An Ant-Mimicking Jumping Spider Achieves Higher Predation Probability with Lower Success Rate When Exposed to Ethanol
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Spider and Fly Maintenance
2.2. ETOH Preference Test
2.3. Predation Probability Test
2.4. Prey Capture Efficiency
2.5. Data Analysis
3. Results
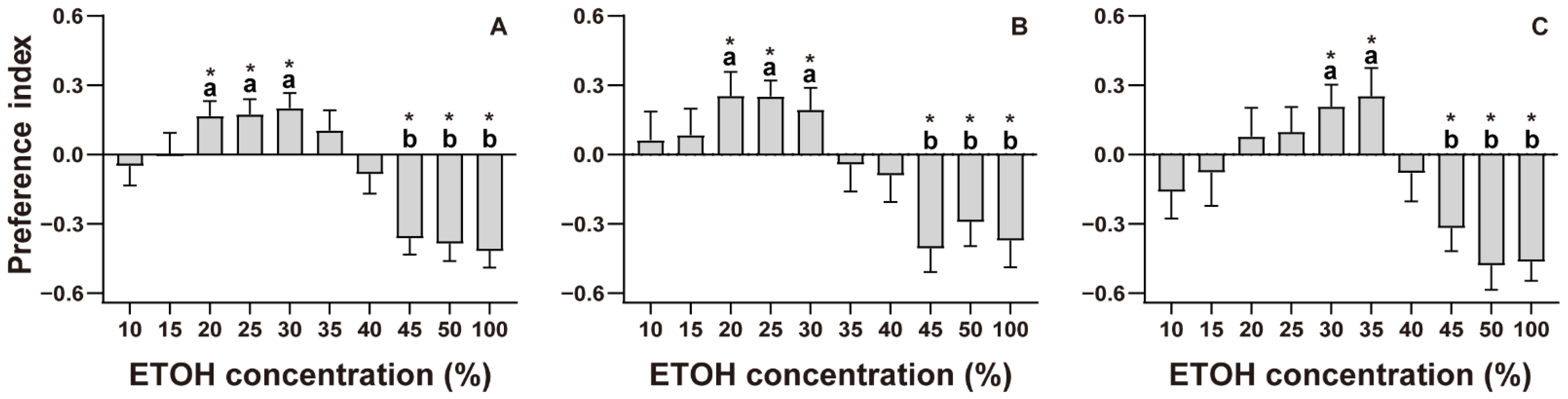
3.1. ETOH Preference Test
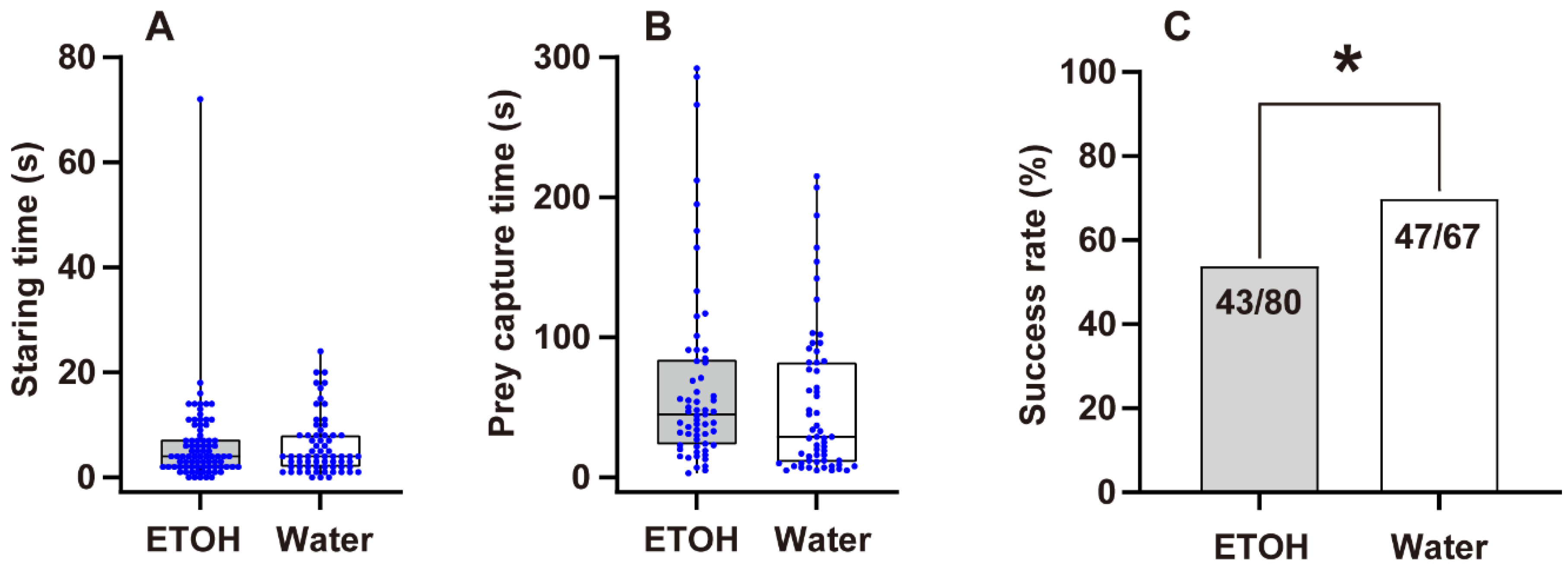
3.2. Predation Probability Test
3.3. Prey Capture Efficiency
4. Discussion
4.1. ETOH Preference
4.2. Predation Probability and Prey Capture Efficiency
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bainton, R.J.; Tsai, L.T.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine, and ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Mustard, J.A.; Edgar, E.A.; Mazade, R.E.; Wu, C.; Lillvis, J.L.; Wright, G.A. Acute ethanol ingestion impairs appetitive olfactory learning and odour discrimination in the honey bee. Neurobiol. Learn. Mem. 2008, 90, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, R.; Maro, A. Human evolution and dietary ethanol. Nutrients 2021, 13, 2419. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.W.; Heberlein, U. Invertebrate models of drug abuse. Dev. Neurobiol. 2003, 54, 161–178. [Google Scholar] [CrossRef]
- Daack, C.W.; Yeh, D.; Busch, M.; Kliethermes, C.L. GABAergic regulation of locomotion before and during an ethanol exposure in Drosophila melanogaster. Behav. Brain Res. 2021, 410, 113369. [Google Scholar] [CrossRef]
- Troutwine, B.; Park, A.; Velez-Hernandez, M.E.; Lew, L.; Mihic, S.J.; Atkinson, N.S. F654A and K558Q mutations in NMDA receptor 1 affect ethanol-induced behaviours in Drosophila. Alcohol. Clin. Exp. Res. 2019, 43, 2480–2493. [Google Scholar] [CrossRef]
- Scaplen, K.M.; Petruccelli, E. Receptors and channels associated with alcohol use: Contributions from Drosophila. Neurosci. Insights 2021, 16, 26331055211007441. [Google Scholar] [CrossRef]
- Rodan, A.R.; Rothenfluh, A. The genetics of behavioural alcohol responses in Drosophila. Int. Rev. Neurobiol. 2010, 91, 25–51. [Google Scholar]
- Abramson, C.I.; Stone, S.M.; Ortez, R.A.; Luccardi, A.; Vann, K.L.; Hanig, K.D.; Rice, J. The development of an ethanol model using social insects I: Behaviour studies of the honey bee (Apis mellifera L.). Alcohol. Clin. Exp. Res. 2000, 24, 1153–1166. [Google Scholar] [CrossRef]
- Singh, C.M.; Heberlein, U. Genetic control of acute ethanol-induced behaviours in Drosophila. Alcohol. Clin. Exp. Res. 2000, 24, 1127–1136. [Google Scholar] [CrossRef]
- Parr, J.; Large, A.; Wang, X.; Fowler, S.C.; Ratzlaff, K.L.; Ruden, D.M. The inebri-actometer: A device for measuring the locomotor activity of Drosophila exposed to ethanol vapor. J. Neurosci. Meth. 2001, 107, 93–99. [Google Scholar] [CrossRef]
- Schumann, I.; Berger, M.; Nowag, N.; Schäfer, Y.; Saumweber, J.; Scholz, H.; Thum, A.S. Ethanol-guided behaviour in Drosophila larvae. Sci. Rep. 2021, 11, 12307. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.W.; Rodan, A.R.; Tsai, L.T.Y.; Heberlein, U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J. Neurosci. 2002, 22, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Devineni, A.V.; Heberlein, U. The evolution of Drosophila melanogaster as a model for alcohol research. Annu. Rev. Neurosci. 2013, 36, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Lin, S.; Waddell, S. Remembering components of food in Drosophila. Front. Integr. Neurosc. 2016, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Abramson, C.I.; Place, A.J.; Aquino, I.S.; Fernandez, A. Development of an ethanol model using social insects: IV. influence of ethanol on the aggression of africanized honey bees (Apis Mellifera L.). Psychol. Rep. 2004, 94, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Creehan, K.M.; de Guglielmo, G.; Roberts, A.J.; Taffe, M.A. Behavioural effects of ethanol in the red swamp crayfish (Procambarus clarkii). J. Exp. Anal. Behav. 2022, 117, 472–492. [Google Scholar] [CrossRef]
- Patt, J.M.; Pfannenstiel, R.S. Odour-based recognition of nectar in cursorial spiders. Entomol. Exp. Appl. 2008, 127, 64–71. [Google Scholar] [CrossRef]
- Cerveira, A.M.; Jackson, R.R. Love is in the air and on the ground: Olfactory and tactile cues elicit visual courtship behaviour by Cyrba males (Araneae: Salticidae). J. Arachnol. 2013, 41, 374–380. [Google Scholar] [CrossRef]
- Nelson, X.J.; Jackson, R.R. Timid spider uses odour and visual cues to actively select protected nesting sites near ants. Behav. Ecol. Sociobiol. 2014, 68, 773–780. [Google Scholar] [CrossRef]
- Hesselberg, T.; Vollrath, F. The effects of neurotoxins on web-geometry and web-building behaviour in Araneus diadematus Cl. Physiol. Behav. 2004, 82, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Benamú, M.A.; Schneider, M.I.; González, A.; Sánchez, N.E. Short and long-term effects of three neurotoxic insecticides on biological and behavioural attributes of the orb-web spider Alpaida veniliae (Araneae, Araneidae): Implications for IPM programs. Ecotoxicology 2013, 22, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Albín, A.; Lacava, M.; Viera, C. Effects of gonyleptidine on the orb-web spider Araneus lathyrinus (Holmberg, 1875). Arachnology 2014, 16, 154–156. [Google Scholar] [CrossRef]
- Pasquet, A.; Tupinier, N.; Mazzia, C.; Capowiez, Y. Exposure to spinosad affects orb-web spider (Agalenatea redii) survival, web construction and prey capture under laboratory conditions. J. Pest. Sci. 2016, 89, 507–515. [Google Scholar] [CrossRef]
- Tietjen, W.J. Pesticides affect the mating behaviour of Rabidosa rabida (Araneae, Lycosidae). J. Arachinol. 2006, 34, 285–288. [Google Scholar] [CrossRef]
- Deng, L.; Dai, J.; Cao, H.; Xu, M. Effects of an organophosphorous insecticide on survival, fecundity, and development of Hylyphantes graminicola (Sundevall) (Araneae: Linyphiidae). Environ. Toxicol. Chem. 2006, 25, 3073–3077. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, B.; Helton, W.S.; Nelson, X.J. Caffeine affects the vigilance decrement of Trite planiceps jumping spiders (salticidae). J. Comp. Psychol. 2019, 133, 551–557. [Google Scholar] [CrossRef]
- World Spider Catalog. World Spider Catalog; Version 23.0; Natural History Museum Bern: Bern, Switzerland, 2022; Available online: http://wsc.nmbe.ch (accessed on 1 October 2022).
- Robledo-Ospina, L.E.; Rao, D. Dangerous visions: A review of visual antipredator strategies in spiders. Evol. Ecol. 2022, 36, 163–180. [Google Scholar] [CrossRef]
- Rubio, G.D.; Stolar, C.E.; Ohashi, D.V.; Baigorria, J.E. Jumping spiders (Araneae: Salticidae) in agroecosystems: A case study to know how friendly some crops can be for native fauna. Stud. Neotrop. Fauna. E 2019, 54, 133–148. [Google Scholar] [CrossRef]
- Cobbold, S.M.; O’Donnell, R.P. Plant structure specialization in Paraphidippus basalis (Araneae: Salticidae), a jumping spider of the Madrean Sky Islands. J. Arachinol. 2021, 49, 159–166. [Google Scholar] [CrossRef]
- Junggebauer, A.; Hartke, T.R.; Ramos, D.; Schaefer, I.; Buchori, D.; Hidayat, P.; Scheu, S.; Drescher, J. Changes in diversity and community assembly of jumping spiders (Araneae: Salticidae) after rainforest conversion to rubber and oil palm plantations. PeerJ 2021, 9, e11012. [Google Scholar] [CrossRef] [PubMed]
- Jasmi, R.A.; Sari, H.P.E.; Janra, M.N. Jumping spider (Arachnida: Salticidae: Araneae) in serang residential area, banten: Inventory study using a photographic approach. J. Biol. Trop. 2022, 22, 30–39. [Google Scholar] [CrossRef]
- Mittelbach, M.; Vannette, R.L. Yeasts in Natural Ecosystems: Ecology; Springer: Cham, Switzerland, 2017; pp. 155–178. [Google Scholar]
- Nespolo, R.F.; Solano-Iguaran, J.J.; Paleo-López, R.; Quintero-Galvis, J.F.; Cubillos, F.A.; Bozinovic, F. Performance, genomic rearrangements, and signatures of adaptive evolution: Lessons from fermentative yeasts. Ecol. Evol. 2020, 10, 5240–5250. [Google Scholar] [CrossRef]
- Madden, A.A.; Epps, M.J.; Fukami, T.; Irwin, R.E.; Sheppard, J.; Sorger, D.M.; Dunn, R.R. The ecology of insect–yeast relationships and its relevance to human industry. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20172733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.R.; Pollard, S.D.; Nelson, X.J.; Edwards, G.B.; Barrion, A.T. Jumping spiders (Araneae: Salticidae) that feed on nectar. J. Zool. 2001, 255, 25–29. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Olesen, J.M. The fruit-wasp route to toxic nectar in Epipactis orchids? Flora 1997, 192, 223–229. [Google Scholar] [CrossRef]
- Jones, P.; Agrawal, A.A. Caffeine and ethanol in nectar interact with flower color impacting bumblebee behaviour. Behav. Ecol. Sociobiol. 2022, 76, 1–11. [Google Scholar]
- Lim, M.L.M.; Land, M.F.; Li, D. Sex-specific UV and fluorescence signals in jumping spiders. Science 2007, 315, 481. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yu, L.; Kwek, B.Z.; Jin, G.; Zeng, H.; Li, D. Sexual selection on jumping spider colour pattern: Investigation with a new quantitative approach. Behav. Ecol. 2021, 32, 695–706. [Google Scholar] [CrossRef]
- Fischer, C.N.; Trautman, E.P.; Crawford, J.M.; Stabb, E.V.; Handelsman, J.; Broderick, N.A. Metabolite exchange between microbiome members produces compounds that influence Drosophila behaviour. eLife 2017, 6, e18855. [Google Scholar] [CrossRef]
- Nelson, X.J.; Jackson, R.R. Vision-based ability of an ant-mimicking jumping spider to discriminate between models, conspecific individuals and prey. Insect. Soc. 2007, 54, 1–4. [Google Scholar] [CrossRef]
- Tedore, C.; Johnsen, S. Weaponry, color, and contest success in the jumping spider Lyssomanes virtdis. Behav. Process 2012, 89, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Kendler, K.S.; Bettinger, J.C.; Davies, A.G.; Grotewiel, M. An assay for evoked locomotor behaviour in Drosophila reveals a role for integrins in ethanol sensitivity and rapid ethanol tolerance. Alcohol. Clin. Exp. Res. 2009, 33, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- Elias, D.O.; Kasumovic, M.M.; Punzalan, D.; Andrade, M.C.; Mason, A.C. Assessment during aggressive contests between male jumping spiders. Anim. Behav. 2008, 76, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Kwek, B.Z.W.; Tan, M.; Yu, L.; Zhou, W.; Chang, C.C.; Li, D. Aggressive males are more attractive and are more likely to win contests in jumping spiders. Anim. Behav. 2021, 179, 51–63. [Google Scholar] [CrossRef]
- Forster, L.M. Visual mechanisms of hunting behaviour in trite planiceps, a jumping spider (Araneae: Salticidae). N. Z. J. Zool. 1979, 6, 79–93. [Google Scholar] [CrossRef]
- Forster, L.M. Vision and prey catching strategies in jumping spiders. Am. Sci. 1982, 70, 165–175. [Google Scholar]
- Devineni, A.V.; Heberlein, U. Preferential ethanol consumption in Drosophila models features of addiction. Curr. Biol. 2009, 19, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Kanno, M.; Hiramatsu, S.; Kondo, S.; Tanimoto, H.; Ichinose, T. Voluntary intake of psychoactive substances is regulated by the dopamine receptor Dop1R1 in Drosophila. Sci. Rep. 2021, 11, 3432. [Google Scholar] [CrossRef]
- Maze, I.S.; Wright, G.A.; Mustard, J.A. Acute ethanol ingestion produces dose-dependent effects on motor behaviour in the honey bee (Apis mellifera). J. Insect Physiol. 2006, 52, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Ja, W.W. Absolute ethanol intake predicts ethanol preference in Drosophila melanogaster. J. Exp. Biol. 2020, 223, jeb224121. [Google Scholar] [CrossRef] [PubMed]
- Lathen, D.R.; Merrill, C.B.; Rothenfluh, A. Flying together: Drosophila as a tool to understand the genetics of human alcoholism. Int. J. Mol. Sci. 2020, 21, 6649. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.; Franke, K.; Fischer, K. Feeding on ripening and over-ripening fruit: Interactions between sugar, ethanol and polyphenol contents in a tropical butterfly. J. Exp. Biol. 2017, 220, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P.; et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Dufaÿ, M.; Hossaert–McKey, M.; Anstett, M.C. When leaves act like flowers: How dwarf palms attract their pollinators. Ecol. Lett. 2003, 6, 28–34. [Google Scholar] [CrossRef]
- Shohat-Ophir, G.; Kaun, K.R.; Azanchi, R.; Mohammed, H.; Heberlein, U. Sexual deprivation increases ethanol intake in Drosophila. Science 2012, 335, 1351–1355. [Google Scholar] [CrossRef] [Green Version]
- Nentwig, W. Non-webbuilding spiders: Prey specialists or generalists? Oecologia 1986, 69, 571–576. [Google Scholar] [CrossRef]
- Su, Q.; Qi, L.; Zhang, W.; Yun, Y.; Zhao, Y.; Peng, Y. Biodiversity survey of flower-visiting spiders based on literature review and field study. Environ. Entomol. 2020, 49, 673–682. [Google Scholar] [CrossRef]
- Cross, F.R.; Jackson, R.R. Odour-mediated response to plants by Evarcha culicivora, a blood-feeding jumping spider from East Africa. N. Z. J. Zool. 2009, 36, 75–80. [Google Scholar] [CrossRef]
- Witt, P.N.; Reed, C.F.; Peakall, D.B. A Spider’s Web: Problems in Regulatory Biology; Springer: New York, NY, USA, 1968. [Google Scholar]
- Japyassú, H.F.; Laland, K.N. Extended spider cognition. Anim. Cogn. 2017, 20, 375–395. [Google Scholar] [CrossRef] [Green Version]
- Namazi, H.R. The complexity based analysis of the correlation between spider’s brain signal and web. ARC J. Neurosci. 2017, 2, 38–44. [Google Scholar]
- Ledford, H. Drunken flies get hypersexual. Nature 2008. Available online: https://www.nature.com/articles/news.2007.402#citeas (accessed on 1 October 2022). [CrossRef]
- Lynch, Z.R.; Schlenke, T.A.; Morran, L.T.; De Roode, J.C. Ethanol confers differential protection against generalist and specialist parasitoids of Drosophila melanogaster. PLoS One 2017, 12, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, E.C.; Allouche, L.; Chapot, P.A.; Vranizan, K.; Moore, M.S.; Heberlein, U.; Wolf, F.W. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol. Clin. Exp. Res. 2010, 34, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Devineni, A.V.; McClure, K.; Guarnieri, D.; Corl, A.; Wolf, F.; Eddison, M.; Heberlein, U. The genetic relationships between ethanol preference, acute ethanol sensitivity, and ethanol tolerance in Drosophila melanogaster. Fly 2011, 5, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustard, J.A.; Oquita, R.; Garza, P.; Stoker, A. Honey bees (Apis mellifera) show a preference for the consumption of ethanol. Alcohol. Clin. Exp. Res. 2019, 43, 26–35. [Google Scholar] [CrossRef]


| Group Number | ETOH | Water |
|---|---|---|
| G1 | 9 | 5 |
| G2 | 15 | 16 |
| G3 | 8 | 8 |
| G4 | 13 | 6 |
| G5 | 19 | 8 |
| G6 | 11 | 4 |
| G7 | 9 | 3 |
| G8 | 6 | 3 |
| G9 | 9 | 2 |
| G10 | 10 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Li, Z.; Zhao, Y.; Liu, J.; Peng, Y. An Ant-Mimicking Jumping Spider Achieves Higher Predation Probability with Lower Success Rate When Exposed to Ethanol. Insects 2022, 13, 1009. https://doi.org/10.3390/insects13111009
Yu G, Li Z, Zhao Y, Liu J, Peng Y. An Ant-Mimicking Jumping Spider Achieves Higher Predation Probability with Lower Success Rate When Exposed to Ethanol. Insects. 2022; 13(11):1009. https://doi.org/10.3390/insects13111009
Chicago/Turabian StyleYu, Guocheng, Zichang Li, Yao Zhao, Jie Liu, and Yu Peng. 2022. "An Ant-Mimicking Jumping Spider Achieves Higher Predation Probability with Lower Success Rate When Exposed to Ethanol" Insects 13, no. 11: 1009. https://doi.org/10.3390/insects13111009






