Comparison of Phototactic Behavior between Two Migratory Pests, Helicoverpa armigera and Spodoptera frugiperda
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. LED Light Sources and Intensity Design
2.3. Phototactic Behavior Tests
2.4. Phototactic Recoveries of Two Moths under Indoor Simulated Conditions
2.5. EC50 and Effective Distances of Two Moths to Different Wavelength Light Sources
2.6. Statistical Analysis
3. Results
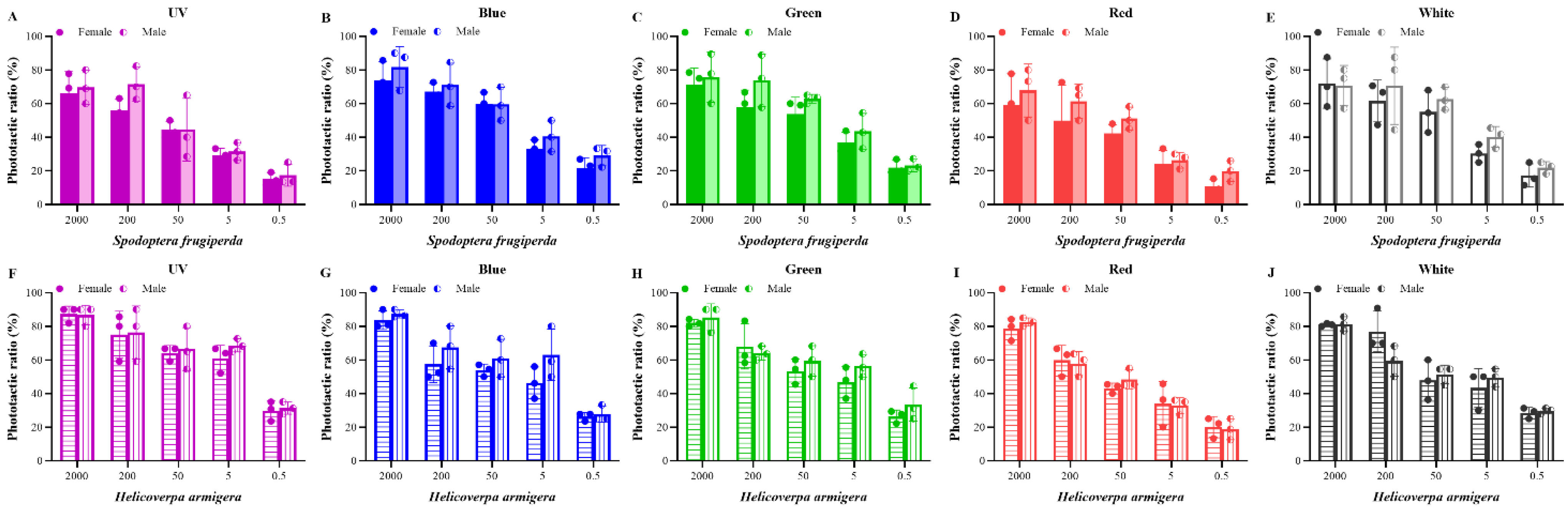
3.1. Phototactic Rates of Two Moths to Different Wavelength Light Sources
3.2. Phototactic Rates of Two Moths to Different Light Intensities
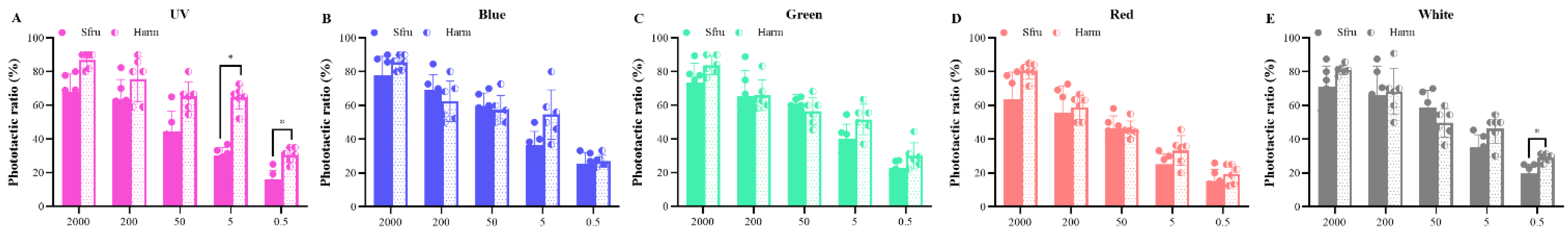
3.3. Comparison of Phototactic Rates between S. frugiperda and H. armigera
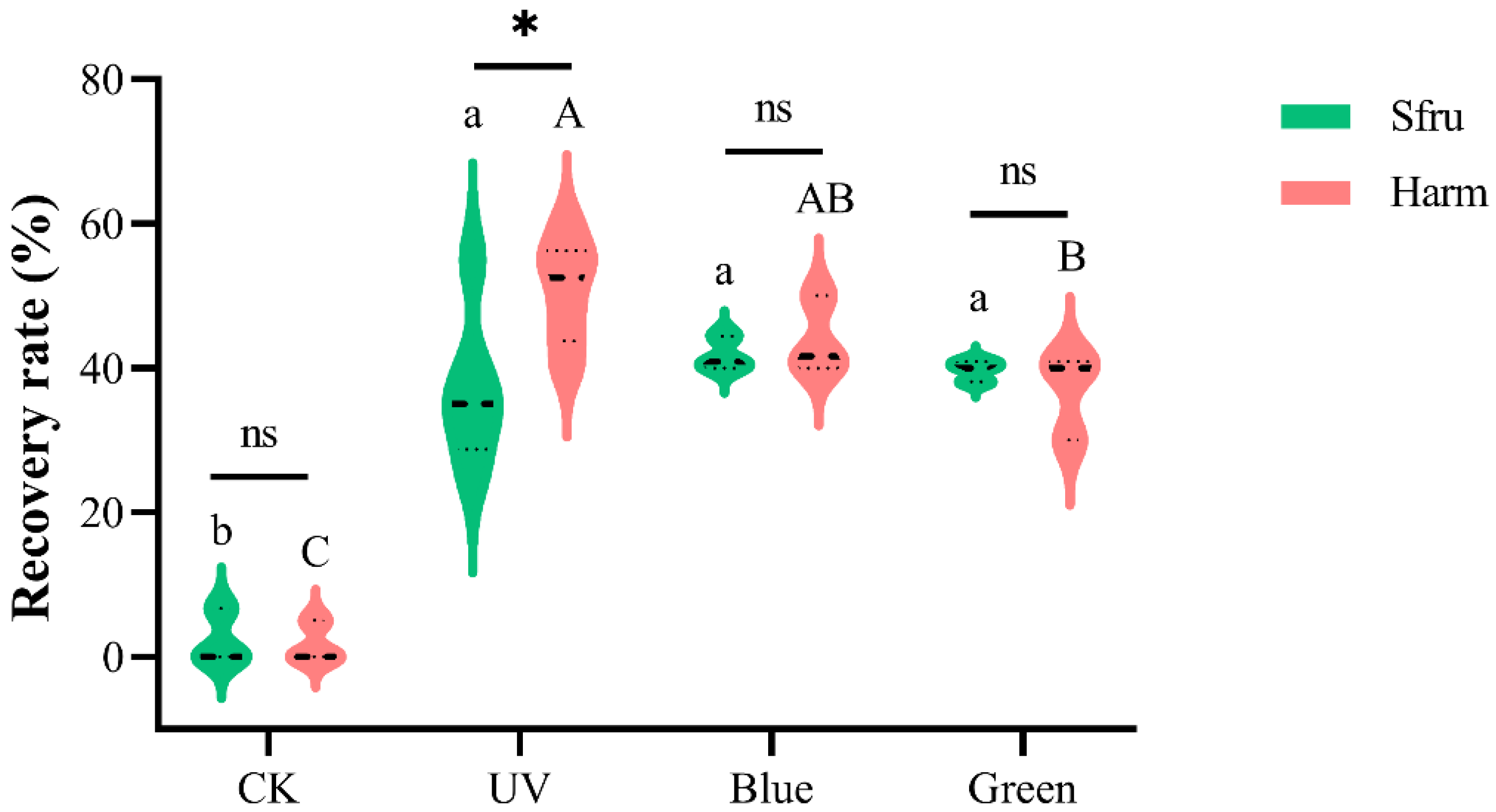
3.4. Phototactic Recovery Rates of Simulated Recovery Experiments for Two Moths
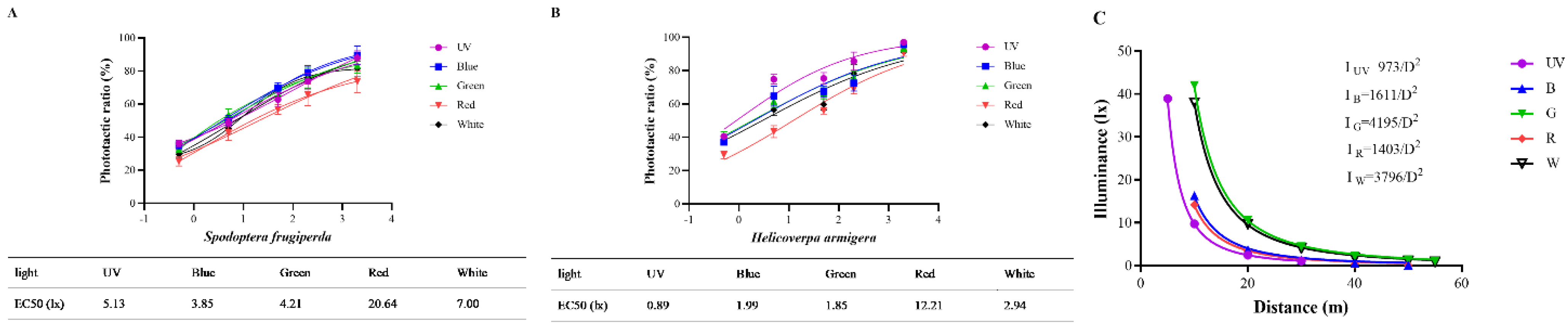
3.5. EC50 and Trapping Distances of Two Moths with Different Light Sources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. Fall Armyworm Symposium: A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Togola, A.; Meseka, S.; Menkir, A.; Badu-Apraku, B.; Boukar, O.; Tamò, M.; Djouaka, R. Measurement of pesticide residues from chemical control of the invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a maize experimental field in Mokwa, Nigeria. Int. J. Environ. Res. Pub. He. 2018, 15, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Huang, C.; Li, C.Y.; Zhou, H.X.; Ren, Y.l.; Li, Z.Y.; Xing, L.S.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agr. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Ge, S.; Sun, X.; He, W.; Wyckhuys, K.A.G.; He, L.; Zhao, S.; Zhang, H.; Wu, K. Potential trade-offs between reproduction and migratory flight in Spodoptera frugiperda. J. Insect Physiol. 2021, 132, 104248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhang, D.D.; Yang, L.Y.; Dong, Y.H.; Liang, G.M.; Philip, D.; Ren, G.W.; Xu, P.J.; Wu, K.M. Analysis of phototactic responses in Spodoptera frugiperda using Helicoverpa armigera as control. J. Integr. Agr. 2021, 20, 821–828. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.D.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.L.; Henri, H.; Vavre, F. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Tendeng, E.; Labou, B.; Diatte, M.; Djiba, S.; Diarra, K. The fall armyworm Spodoptera frugiperda (JE Smith), a new pest of maize in Africa: Biology and first native natural enemies detected. Int. J. Biol. Chem. Sci. 2019, 13, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Kasoma, C.; Shimelis, H.; Laing, M.D. Fall armyworm invasion in Africa: Implications for maize production and breeding. J. Crop Improv. 2020, 35, 111–146. [Google Scholar] [CrossRef]
- van Grunsven, R.H.A.; Donners, M.; Boekee, K.; Tichelaar, I.; van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E.M. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Pan, H.; Liang, G.; Lu, Y. Response of Different Insect Groups to Various Wavelengths of Light under Field Conditions. Insects 2021, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; Ffrench-Constant, R.H. Shedding light on moths: Shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 2013, 9, 20130376. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Lee, H.-S. Phototactic behavior 5: Attractive effects of the angoumois grain moth, Sitotroga cerealella, to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 259–262. [Google Scholar] [CrossRef]
- Kim, K.N.; Song, H.S.; Li, C.S.; Huang, Q.Y.; Lei, C.H. Effect of several factors on the phototactic response of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2018, 21, 952–957. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yan, S.; Shen, Z.J.; Li, Z.; Zhang, X.F.; Liu, X.M.; Zhang, Q.W.; Liu, X.X. The expression of three opsin genes and phototactic behavior of Spodoptera exigua (Lepidoptera: Noctuidae): Evidence for visual function of opsin in phototaxis. Insect Biochem. Mol. Biol. 2018, 96, 27–35. [Google Scholar] [CrossRef]
- Cowan, T.; Gries, G. Ultraviolet and violet light: Attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomol. Exp. Appl. 2009, 131, 148–158. [Google Scholar] [CrossRef]
- Pan, H.S.; Xu, Y.L.; Liang, G.M.; Wyckhuys, K.A.; Yang, Y.Z.; Lu, Y.H. Field evaluation of light-emitting diodes to trap the cotton bollworm, Helicoverpa armigera. Crop Prot. 2020, 137, 105267. [Google Scholar] [CrossRef]
- Garris, H.W.; Snyder, J.A.J.S.N. Sex-specific attraction of moth species to ultraviolet light traps. Southeast. Nat. 2010, 9, 427–434. [Google Scholar] [CrossRef]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest. Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Paris, T.M.; Allan, S.A.; Udell, B.J.; Stansly, P.A. Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid. Insects 2017, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.K.; Pan, X.H.; Liu, W.; Wei, J.L.; Huang, C.H.; Goebel, F.; Nikpay, A. Effect of spectral sensitivity and light intensity response on the phototactic behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a pest of sugarcane in China. Agronomy 2022, 12, 481. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Zhou, J.X.; Dou, H.; Kuang, R.P. Phototactic behaviour of Pachyneuron aphidis (Hymenoptera: Pteromalidae)—Hyperparasitoid of Myzus persicae (Hemiptera: Aphidiae). Biocontrol. Sci. Techn. 2014, 24, 1469–1480. [Google Scholar] [CrossRef]
- Wen, C.; Ji, Y.C.; Zhang, G.Y.; Tan, S.B.; Wen, J.B. Phototactic behaviour of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) adults. Biocontrol Sci. Technol. 2018, 28, 544–561. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, J.; Zhang, R.P.; Li, H.M.; Kuang, R.P. Spectral sensitivity and response to light intensity of Thrips tabaci Lindeman (Thysanoptera:Thripidae). J. Henan Agr. Sci. 2020, 49, 98–104. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, M.; Zhang, H.; Sun, H.; Wang, H.; Miao, L.; Li, M.; Shu, R.; Qin, Q. A greenhouse test to explore and evaluate light-emitting diode (LED) insect traps in the monitoring and control of Trialeurodes vaporariorum. Insects 2020, 11, 94. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Y.; Liu, J.; Yang, J.J.; Zhao, W.X.; Yin, L.; Liu, Y.; Ye, S.F.; Qin, B.Q.; Song, L.D. Trapping effect of searchlight-trap and light trap for the moth of Spodoptera frugiperda in 2019. Plant Prot. 2020, 46, 118–122. [Google Scholar]
- Dent, D.R.; Pawar, C.S. The influence of moonlight and weather on catches of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) in light and pheromone traps. B Entomol. Res. 1988, 78, 365–377. [Google Scholar] [CrossRef]
- Vilarinho, E.C.; Fernandes, O.A.; Hunt, T.E.; Caixeta, D.F. Movement of Spodoptera frugiperda adults (Lepidoptera: Noctuidae) in maize in Brazil. Fla. Entomol. 2011, 94, 480–488. [Google Scholar] [CrossRef]
- Johansen, N.S.; Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Shipp, L. In the light of new greenhouse technologies: 2. Direct effects of artificial lighting on arthropods and integrated pest management in greenhouse crops. Ann. Appl. Biol. 2011, 159, 1–27. [Google Scholar] [CrossRef]
- Colvin, J.; Gatehouse, A.G. The reproduction-flight syndrome and the inheritance of tethered-flight activity in the cotton-bollworm moth, Heliothis armigera. Physiol. Entomol. 1993, 18, 16–22. [Google Scholar] [CrossRef]
- Wei, G.S.; Zhang, Q.W.; Zhou, M.Z. Assessment of the control effects and effective radi of four kinds of traps for cotton bollworm in cotton fields. Acta. Phytophy Sin. 2001, 28, 157–162. [Google Scholar]
- Ullah, F.; Ali, M.; Ahmad, S.; Badshah, H. Impact of light traps on population density of gram pod borer, Helicoverpa armigera (Hub) (Lepidoptera: Noctuidae) and its larval parasitoid (Campoletis chlorideae Uchida) in Rod Kohi area of Dera Ismail Khan, Pakistan. J. Entomol. Zool. Stud. 2015, 2, 203–207. [Google Scholar]
- King, A.B.S.; Armes, N.J.; Pedgley, D.E. A mark-capture study of Helicoverpa armigera dispersal from pigeonpea in southern India. Entomol. Exp. Appl. 1990, 55, 257–266. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Marchetti, E.; Nasi, S.; Ferrari, R.; Conti, E. Improved Captures of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys, Using a Novel Multimodal Trap. Insects 2022, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Liu, Z.X.; Chen, Y.T.; Wang, Y.; Chen, J.Z.; Fu, S.; Ma, W.F.; Xia, S.; Liu, D.; Wu, T.; et al. CRISPR/Cas9-mediated knockout of LW-opsin reduces the efficiency of phototaxis in the diamondback moth Plutella xylostella. Pest. Manag. Sci. 2021, 77, 3519–3528. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Yuan, F.; Savage, W.K.; Bernard, G.D.; Mullen, S.P.; Briscoe, A.D. Opsin clines in butterflies suggest novel roles for insect photopigments. Mol. Biol. Evol. 2015, 32, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.J.; Feuda, R.; Lu, B.; Xiao, H.J.; Graham, R.I.; Wu, K.M. Functional opsin retrogene in nocturnal moth. Mob. DNA 2016, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuda, R.; Marletaz, F.; Bentley, M.A.; Holland, P.W. Conservation, duplication, and divergence of five opsin genes in insect evolution. Genome Biol. Evol. 2016, 8, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Lee, H.S. Phototactic behavioral response of agricultural insects and stored-product insects to light-emitting diodes (LEDs). Appl. Biol. Chem. 2017, 60, 137–144. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, H.S. Visual preference of diamondback moth, Plutella xylostella, to light-emitting diodes. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 681–684. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.M.; Lee, S.G.; Lee, H.S. Attractive Effects Efficiency of LED Trap on Controlling Plutella xylostella Adults in Greenhouse. J. Appl. Biol. Chem. 2014, 57, 255–257. [Google Scholar] [CrossRef]
- Chen, Y.W. Capture Effect of Different Wavelengths and Intensities of LED Light on Plutella Xylostella and Capture Device Development. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Kecskeméti, S.; Geösel, A.; Fail, J.; Egri, Á. In search of the spectral composition of an effective light trap for the mushroom pest Lycoriella ingenua (Diptera: Sciaridae). Sci. Rep. 2021, 11, 12770. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S. The Optics of Life. In The Optics of Life, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2012; pp. 10–41. [Google Scholar]
- Cheng, W.J.; Zheng, X.L.; Wang, P.; Zhou, L.L.; Si, S.Y.; Wang, X.P. Male-Biased capture in light traps in Spodoptera exigua (Lepidoptera: Noctuidae): Results from the studies of reproductive activities. J. Insect Behav. 2016, 29, 368–378. [Google Scholar] [CrossRef]
- Nowinszky, L.; Puskás, J. Sex ratio analysis of some Macrolepidoptera species collected by Hungarian forestry light traps. Acta Silv. Lignaria Hung. 2015, 11, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gao, Z.; Deng, S.Z.; Cao, F.Q.; Lu, Y.Y. The photokinesis of oriental fruit flies, Bactrocera dorsalis, to LED lights of various wavelengths. Entomol. Exp. Appl. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Truxa, C.; Fiedler, K. Attraction to light—From how far do moths (Lepidoptera) return to weak artificial sources of light? Eur. J. Entomol. 2012, 109, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Bowden, J.; Morris, M.G. The influence of moonlight on catches of insects in light-traps in Africa. III. The effective radius of a mercury-vapour light-trap and the analysis of catches using effective radius. B Entomol. Res. 1975, 65, 303–348. [Google Scholar] [CrossRef]
- Baker, R.R.; Sadovy, Y. The distance and nature of the light-trap response of moths. Nature 1978, 276, 818–821. [Google Scholar] [CrossRef]
- Agee, H.R. Sensory response of the compound eye of adult Heliothis zea and H. virescens to ultraviolet stimuli. Ann. Entomol. Soc. Am. 1972, 65, 701–705. [Google Scholar] [CrossRef]
- Nowinszky, L.; Puskás, J. Light-trap catch of harmful Microlepidoptera species in connection with polarized moonlight and collecting distance. J. Adv. Lab. Res. Biol. 2013, 4, 108–117. [Google Scholar]
- Bowden, J. An analysis of factors affecting catches of insects in light-traps. B Entomol. Res. 1982, 72, 535–556. [Google Scholar] [CrossRef]
- Merckx, T.; Slade, E.M. Macro-moth families differ in their attraction to light: Implications for light-trap monitoring programmes. Insect Conserv. Diver. 2014, 7, 453–461. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Miao, J.; Gong, Z.; Li, T.; Duan, Y.; Wu, Y. Photoreceptive reaction spectrum effect and phototactic activity intensity of locusts’ visual display characteristics stimulated by spectral light. Int. J. Agric. Biol. Eng. 2021, 14, 19–25. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.-M.; Zhou, C.-L. Phototactic Behavior of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Coleopts. Bull. 2020, 74, 513–522. [Google Scholar] [CrossRef]
- Liu, Q.H.; Xin, Z.; Zhou, Q. Visual reaction effects induced and stimulated by different lights on phototactic bio-behaviors in Locusta migratoria manilensis. Int. J. Agric. Biol. Eng. 2017, 10, 173–181. [Google Scholar] [CrossRef]
- Song, J.; Jeong, E.-Y.; Lee, H.-S. Phototactic behavior 9: Phototactic behavioral response of Tribolium castaneum (Herbst) to light-emitting diodes of seven different wavelengths. J. Appl. Biol. Chem. 2016, 59, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.-W.; Chen, Y. Phototactic Behavior of Scleroderma guani (Hymenoptera: Bethylidae)—Parasitoid of Pissodes punctatus (Coleoptera: Curculionidae). J. Insect Behav. 2016, 29, 605–614. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, R.; Kuang, R.p.; Sun, R. Phototactic behaviour of the parasitoid Encarsia Formosa (Hymenoptera: Aphelinidae). Biocontrol Sci. Techn. 2016, 26, 250–262. [Google Scholar] [CrossRef]


| Species | Light Source | P1 (%) | P3 (%) | Distance (m) |
|---|---|---|---|---|
| S. frugiperda | blue | 50 | 87.5 | 36 |
| 10 * | 27.1 | 108 | ||
| H. armigera | UV | 50 | 87.5 | 54 |
| 10 * | 27.1 | 163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chang, Y.; Zhang, S.; Jiang, X.; Yang, B.; Wang, G. Comparison of Phototactic Behavior between Two Migratory Pests, Helicoverpa armigera and Spodoptera frugiperda. Insects 2022, 13, 917. https://doi.org/10.3390/insects13100917
Wang Y, Chang Y, Zhang S, Jiang X, Yang B, Wang G. Comparison of Phototactic Behavior between Two Migratory Pests, Helicoverpa armigera and Spodoptera frugiperda. Insects. 2022; 13(10):917. https://doi.org/10.3390/insects13100917
Chicago/Turabian StyleWang, Yong, Yajun Chang, Sai Zhang, Xingchuan Jiang, Bin Yang, and Guirong Wang. 2022. "Comparison of Phototactic Behavior between Two Migratory Pests, Helicoverpa armigera and Spodoptera frugiperda" Insects 13, no. 10: 917. https://doi.org/10.3390/insects13100917






