Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Insects Culture
2.3. Chemical Synthesis of CuO NPs
2.4. Characterization of CuO NPs
2.5. Larvicidal Activity
2.6. Antifeedant Activity
2.7. Total Hemocyte Count (THC)
2.8. Acetylcholinesterase Assay
2.9. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy (SEM) Analysis
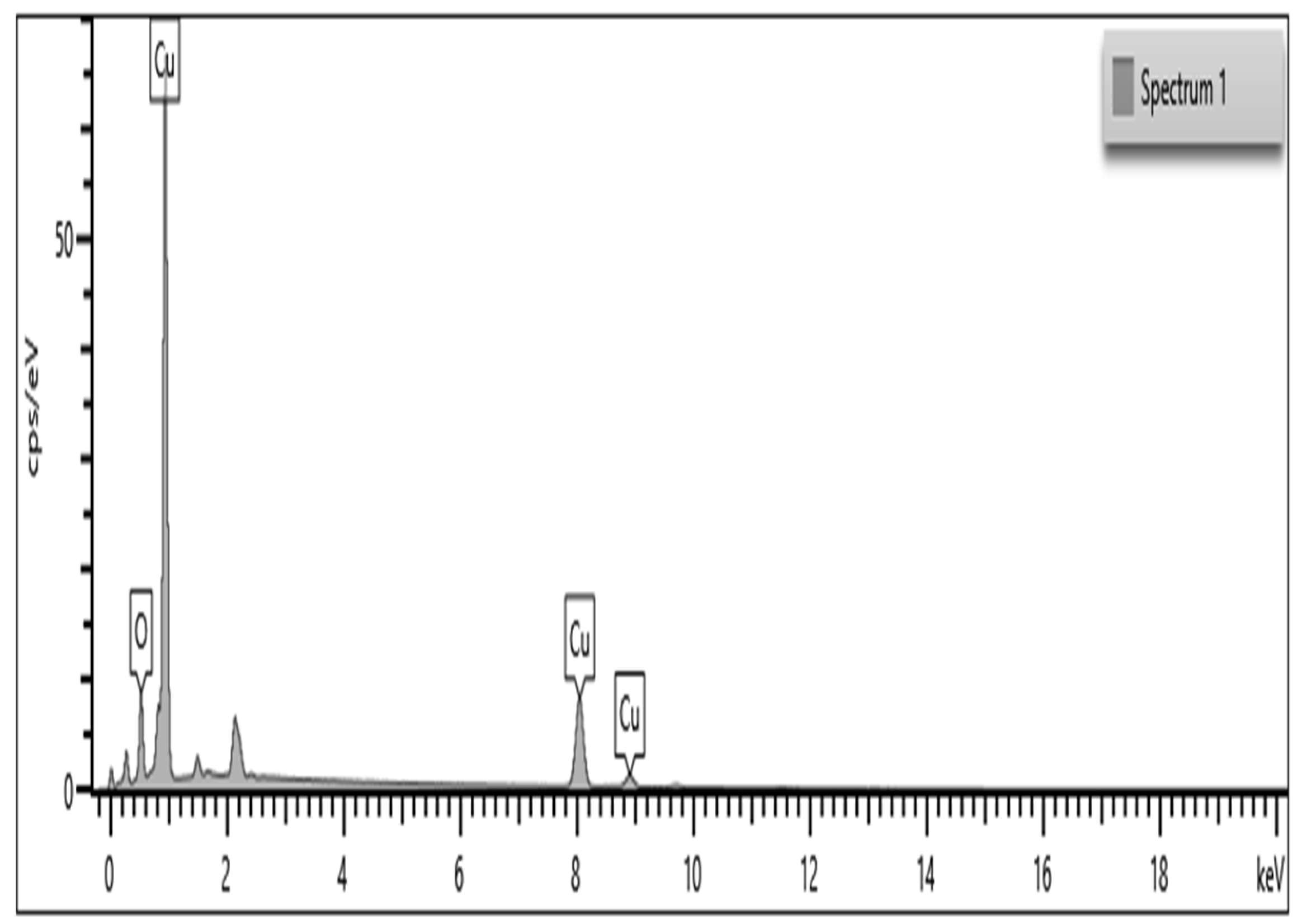
3.2. Energy Dispersive X-ray Spectroscopy
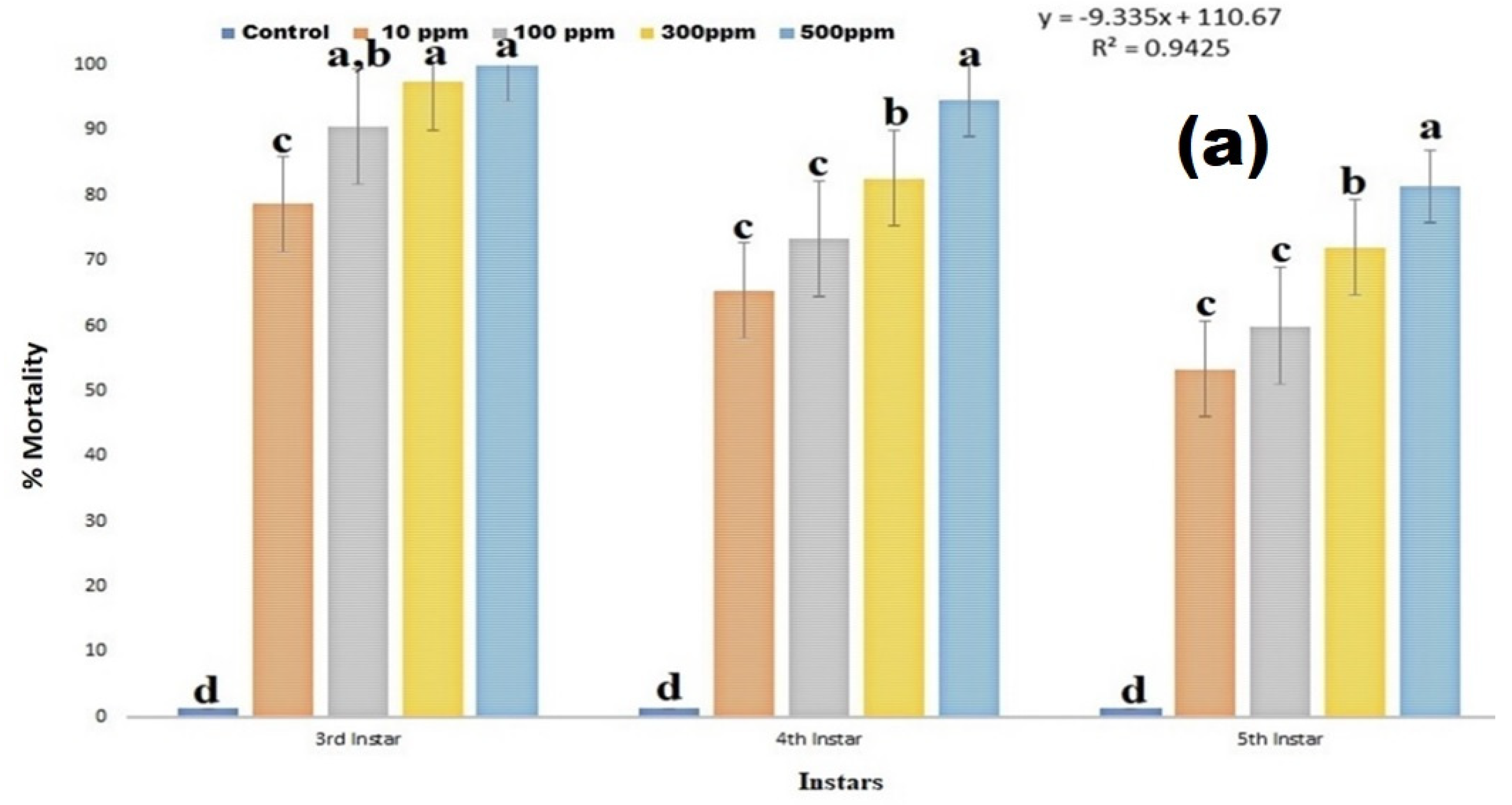
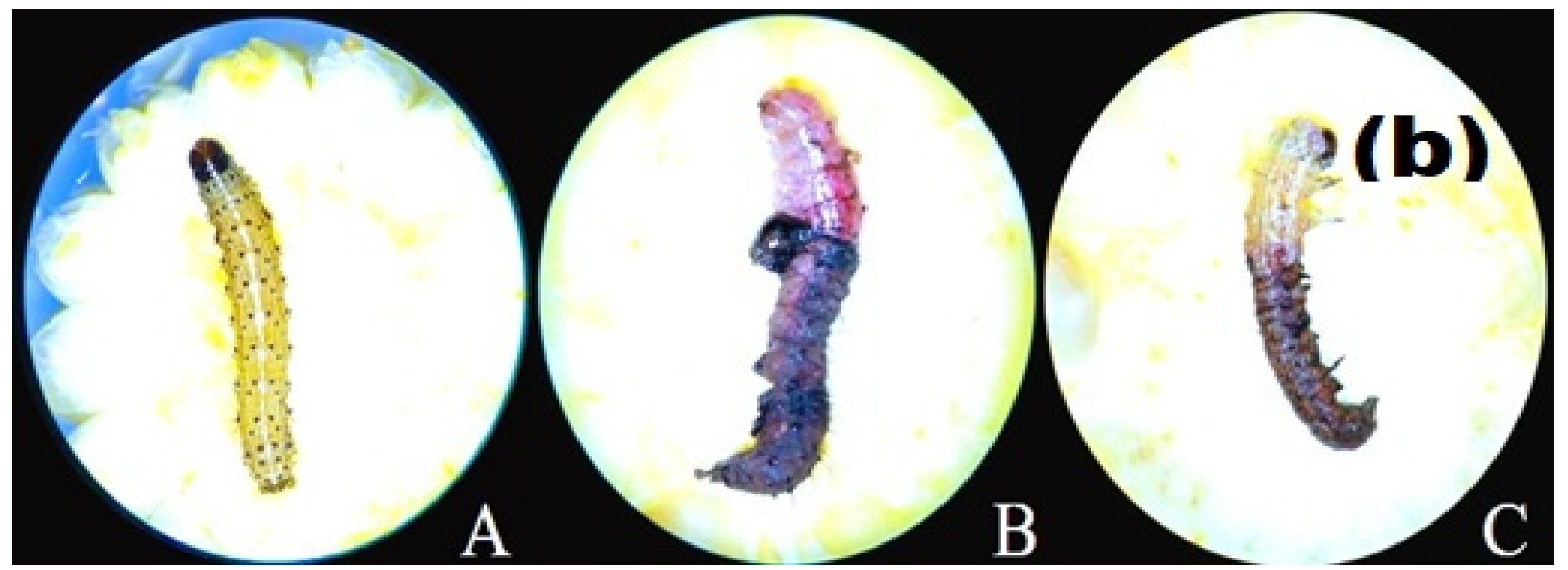
3.3. Larvicidal Effect
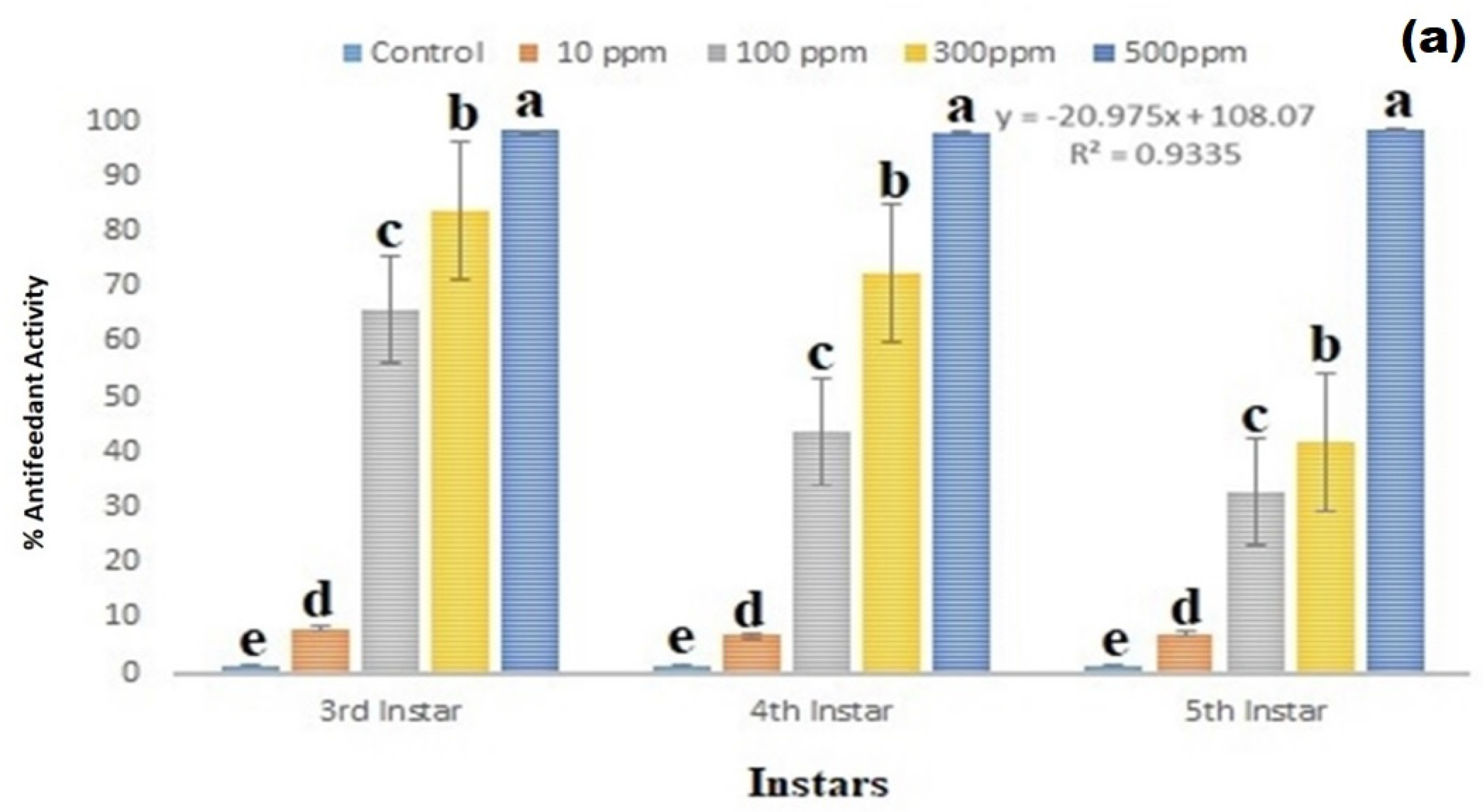
3.4. Antifeedant Effect
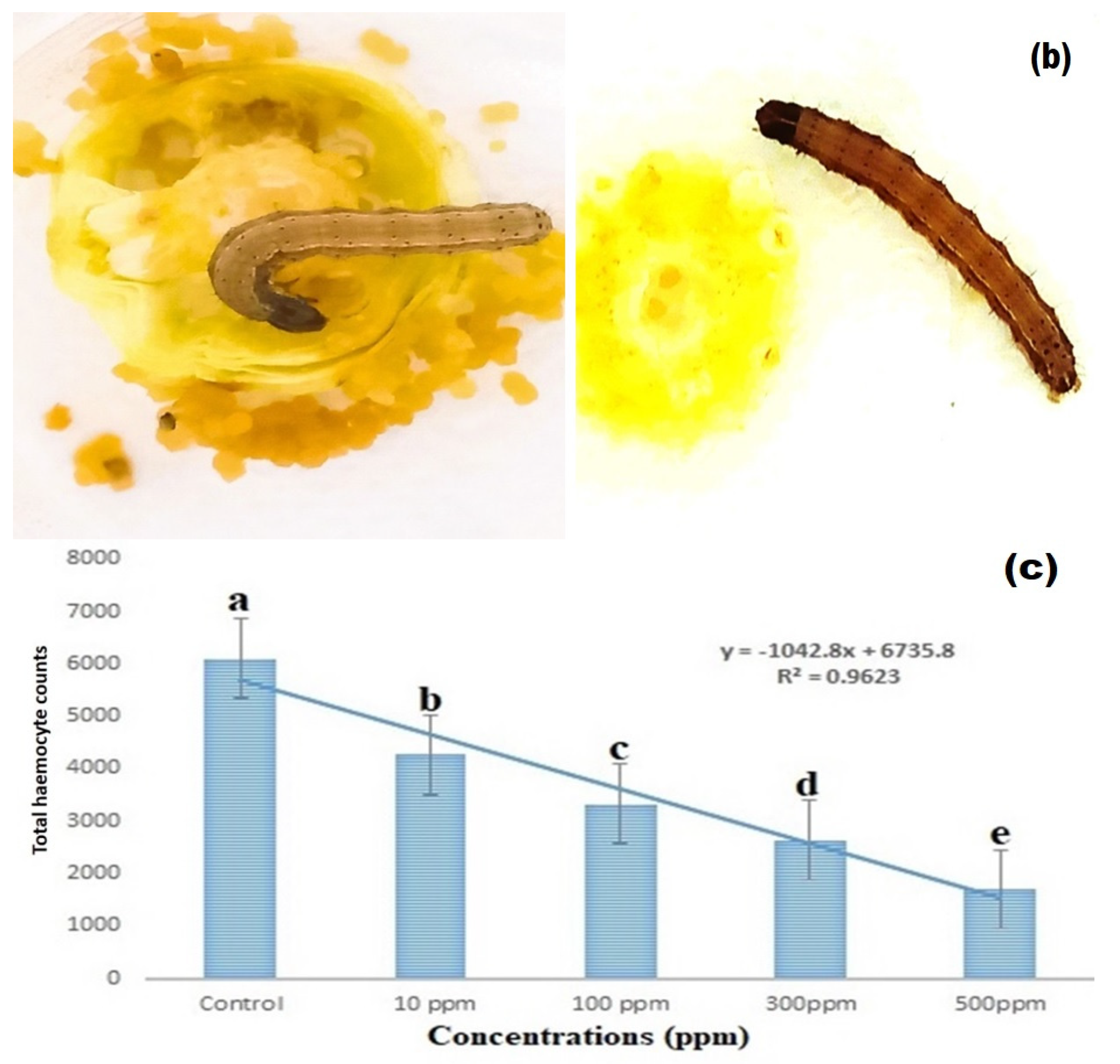
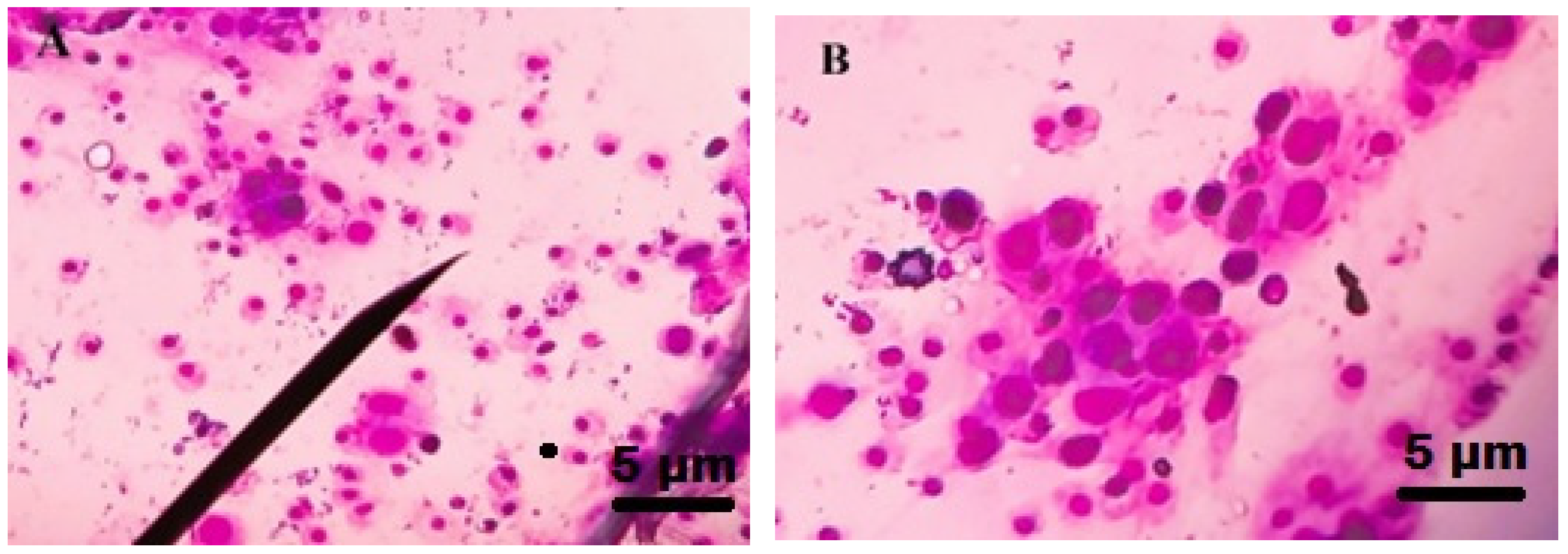
3.5. Total Haemocyte Count
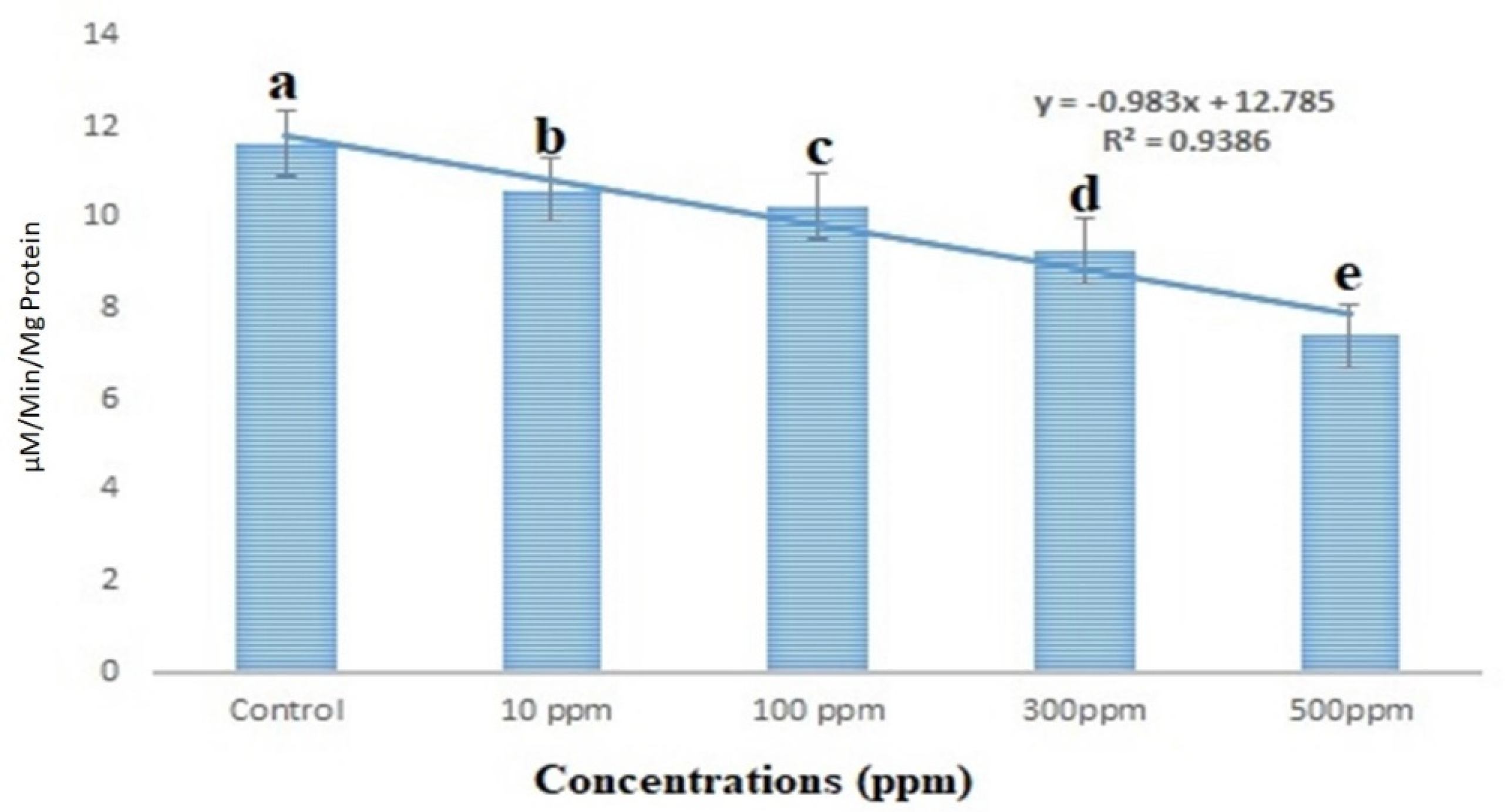
3.6. Acetylcholinesterase Enzyme
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosegrant, M.W.; Ringler, C.; Sulser, T.B.; Ewing, M.; Palazzo, A.; Zhu, T.; Nelson, G.C.; Koo, J.; Robertson, R.; Msangi, S.; et al. Agriculture and Food Security under Global Change: Prospects for 2025/2050; International Food Policy Research Institute: Washington, DC, USA, 2009; p. 80. [Google Scholar]
- Dubovskiy, I.M.; Whitten, M.M.A.; Yaroslavtseva, O.N.; Greig, C.; Kryukov, V.Y.; Grizanova, E.V.; Mukherjee, K.; Vilcinskas, A.; Glupov, V.V.; Butt, T.M. Can Insects Develop Resistance to Insect Pathogenic Fungi? PLoS ONE 2013, 8, e60248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D.; Xiao, Y.T.; Xu, P.J.; Yang, X.M.; Wu, Q.L.; Wu, K.M. Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J. Integr. Agric. 2021, 20, 783–791. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Thendralmanikandan, A.; Kweka, E.J.; Mahande, A.M. Resistance to temephos in Anopheles stephensi larvae is associated with increased cytochrome P450 and α-esterase genes overexpression. Int. J. Trop. Insect Sci. 2021, 41, 2543–2548. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. Int. J. Trop. Insect. Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Pogue, G.M. A world revision of the genus Spodoptera Guenee (Lepidoptera: Noctuidae). Mem. Am. Entomol. Soc. 2005, 43, 117–124. [Google Scholar]
- Nagoshi, R.N. Can the amount of corn acreage predict fall armyworm (Lepidoptera: Noctuidae) infestation levels in nearby cotton? J. Econ. Entomol. 2009, 102, 210–218. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Khooshe-Bast, Z.; Sahebzadeh, N.; Ghaffari-Moghaddam, M.; Mirshekar, A. Insecticidal effects of zinc oxide nanoparticles and Beauveria bassiana TS11 on Trialeurodes vaporariorum (Westwood, 1856) (Hemiptera: Aleyrodidae). Acta Agric. Slov. 2016, 107, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Clark, P.L.; Molina-Ochoa, J.; Martinelli, S.; Skoda, S.R.; Isenhr, D.J.; Lee, D.J.; Krumm, J.T.; Foster, J.E. Population variation of the fall armyworm, Spodoptera frugiperda, in the Western Hemisphere. J. Insect Sci. 2007, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Bateman, M.L.; Day, R.K.; Luke, B.; Edgington, S.; Kuhlmann, U.; Cock, M.J.W. Assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J. Appl. Entomol. 2018, 142, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- CABI. Spodoptera frugiperda (fall armyworm). In CABI: Invasive Species Compendium; CABI: Wallingford, UK, 2019. [Google Scholar]
- Nelly, N.; Lina, E.C.; Hamid, H.; Yunisman, Y. Distribution and genetic diversity of Spodoptera frugiperda JE Smith (Noctuidae: Lepidoptera) on maize in West Sumatra, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22. Available online: https://smujo.id/biodiv/article/view/7912 (accessed on 16 September 2022).
- Bahadur, L.C.; Bikram, A. Fall armyworm (Spodoptera frugiperda): A threat to food security for south Asian country: Control and management options: A review. Farming Manag. 2019, 4, 38–44. [Google Scholar] [CrossRef]
- Bhusal, S.; Chapagain, E. Threats of fall armyworm (Spodoptera frugiperda) incidence in Nepal and it’s integrated management-A review. Agric. Nat. Resour. 2020, 3, 345–359. [Google Scholar] [CrossRef]
- Hassan, A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Norris, E.J.; Johnson, J.B.; Gross, A.D.; Bartholomay, L.C.; Coats, J.R. Plant essential oils enhance diverse pyrethroids against multiple strains of mosquitoes and inhibit detoxification enzyme processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Azeem, M.; Zaman, T.; Tahir, M.; Haris, A.; Iqbal, Z.; Binyameen, M.; Nazir, A.; Shad, S.A.; Majeed, S.; Mozūraitis, R. Chemical composition and repellent activity of native plants essential oils against dengue mosquito, Aedes aegypti. Ind. Crops Prod. 2019, 140, 111609. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Johnson, E.J.; Rault, L.C.; Anderson, T.D. Vapor delivery of plant essential oils alters pyrethroid efficacy and detoxification enzyme activity in mosquitoes. Pestic. Biochem. Physiol. 2019, 157, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Usha-Raja-Nanthini, A.; Valli, G.; Subramanian, S.M. Comparative efficacy of Eucalyptus globulus (Labill) hydrodistilled essential oil and temephos as mosquito larvicide. Nat. Prod. Res. 2020, 34, 2626–2629. [Google Scholar] [CrossRef]
- Crow, J.F. Genetics of insect resistance to chemicals. Annu. Rev. Entomol. 1957, 2, 227–246. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop. Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Rani, P.U.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest. Sci. 2018, 91, 1–15. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, M.; Fan, C.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Development of novel ure-ase-responsive pendimethalin microcapsules using silica-IPTS-PEI as controlled release carrier materials. ACS Sustain. Chem. Eng. 2017, 5, 4802–4810. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Nanotechnology applications in pollution sensing and degradation in agriculture: A review. Environ. Chem. Lett. 2009, 7, 191–204. [Google Scholar] [CrossRef]
- Kumar, R.; Sharon, M.; Choudhary, A.K. Nanotechnology in agricultural diseases and food safety. J. Phytol. 2010, 2, 83–92. [Google Scholar]
- Sooresh, A.; Kwon, H.; Taylor, R.; Pietrantonio, P.; Pine, M.; Sayes, C.M. Surface functionalization of silver nanoparticles: Novel applications for insect vector control. ACS. Appl. Mater. Interfaces 2011, 3, 3779–3787. [Google Scholar] [CrossRef]
- Barik, T.K.; Kamaraju, R.; Gowswami, A. Silica nanoparticle: A potential new insecticide for mosquito vector control. Parasitol Res. 2012, 111, 1075–1083. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, B.; Li, F.; Ma, L.; Ni, M.; Shen, W.; Hong, F.; Li, B. Molecular mechanisms of reduced nerve toxicity by titanium dioxide nanoparticles in the phoxim-exposed brain of Bombyx mori. PLoS ONE 2014, 9, 101062. [Google Scholar] [CrossRef] [Green Version]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Nicoletti, M.; Madhiyazhagan, P.; Dinesh, D.; Suresh, U.; Khater, H.F.; Wei, H.; et al. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 2015, 121, 31–38. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Deepa, S.; Kweka, E.J.; Shivakumar, M.S. Toxicity of Fusarium oxysporum-VKFO-01 derived silver nano-particles as potential inseciticide against three mosquito vector species (Diptera: Culicidae). J. Clust. Sci. 2018, 29, 1139–1149. [Google Scholar] [CrossRef]
- Parthiban, E.; Ramachandran, M.; Jayakumar, M.; Ramanibai, R. Biocompatible green synthesized silver nanoparticles impact on insecticides resistant developing enzymes of dengue transmitted mosquito vector. SN Appl. Sci. 2019, 1, 1282. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Lukehart, C.M. Applications of green-synthesized nanoparticles in pharmacology, parasitology and entomology. J. Clust. Sci. 2017, 28, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Banumathi, B.; Vaseeharan, B.; Rajasekar, P.; Prabhu, N.M.; Ramasamy, P.; Murugan, K.; Canale, A.; Benelli, G. Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick, Rhipicephalus (Boophilus) microplus—A review. Vet. Parasitol. 2017, 244, 102–110. [Google Scholar] [CrossRef]
- Benelli, G. Gold nanoparticles–against parasites and insect vectors. Acta Trop. 2018, 178, 73–80. [Google Scholar] [CrossRef]
- Amerasan, D.; Nataraj, T.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Nicoletti, M.; Benelli, G. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J. Pest Sci. 2016, 89, 249–256. [Google Scholar] [CrossRef]
- Shaker, A.M.; Zaki, A.H.; Abdel-Rahim, E.H.F.; Khedr, M.H. Photocatalytic Degradation of Carbamate Pesticide (methomyl) Using Synthesized TiO2 Nanoparticles against the Cotton Leafworm S. littoralis. Egypt. Acad. J. Biol. Sci. 2017, 9, 157–168. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Jones, J.C. Current concepts concerning insect hemocytes. Am. Zool. 1962, 2, 209–246. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Poopathi, S.; De Britto, L.J.; Praba, V.L.; Mani, C.; Praveen, M. Synthesis of silver nanoparticles from Azadirachta indica—A most effective method for mosquito control. Environ. Sci. Pollut. Res. 2015, 22, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: Discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Yamanaka, Y.J.; Leong, K.W. Engineering strategies to enhance nanoparticle-mediated oral delivery. J. Biomater. Sci. Polym. Ed. 2008, 19, 1549–1570. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D. Nanotoxicology: Laying a firm foundation for sustainable nanotechnologies. In Nanotoxicology; CRC Press: Boca Raton, FL, USA, 2007; pp. 17–22. [Google Scholar]
- Debnath, N.; Das, S.; Seth, D.; Chandra, R.; Bhattacharya, S.C.; Goswami, A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J. Pest. Sci. 2011, 84, 99–105. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal efficacy of microbial-mediated synthesized copper nano-pesticide against insect pests and non-target organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Stadler, T.; Buteler, M.; Weaver, D.K.; Sofie, S. Comparative toxicity of nanostructured alumina and a commercial inert dust for Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) at varying ambient humidity levels. J. Stored Prod. Res. 2012, 48, 81–90. [Google Scholar] [CrossRef]
- Ki, H.Y.; Kim, J.H.; Kwon, S.C.; Jeong, S.H. A study on multifunctional wool textiles treated with nano-sized silver. J. Mater. Sci. 2007, 42, 8020–8024. [Google Scholar] [CrossRef]
- Yasur, J.; Rani, P.U. Lepidopteran insect susceptibility to silver nanoparticles and measurement of changes in their growth, development and physiology. Chemosphere 2015, 124, 92–102. [Google Scholar] [CrossRef]
- Murugan, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasit. 2015, 153, 129–138. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Bedini, S.; Shivakumar, M.S. Isolation and identification of entomopathogenic fungus from Eastern Ghats of South Indian forest soil and their efficacy as biopesticide for mosquito control. Parasitol. Int. 2020, 76, 102099. [Google Scholar] [CrossRef]
- Rouhani, M.; Samih, M.A.; Kalantari, S. Insecticied effect of silver and zinc nanoparticles against Aphis nerii Boyer of fonsco-lombe (Hemiptera: Aphididae). Chil. J. Agric. Res. 2012, 72, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Raykar, V.S. Microwave synthesis of silver nanofluids with polyvinylpyrrolidone (PVP) and their transport prop-erties. Coll. Polym. Sci. 2008, 286, 1667–1673. [Google Scholar] [CrossRef]
- Gintenreiter, S.; Ortel, J.; Nopp, H.J. Bioaccumulation of cadmium, lead, copper, and zinc in successive developmental stages of Lymantria dispar L. (Lymantriidae, Lepid)—A life cycle study. Arch. Environ. Contam. Toxicol. 1993, 25, 55–61. [Google Scholar] [CrossRef]
- Kramarz, P.; Kafel, A. The respiration rate of the beet armyworm pupae (Spodoptera exigua) after multigeneration intoxication with cadmium and zinc. Environ. Pollut. 2003, 126, 1–3. [Google Scholar] [CrossRef]
- Stone, D.; Jepson, P.; Laskowski, R. Trends in detoxification enzymes and heavy metal accumulation in ground beetles (Cole-optera: Carabidae) inhabiting a gradient of pollution. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 105–112. [Google Scholar] [CrossRef]
- Ballan-Dufrancais, C. Localization of metals in cells of pterygote insects. Microsc. Res. Tech. 2002, 56, 403–420. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Karthi, S.; Shivakumar, M.S.; Benelli, G. Synergistic effect of entomopathogenic fungus Fusarium oxysporum extract in combination with temephos against three major mosquito vectors. Pathog. Glob. Health 2018, 112, 37–46. [Google Scholar] [CrossRef]
- Pigino, G.; Migliorini, M.; Paccagnini, E.; Bernini, F.; Leonzio, C. Fine structure of the midgut and Malpighian papillae in Campodea (Monocampa) quilisi Silvestri, 1932 (Hexapoda, Diplura) with special reference to the metal composition and physi-ological significance of midgut intracellular electron-densegranules. Tissue Cell 2005, 37, 223–232. [Google Scholar] [CrossRef]
- Thabet, A.F.; Boraei, H.A.; Galal, O.A.; El-Samahy, M.F.; Mousa, K.M.; Zhang, Y.Z.; Tuda, M.; Helmy, E.A.; Wen, J.; Nozaki, T. Silica nanoparticles as pesticide against insects of different feeding types and their non-target attraction of predators. Sci. Rep. 2021, 11, 14484. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Alford, L.; Pittarate, S.; Subala, S.P.R.R.; Mekchay, S.; Elangovan, D.; Krutmuang, P. Toxicity of Metarhizium flavoviride conidia virulence against Spodoptera litura (Lepidoptera: Noctuidae) and its impact on physiological and biochemical activities. Sci. Rep. 2022, 12, 16775. [Google Scholar] [CrossRef] [PubMed]
- Anreddy, R.N.R. Copper oxide nanoparticles induces oxidative stress and liver toxicity in rats following oral exposure. Toxicol. Rep. 2018, 5, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Assadian, E.; Zarei, M.H.; Gilani, A.G.; Farshin, M.; Degampanah, H.; Pourahmad, J. Toxicity of copper oxide (CuO) nanoparticles on human blood lymphocytes. Biol. Trace Elem. Res. 2018, 184, 350–357. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Pittarate, S.; Perumal, V.; Rajula, J.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response. Insects 2022, 13, 1030. https://doi.org/10.3390/insects13111030
Rahman A, Pittarate S, Perumal V, Rajula J, Thungrabeab M, Mekchay S, Krutmuang P. Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response. Insects. 2022; 13(11):1030. https://doi.org/10.3390/insects13111030
Chicago/Turabian StyleRahman, Afroja, Sarayut Pittarate, Vivekanandhan Perumal, Julius Rajula, Malee Thungrabeab, Supamit Mekchay, and Patcharin Krutmuang. 2022. "Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response" Insects 13, no. 11: 1030. https://doi.org/10.3390/insects13111030





