Evaluating the Toxic Effects of Tannic Acid Treatment on Hyphantria cunea Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Tannic Acid Treatment
2.3. Determination of Mortality, Growth, and Nutrition Utilization
(1 − corrected water loss rate);
(food intake − weight of feces) × 100%;
2.4. Determination of MDA and H2O2 Contents
2.5. Determination of Antioxidant and Detoxification Capacities in the Larval Midgut
2.6. Gut Microbiological Analysis
2.7. Statistical Analysis
3. Results
3.1. Larval Mortality, Growth, and Food Utilization
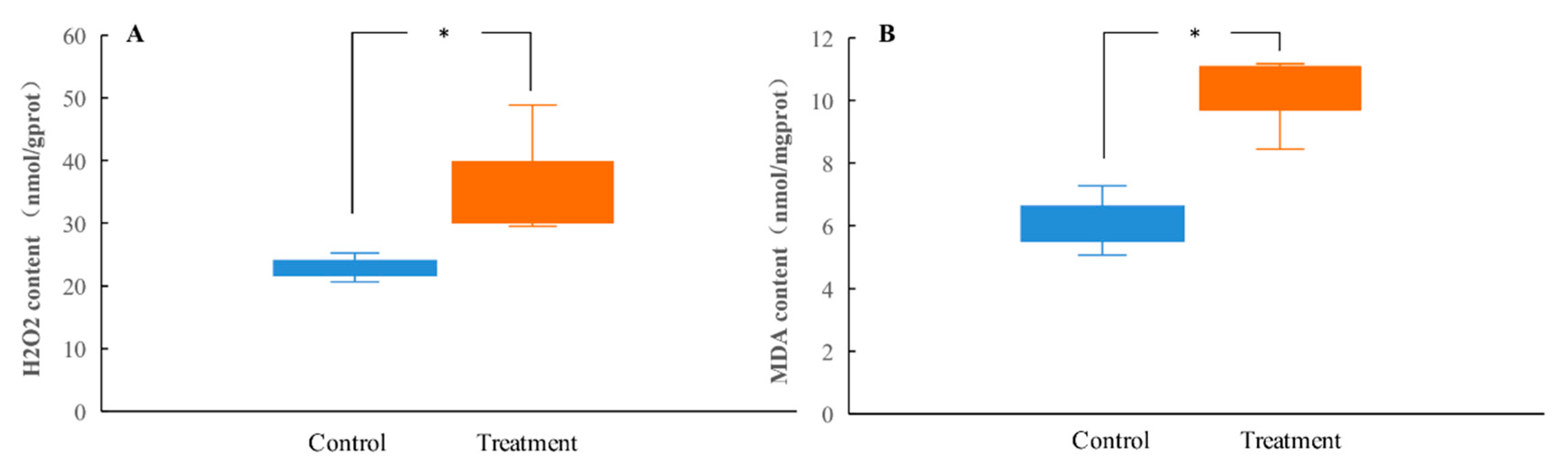
3.2. Evaluation of Oxidative Damage in Midgut tissues
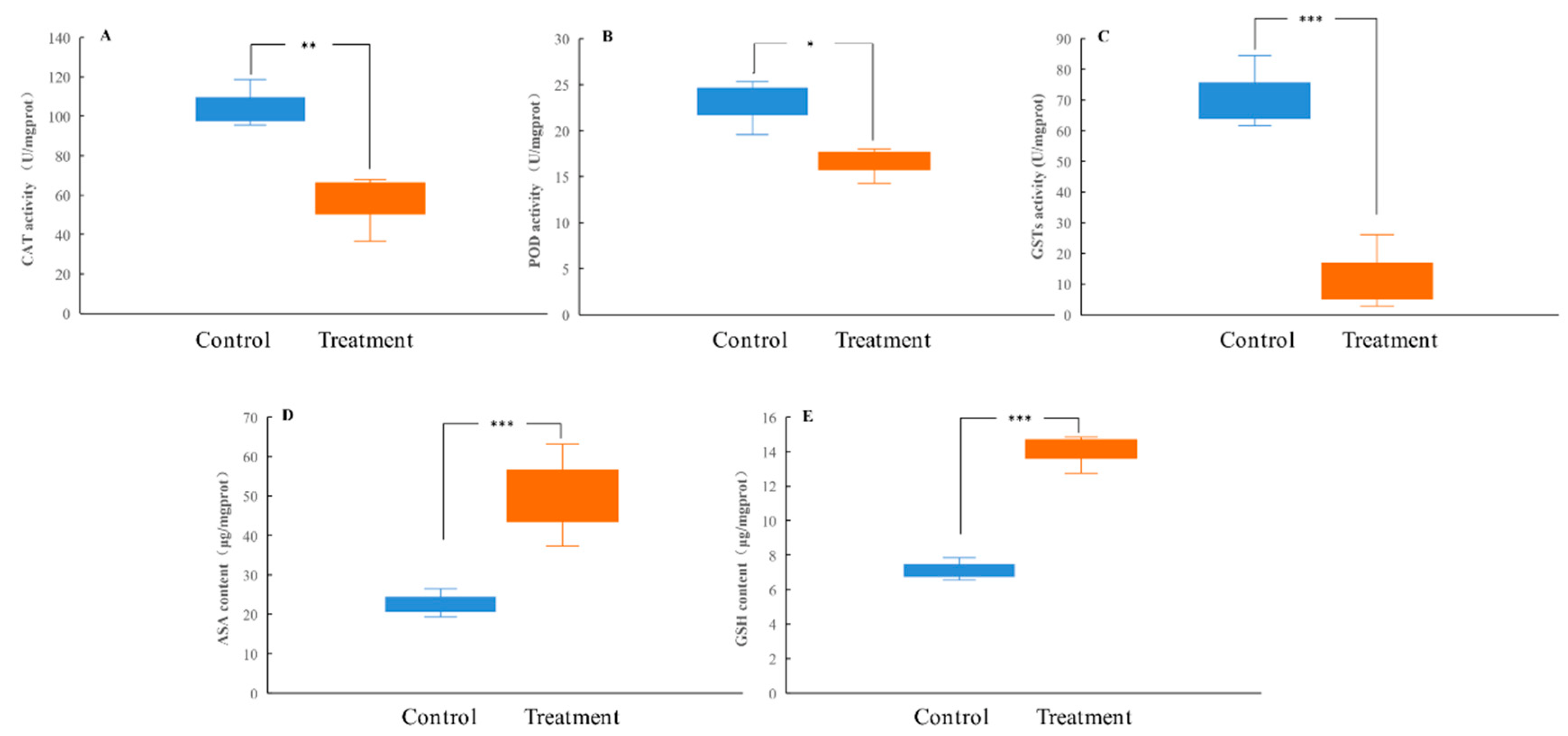
3.3. Larval Antioxidation and Detoxification Abilities
3.4. Analysis of the Microbial Diversity in the Larval Gut
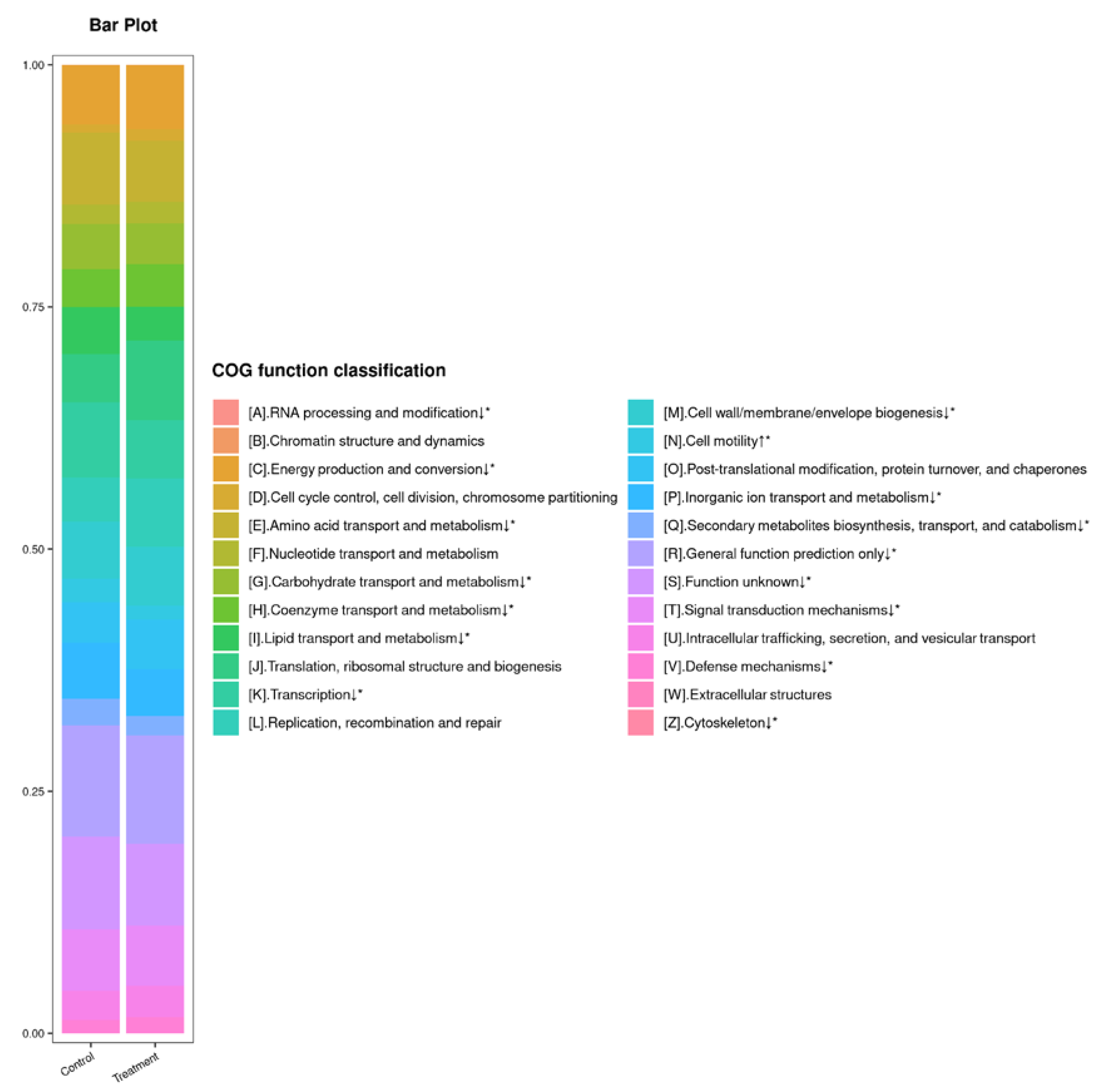
3.5. Taxonomic Composition of the Gut Microbiota
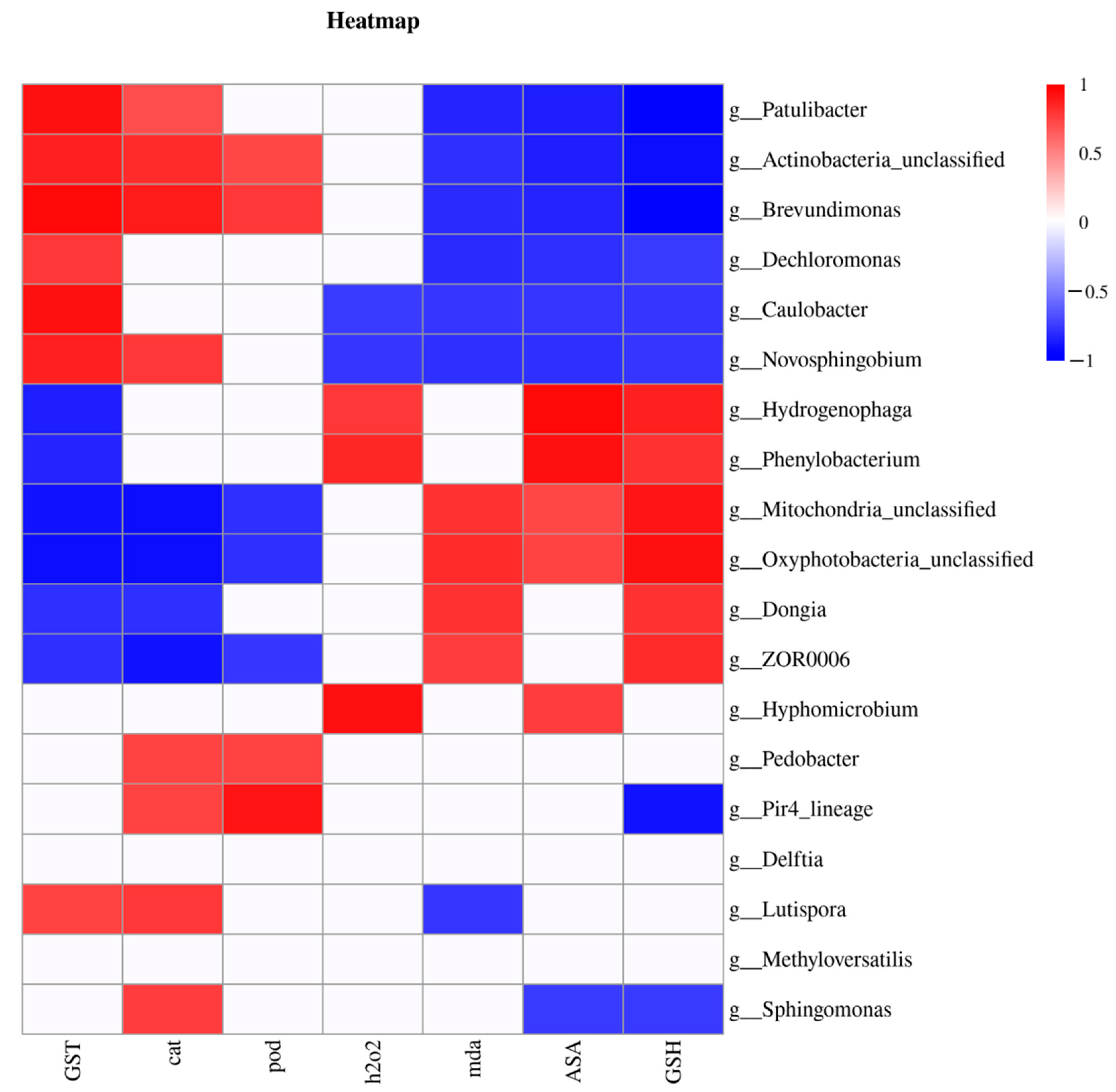
3.6. Correlation between Gut Microflora and Biochemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, G.A.; Herde, M. Interaction of plant defense compounds with the insect gut: New insights from genomic and molecular analyses. Curr. Opin. Insect. Sci. 2015, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ren, L.; Chen, F.; Feng, Y.; Luo, Y. Antifeedant Activity of Ginkgo biloba secondary metabolites against Hyphantria cunea larvae: Mechanisms and applications. PLoS ONE 2016, 11, e0155682. [Google Scholar] [CrossRef]
- Dixit, G.; Praveen, A.; Tripathi, T.; Yadav, V.K.; Verma, P.C. Herbivore-responsive cotton phenolics and their impact on insect performance and biochemistry. J. Asia-Pac. Entomol. 2017, 20, 341–351. [Google Scholar] [CrossRef]
- Sun, L.L.; Hou, W.H.; Zhang, J.J.; Dang, Y.L.; Yang, Q.Y.; Zhao, X.C.; Ma, Y.; Tang, Q.B. Plant metabolites drive different responses in caterpillars of two closely related Helicoverpa species. Front. Physiol. 2021, 12, 662978. [Google Scholar] [CrossRef]
- Chen, C.Y.; Han, P.; Yan, W.Y.; Wang, S.Y.; Shi, X.Y.; Zhou, X.G.; Desneux, N.; Gao, X.W. Uptake of quercetin reduces larval sensitivity to lambda-cyhalothrin in Helicoverpa armigera. J. Pest. Sci. 2018, 91, 919–926. [Google Scholar] [CrossRef]
- Kousar, B.; Bano, A.; Khan, N. PGPR Modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy 2020, 10, 778. [Google Scholar] [CrossRef]
- Yan, J.; Lipka, A.E.; Schmelz, E.A. Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress. J. Exp. Bot. 2015, 66, 593–602. [Google Scholar] [CrossRef]
- Roy, A.; Walker, W.B.; Vogel, H.; Chattington, S.; Larsson, M.C.; Anderson, P.; Heckel, D.G.; Schlyter, F. Diet dependent metabolic responses in three generalist insect herbivores Spodoptera spp. Insect Biochem. Molec. 2016, 71, 91–105. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends. Plant. Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Moraes, M.C.B.; Laumann, R.A.; Aquino, M.F.S.; Paula, D.P.; Borges, M. Effect of Bt genetic engineering on indirect defense in cotton via a tritrophic interaction. Transgenic. Res. 2011, 20, 99–107. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Yousaf, H.K.; Jan, S.; Wang, R.L.; Fernández-Grandon, G.M.; Li, X.; Gulzar, A.; Ali, B.; Rehman, M.; et al. RNA interference-mediated knockdown of a cytochrome P450 gene enhanced the toxicity of α-cypermethrin in xanthotoxin-fed larvae of Spodoptera exigua (Hübner). Pestic. Biochem. Phys. 2020, 162, 6–14. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Gao, X.; Luo, J.; et al. Silencing of cytochrome P450 gene CYP321A1 effects tannin detoxification and metabolism in Spodoptera litura. Int. Biol. Macromol. 2022, 194, 895–902. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, S.; Tan, M.; Wang, Q.; Zheng, L.; Yan, S. The high adaptability of Hyphantria cunea larvae to cinnamic acid involves in detoxification, antioxidation and gut microbiota response. Pestic. Biochem. Phys. 2021, 174, 104805. [Google Scholar] [CrossRef]
- Altuntas, H.; Duman, E.; Kilic, G. Juglone induced oxidative and genotoxic stress in the model insect Galleria mellonella L. (Pyralidae: Lepidoptera). Int. J. Trop. Insect. Sci. 2020, 40, 611–619. [Google Scholar] [CrossRef]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; LeGoff, G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): Responses to plant allelochemicals and pesticides. Insect. Mol. Biol. 2015, 24, 115–128. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Ma, H.J.; Feng, D.D.; Lai, X.F.; Chen, Z.M.; Xu, M.Y.; Yu, Q.Y.; Zhang, Z. Induction of detoxification enzymes by quercetin in the silkworm. J. Econ. Entomol. 2012, 105, 1034–1042. [Google Scholar] [CrossRef]
- Berasategui, A.; Salem, H.; Paetz, C.; Santoro, M.; Gershenzon, J.; Kaltenpoth, M.; Schmidt, A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 2017, 26, 4099–4110. [Google Scholar] [CrossRef]
- Guo, S.H.; Yi, X.F. Gut bacterial composition of two Curculio species and their adaptation to high-tannin food. Acta. Microbiologica. Sin. 2019, 59, 657–667. [Google Scholar]
- Jing, T.Z.; Qi, F.H.; Wang, Z.Y. Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 2020, 8, 38. [Google Scholar] [CrossRef]
- Xu, L.T.; Lou, Q.Z.; Cheng, C.H.; Lu, M.; Sun, J.H. Gut-associated bacteria of Dendroctonus valens and their involvement in verbenone production. Microb. Ecol. 2015, 70, 1012–1023. [Google Scholar]
- Zhang, S.K.; Wang, Y.; Li, Z.K.; Xue, H.J.; Zhou, X.D.; Huang, J.H. Two Apriona species sharing a host niche have different gut microbiome diversity. Microb. Ecol. 2021, 4, 1799. [Google Scholar] [CrossRef]
- Sun, L.L.; Ma, H.T.; Gao, Y.; Cao, C.Y. Functional identification and characterization of leucokinin and its Receptor in the fall webworm, Hyphantria cunea. Front. Physiol. 2021, 12, 741362. [Google Scholar]
- Feng, K.; Luo, J.; Ding, X.; Tang, F. Transcriptome analysis and response of three important detoxifying enzymes to Serratia marcescens Bizio (SM1) in Hyphantria cunea (Drury) (Lepidoptera: Noctuidae). Pestic. Biochem. Phys. 2021, 178, 104922. [Google Scholar]
- Topkara, E.F.; Yanar, O.; Solmaz, F.G. Effects of gallic acid and Zn, Cu, and Ni on antioxidant enzyme activities of Hyphantria cunea larvae infected with Bacillus thuringiensis. Ecotoxicology 2022, 31, 440–446. [Google Scholar] [PubMed]
- Wang, Z.; Feng, K.; Tang, F.; Xu, M. Activation of the host immune response in Hyphantria cunea (Drury) (Lepidoptera: Noctuidae) induced by Serratia marcescens Bizio. Insects 2021, 12, 983. [Google Scholar] [CrossRef]
- Edosa, T.T.; Jo, Y.H.; Keshavarz, M.; Anh, Y.S.; Noh, M.Y.; Han, Y.S. Current status of the management of fall webworm, Hyphantria cunea: Towards the integrated pest management development. J. Appl. Entomol. 2019, 143, 1–10. [Google Scholar]
- Sun, Z.; Lv, M.; Huang, W.; Li, T.; Xu, H. Development of botanical pesticides: Exploration on the phenotype of vestigial wings of insect pests induced by plant natural products or their derivatives by blocking tyrosine phosphorylation of insulin receptor 1. J. Agr. Food. Chem. 2022, 70, 2117–2126. [Google Scholar]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and prospects of botanical biopesticides in Europe and Mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar]
- Zhao, J.; Liang, D.; Li, W.; Yan, X.; Qiao, J.; Caiyin, Q. Research progress on the synthetic biology of botanical biopesticides. Bioengineering 2022, 9, 207. [Google Scholar]
- Yan, X.E.; Liu, Y.; Li, Z. Research progress of plant-derived aconitine as insecticide. ScienceAsia 2022, 48, 119–127. [Google Scholar] [CrossRef]
- Macauley, B.J.; Fox, L.R. Variation in total phenols and condensed tannins in leaf phenology and insect grazing. Austral. Ecol. 2010, 5, 31–35. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Cui, K.; Lu, Q.; Wang, C.; Wu, H.; Yang, Z.; Ding, W.; Shao, S.; Wang, H.; et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions. Sci. Rep. 2018, 8, 9841. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Tu, X.; Zhang, Z. Effects of four plant-derived compounds on the survival rate and activities of detoxification enzymes and protective enzymes in the grasshopper Oedaleus asiaticus. J. Plant Prot. 2021, 48, 158–164. [Google Scholar]
- Kim, J.; Park, G.G. Effects of persimmon tannin on survival and reproduction of Halyomorpha halys (Hemiptera: Pentatomidae). Entomol. Res. 2015, 45, 71–76. [Google Scholar] [CrossRef]
- Kumbasli, M.; Bauce, E.; Rochefort, S.; Creplin, M. Effects of tree age and stand thinning related variations in balsam fir secondary compounds on spruce budworm Choristoneura fumiferana development, growth and food utilization. Agr. Forest. Entomol. 2011, 13, 131–141. [Google Scholar] [CrossRef]
- Tong, L.L.; Yan, S.C.; Wang, Q.; Xu, B. Relationships of condenses tannin content in larch needles with larch stand age and its family. Chin. J. Ecol. 2010, 29, 221–225. [Google Scholar]
- Meng, Z.J.; Zhou, Y.Q.; Yan, S.C.; Jin, H.; Hu, X. Effects of exogenous jasmonates on tannin content in needles of two larch species. Sci. Silvae Sin. 2010, 46, 96–104. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Nahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.F.; Li, L.S.; Zhao, J.F.; Chen, M. Effect of tannic acid on nutrition and activities of detoxification enzymes and acetylcholinesterase of the fall webworm (Lepidoptera: Arctiidae). J. Insect. Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Gajger, I.T.; Dar, S.A. Plant allelochemicals as sources of insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Perkovich, C.; Ward, D. Protein: Carbohydrate ratios in the diet of gypsy moth Lymantria dispar affect its ability to tolerate tannin. J. Chem. Ecol. 2020, 46, 299–307. [Google Scholar] [PubMed]
- Zhang, W.H.; Liu, G.J. A review on plant secondary substances in plant resistance to insect pests. Chin. Bull. Bot. 2003, 20, 522–530. [Google Scholar]
- Jiang, D.; Zhou, Y.T.; Tan, M.T.; Zhang, J.; Guo, Q.X.; Yan, S.C. Cd exposure-induced growth retardation involves in energy metabolism disorder of midgut tissues in the gypsy moth larvae. Environ. Pollut. 2020, 066, 115173. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Tree resistance to Lymantria dispar caterpillars: Importance and limitations of foliar tannin composition. Oecologia 2009, 159, 777–788. [Google Scholar] [CrossRef]
- Chen, F.; Gao, X.; Lei, M.; Zheng, B. Effects of tannic acid on glutathione S-transferases in Helicoverpa armigera. Acta Entomol. Sin. 2003, 6, 4. [Google Scholar]
- Ma, J.; Zhu, D.; Chen, Q.L.; Ding, J.; Zhu, Y.G.; Sheng, G.D.; Qiu, Y.P. Exposure to tetracycline perturbs the microbiome of soil oligochaete Enchytraeus crypticus. Sci. Total Environ. 2019, 654, 643–650. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.; Xu, X.; Cai, R.; Xie, S. Effects of heavy metals on the bioaccumulation, excretion and gut microbiome of black soldier fly larvae (Hermetia illucens). Ecotox. Environ. Saf. 2020, 192, 110323. [Google Scholar] [CrossRef]
- Chakraborty, A.; Picardal, F. Neutrophilic, nitrate-dependent, Fe(II) oxidation by a Dechloromonas species. World J. Microb. Biot. 2013, 29, 617–623. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, P.; Zhang, H. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). Int. J. Mol. Sci. 2012, 13, 2563–2577. [Google Scholar] [CrossRef]
- Chen, Y.; Chai, L.; Tang, C.; Yang, Z.; Zheng, Y.; Shi, Y.; Zhang, H. Kraft lignin biodegradation by Novosphingobium sp. B-7 and analysis of the degradation process. Bioresour. Technol. 2012, 123, 682–685. [Google Scholar] [CrossRef]
- Guo, T.; Zhu, M.; Li, J.; Guo, X.; He, H. Composition and functions of cultural bacteria in the larval guts of Orthosia songi (Lepidoptera: Noctuidae). Sci. Silvae Sin. 2020, 56, 124–133. [Google Scholar]
- Graevenitz, A.V. Acinetobacter, Alcaligenes, Moraxella, and other nonfermentative gram-negative bacteria. Man. Clin. Microbiol. 1995, 4, 520–532. [Google Scholar]
- Smalley, N.E.; Taipale, S.; Marco, P.D.; Doronina, N.V.; Kyrpides, N.; Shapiro, N.; Woyke, T.; Kalyuzhnaya, M.G. Functional and genomic diversity of methylotrophic Rhodocyclaceae: Description of Methyloversatilis discipulorum sp. Nov. Int. J. Syst. Evol. Micr. 2015, 65, 2227–2233. [Google Scholar] [CrossRef]
- Xia, X.J.; Li, G.N.; Liao, F.R.; Zhang, F.S.; Zheng, J.; Kan, J.Q. Granular structure and physicochemical properties of starches from amaranth grain. Int. J. Food. Prop. 2015, 18, 1029–1037. [Google Scholar] [CrossRef]
- Li, F.C.; Li, M.X.; Mao, T.T.; Wang, H.; Chen, J.; Lu, Z.T.; Qu, J.W.; Fang, Y.L.; Gu, Z.Y.; Li, B. Effects of phoxim exposure on gut microbial composition in the silkworm, Bombyx mori. Ecotox. Environ. Saf. 2020, 189, 110011. [Google Scholar] [CrossRef]


| Control | Treatment (12.5 mg/g) | ||
|---|---|---|---|
| Larval mortality rate | 4th instar | 0.00 ± 0.00% a | 6.67 ± 1.76% b |
| 5th instar | 0.00 ± 0.00% a | 19.33 ± 2.91% b | |
| 6th instar | 0.00 ± 0.00% a | 45.67 ± 4.37% b | |
| Mature larvae | 0.00 ± 0.00% a | 100.00 ± 0.00% b | |
| Pupation rate Emergence rate | 70.00 ± 5.78% a | 0.00 ± 0.00% b | |
| 80.95 ± 6.67% a | 0.00 ± 0.00% b | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Wu, H.; Yan, S.; Jiang, D. Evaluating the Toxic Effects of Tannic Acid Treatment on Hyphantria cunea Larvae. Insects 2022, 13, 872. https://doi.org/10.3390/insects13100872
Tan M, Wu H, Yan S, Jiang D. Evaluating the Toxic Effects of Tannic Acid Treatment on Hyphantria cunea Larvae. Insects. 2022; 13(10):872. https://doi.org/10.3390/insects13100872
Chicago/Turabian StyleTan, Mingtao, Hongfei Wu, Shanchun Yan, and Dun Jiang. 2022. "Evaluating the Toxic Effects of Tannic Acid Treatment on Hyphantria cunea Larvae" Insects 13, no. 10: 872. https://doi.org/10.3390/insects13100872




