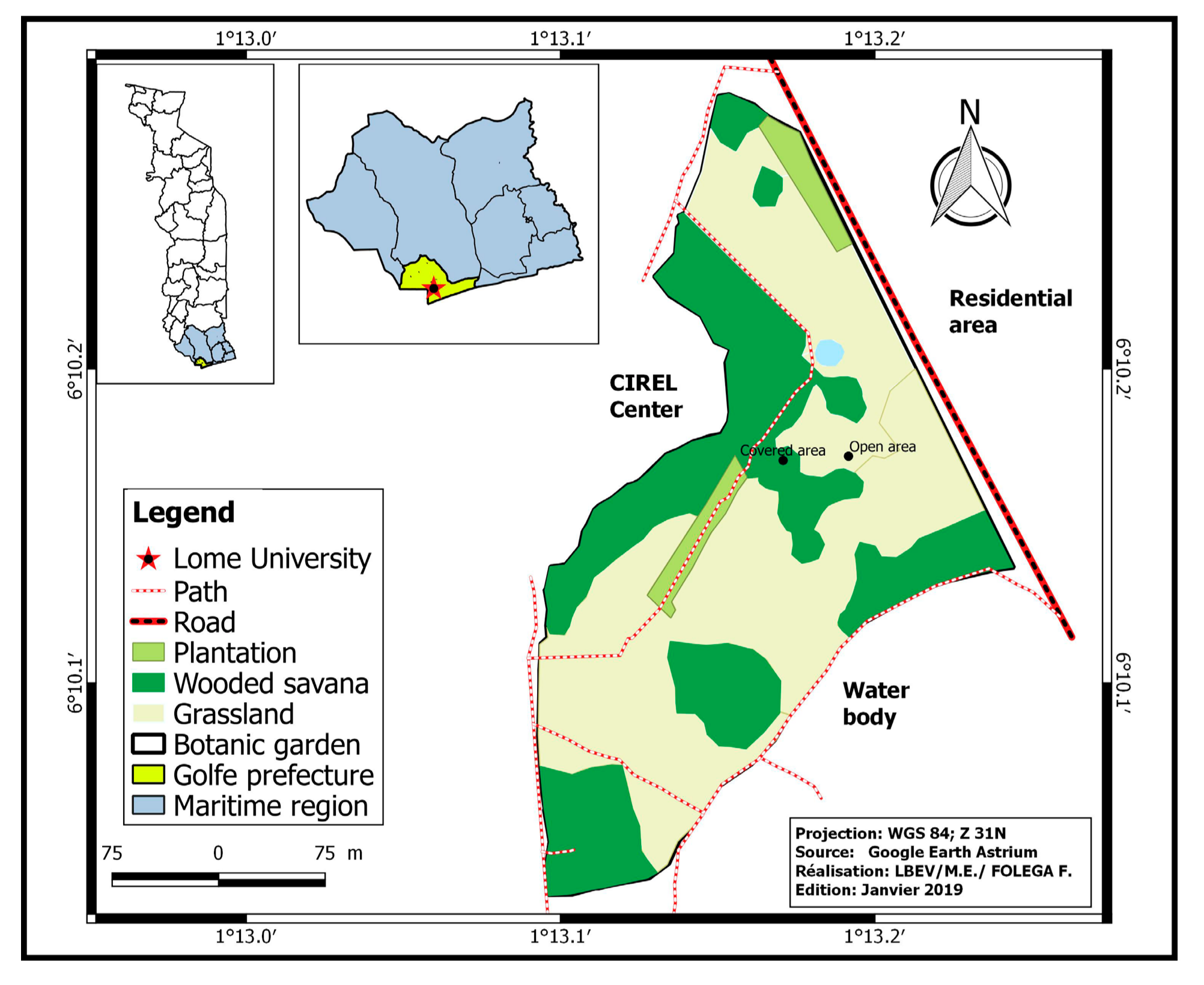
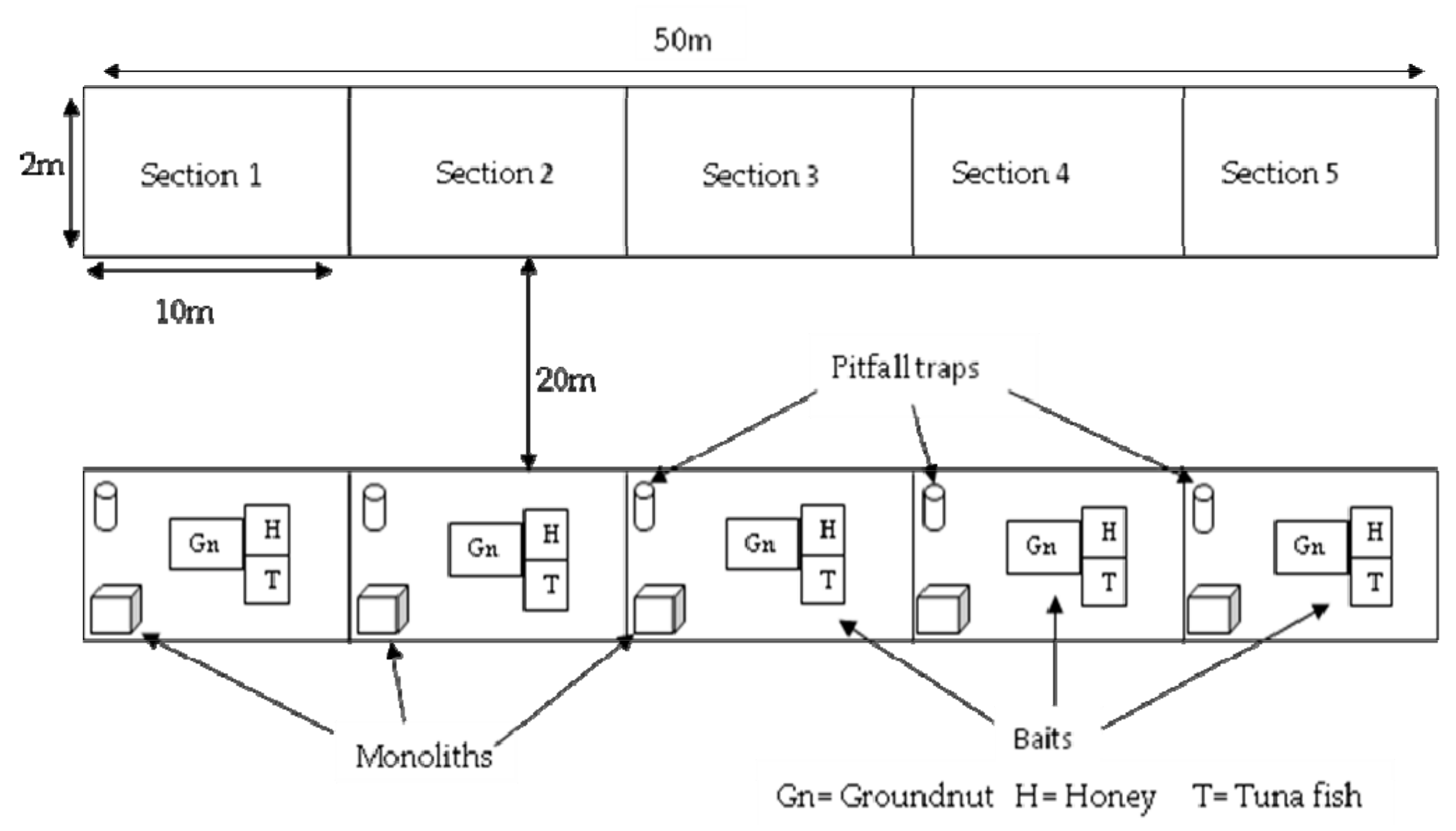
| Habitats | Plant Species |
|---|---|
| Covered area | Acacia auriculiformis A.Cunn. ex Benth (Fabaceae) |
| Azadirachta indica (Meliaceae) | |
| Blighia sapida K. D. Koenig (Sapindaceae) | |
| Delonix regia (Boj.ex Hook.) Raf. (Caesalpiniaceae) | |
| Ficus sp (Moraceae) | |
| Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill. (Irvingiaceae) | |
| Khaya senegalensis (Desr.) A. Juss. (Meliaceae) | |
| Mangifera indica L. (Anacardiaceae) | |
| Polyalthia longifolia Sonn. (Annonaceae) | |
| Senna Siamea (Lam.) Irwin et Barneby (Fabaceae) | |
| Open area | Azadirachta indica (Meliaceae) Heteropogon contortus (L.) P. Beauv. Ex Roem. & Shult (Poaceae) |
| Sub-Family (Percentage of Species) | Species | Total Occurrences | Methods | |
|---|---|---|---|---|
| Open Area | Covered Area | |||
| Dolichoderinae (4.65%) | Tapinoma lugubre (Santschi, 1917) | 8 | 3 | M, P |
| Tapinoma melanocephalum (Fabricius, 1793) | - | 2 | P | |
| Dorylinae (2.33%) | Dorylus sp.1 | 1 | 2 | M, P |
| Formicinae (30.23%) | Camponotus acvapimensis (Donisthorpe, 1945) | 1 | 2 | P, D |
| Camponotus controversus (Santschi, 1916) | 4 | - | M, P, D | |
| Camponotus sp.1 | - | - | *D | |
| Camponotus maculatus (Fabricius, 1782) | 3 | - | M, P, D | |
| Camponotus sericeus (Fabricius, 1798) | 1 | 2 | P, D | |
| Camponotus vividus (Smith, F, 1858) | - | - | # D | |
| Lepisiota guineensis (Mayr, 1902) | 3 | 1 | M | |
| Lepisiota laevis (Santschi, 1913) | - | 1 | M, D | |
| Lepisiota sp.1 | 10 | 2 | M, H, P, D | |
| Lepisiota sp.2 | - | 1 | M, D | |
| Nylanderia boltoni ** (La Polla & Fisher, 2011) | 4 | - | M, P | |
| Oecophylla longinoda (Latreille, 1802) | - | 1 | P, D | |
| Paratrechina longicornis ** (Latreille, 1802) | 1 | - | P | |
| Myrmicinae (51.16%) | Cataulacus traegaordhi (Santschi, 1914) | - | 1 | M, D |
| Cardiocondyla emeryi (Forel,1881) | 3 | 1 | M, P | |
| Carebara sp.1 | 1 | - | M | |
| Carebara sp.2 | - | 1 | P | |
| Crematogaster sp.1 | - | 3 | M, D | |
| Crematogaster sp.2 | - | 2 | M, D | |
| Monomorium bicolor (Emery, 1877) | 2 | - | P | |
| Monomorium exiguum (Forel, 1894) | - | 1 | P | |
| Monomorium sp.1 | 2 | 2 | M, P, D | |
| Pheidole excellens (Mayr,1862) | - | - | # D | |
| Pheidole impressifrons (Wasmann, 1905) | 3 | 12 | M, P, D | |
| Pheidole senilifrons (Wheeler, 1922) | 25 | 12 | G, M, P, D | |
| Pheidole tenuinodis (Mayr, 1901) | 36 | 31 | G, M, H, P, T, D | |
| Pheidole sp.1 | 5 | 7 | M, P, D | |
| Pheidole sp.2 | - | 1 | P | |
| Pheidole sp.3 | - | 1 | P | |
| Strumigenys bernardi * (Brown, 1960) | - | 2 | P | |
| Strumigenys sistrura * (Bolton, 1983) | - | 1 | P | |
| Tetramorium anxium (Santschi, 1914) | - | 1 | M | |
| Tetramorium sericeiventre (Emery, 1877) | 2 | - | P | |
| Tetramorium simillimum (Smith, F., 1851) | - | 2 | P, M | |
| Trichomyrmex oscaris **(Forel, 1894) | 8 | - | G, M, H, P, T, D | |
| Ponerinae (9.3%) | Brachyponera sennaarensis ** (Mayr, 1862) | 1 | - | M |
| Hypoponera sp.1 | - | 1 | M | |
| Hypoponera sp.2 | 2 | - | M | |
| Hypoponera sp.3 | 1 | - | M | |
| Pseudomyrmicinae (2.33%) | Tetraponera mocquerysi (André, 1890) | - | 2 | P, D |
| Standard Deviation | ||
|---|---|---|
| Observed species (S) | 40 | |
| Chao 2 | 52.54 | 6.87 |
| Jacknife 1 | 53.5 | 2.5 |
| Jacknife 2 | 53.5 | |
| Bootstrap | 46.75 |
| Habitats | Overall Occurrence | S | Simpson 1-D | Shannon |
|---|---|---|---|---|
| Open area | 128 | 24 | 0.8617 | 2.48 |
| Covered area | 101 | 29 | 0.8658 | 2.633 |
| Shannon Index (H) | Simpson Index (D) | |
|---|---|---|
| Open area | 2.4798 ± 0.011071 | 0.13831 ± 0.00037821 |
| Covered area | 2.6333 ± 0.017097 | 0.1342 ± 0.0060072 |
| t = −0.91421, df = 206, p(same) = 0.36168 | t = 0.13114, df = 204.31, p(same) = 0.89579 |
| Sample | S | Shared Species | Chao Shared Estimates | Jaccard Classic (%) | Sorensen Classic (%) |
|---|---|---|---|---|---|
| Open area | 24 | 12 | 19.2 | 32.5 | 49 |
| Covered area | 29 |
| Sub-Family | Termites Species | Feeding Group | Occurrence | ||
|---|---|---|---|---|---|
| Open Area | Covered Area | Total | |||
| Apicotermitinae | Adaiphrotermes sp | S | 19 | 7 | 26 |
| Astalotermes sp | S | 8 | 5 | 13 | |
| Cubitermitinae | Basidentitermes mactus * (Sjöstedt, 1911) | S | 0 | 24 | 24 |
| Macrotermitinae | Allodontermes sp | F | 5 | 24 | 29 |
| Ancistrotermes cavithorax ( Sjöstedt, 1899) | F | 10 | 8 | 18 | |
| Ancistrotremes crucifer (Sjöstedt, 1897) | F | 3 | 6 | 9 | |
| Macrotermes subhyalinus (Rambur, 1842) | F | 16 | 5 | 21 | |
| Microtermes comprehensa (Silvestri, 1914) | F | 11 | 1 | 12 | |
| Microtermes grassei (Ghidini, 1955) | F | 1 | 9 | 10 | |
| Microtermes tumodiensis (Grassé, 1937) | F | 0 | 2 | 2 | |
| Termitinae | Amitermes evuncifer (Silvestri, 1912) | W | 2 | 0 | 2 |
| Microcerotermes sp.1 | W | 4 | 4 | 8 | |
| Microcerotermes sp.2 | W | 0 | 13 | 13 | |
| Nasutitermitinae | Trinervitermes geminatus ** (Wasmann, 1897) | G | 16 | 0 | 16 |
| Trinertermes oeconomus ** (Trägårdh, 1904) | G | 22 | 1 | 23 | |
| Trinervitermes togoensis** (Sjöstedt, 1899) | G | 24 | 0 | 24 | |
| Trinervitermes sp ** | G | 13 | 0 | 13 | |
| Total | 154 | 109 | 263 | ||
| Standard Deviation | ||
|---|---|---|
| Observed S | 17 | |
| Chao 2 | 17.96 | 1.28 |
| Jacknife 1 | 20.5 | 0.5 |
| Jacknife 2 | 20.5 | |
| Bootstrap | 18.75 |
| Habitat | Overall Occurrence | S | Simpson 1-D | Shannon |
|---|---|---|---|---|
| Open area | 154 | 14 | 0.897 | 2.397 |
| Covered area | 109 | 13 | 0.863 | 2.217 |
| Shannon Index (H) | Simpson Index (D) | |
|---|---|---|
| Open area | 2.3967 ± 0.00244 | 0.10297 ± 5.06 × 10−5 |
| Covered area | 2.217 ± 0.00567 | 0.1366 ± 0.000239 |
| t = 1.9946, df = 197.18, p(same) = 0.047459 | t = −1.9759, df = 155.18, p (same) = 0.049937 |
| Sample | S | Shared Species | Chao Shared Estimates | Jaccard Classic (%) | Sorensen Classic (%) |
|---|---|---|---|---|---|
| Open area | 14 | 10 | 10.67 | 58.8 | 74 |
| Covered area | 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasseney, B.D.; N’tie, T.B.; Nuto, Y.; Wouter, D.; Yeo, K.; Glitho, I.A. Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo. Insects 2019, 10, 218. https://doi.org/10.3390/insects10070218
Kasseney BD, N’tie TB, Nuto Y, Wouter D, Yeo K, Glitho IA. Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo. Insects. 2019; 10(7):218. https://doi.org/10.3390/insects10070218
Chicago/Turabian StyleKasseney, Boris Dodji, Titati Bassouo N’tie, Yaovi Nuto, Dekoninck Wouter, Kolo Yeo, and Isabelle Adolé Glitho. 2019. "Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo" Insects 10, no. 7: 218. https://doi.org/10.3390/insects10070218





